
History of quantum mechanics
Encyclopedia
The history of quantum mechanics
, as it interlaces with the history of quantum chemistry
, began essentially with a number of different scientific discoveries: the 1838 discovery of cathode rays by Michael Faraday
; the 1859-1860 winter statement of the black body radiation problem by Gustav Kirchhoff
; the 1877 suggestion by Ludwig Boltzmann
that the energy states of a physical system could be discrete; the discovery of the photoelectric effect
by Heinrich Hertz in 1887; and the 1900 quantum hypothesis by Max Planck
that any energy-radiating atomic system can theoretically be divided into a number of discrete "energy elements" ε (epsilon
) such that each of these energy elements is proportional to the frequency
ν with which each of them individually radiate energy
, as defined by the following formula:
where h is a numerical value called Planck's constant.
Then, Albert Einstein
in 1905, in order to explain the photoelectric effect
previously reported by Heinrich Hertz in 1887, postulated consistently with Max Planck's quantum hypothesis that light
itself is made of individual quantum particles, which in 1926 came to be called photons by Gilbert N. Lewis. The photoelectric effect was observed upon shining light of particular wavelengths on certain materials, such as metals, which caused electrons to be ejected from those materials only if the light quantum energy was greater than the Fermi level (work function
) in the metal.
together with the mathematicians Gustav von Escherich
and Emil Müller
. Boltzmann's rationale for the presence of discrete energy levels in molecules such as those of iodine gas had its origins in his statistical thermodynamics and statistical mechanics
theories, and was backed up by mathematical arguments, as it will also be the case twenty years later with the first quantum theory
put forward by Max Planck
.
Thus, in 1900, the German physicist Max Planck reluctantly introduced the idea that energy is quantized, to derive a formula for the observed frequency dependence of the energy emitted by a black body
, called Planck's Law, that included a Boltzmann distribution
(applicable in the classical limit). Planck's law can be stated as follows: where:
where:
The earlier Wien approximation may be derived from Planck's law by assuming .
.
Moreover, the application of Planck's quantum theory to the electron allowed Ștefan Procopiu
in 1911—1913, and subsequently Niels Bohr
in 1913, to calculate the magnetic moment
of the electron, which was later called the ``magneton"; similar quantum computations, but with numerically quite different values, were subsequently made possible for both the magnetic moments of the proton
and the neutron
that are three orders of magnitude smaller than that of the electron.
In 1905, Einstein explained the photoelectric effect
by postulating that light, or more generally all electromagnetic radiation
, can be divided into a finite number of "energy quanta" that are localized points in space. From the introduction section of his March 1905 quantum paper, “On a heuristic viewpoint concerning the emission and transformation of light”, Einstein states:
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
, as it interlaces with the history of quantum chemistry
Quantum chemistry
Quantum chemistry is a branch of chemistry whose primary focus is the application of quantum mechanics in physical models and experiments of chemical systems...
, began essentially with a number of different scientific discoveries: the 1838 discovery of cathode rays by Michael Faraday
Michael Faraday
Michael Faraday, FRS was an English chemist and physicist who contributed to the fields of electromagnetism and electrochemistry....
; the 1859-1860 winter statement of the black body radiation problem by Gustav Kirchhoff
Gustav Kirchhoff
Gustav Robert Kirchhoff was a German physicist who contributed to the fundamental understanding of electrical circuits, spectroscopy, and the emission of black-body radiation by heated objects...
; the 1877 suggestion by Ludwig Boltzmann
Ludwig Boltzmann
Ludwig Eduard Boltzmann was an Austrian physicist famous for his founding contributions in the fields of statistical mechanics and statistical thermodynamics...
that the energy states of a physical system could be discrete; the discovery of the photoelectric effect
Photoelectric effect
In the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons...
by Heinrich Hertz in 1887; and the 1900 quantum hypothesis by Max Planck
Max Planck
Max Karl Ernst Ludwig Planck, ForMemRS, was a German physicist who actualized the quantum physics, initiating a revolution in natural science and philosophy. He is regarded as the founder of the quantum theory, for which he received the Nobel Prize in Physics in 1918.-Life and career:Planck came...
that any energy-radiating atomic system can theoretically be divided into a number of discrete "energy elements" ε (epsilon
Epsilon
Epsilon is the fifth letter of the Greek alphabet, corresponding phonetically to a close-mid front unrounded vowel . In the system of Greek numerals it has a value of 5. It was derived from the Phoenician letter He...
) such that each of these energy elements is proportional to the frequency
Frequency
Frequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
ν with which each of them individually radiate energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
, as defined by the following formula:

where h is a numerical value called Planck's constant.
Then, Albert Einstein
Albert Einstein
Albert Einstein was a German-born theoretical physicist who developed the theory of general relativity, effecting a revolution in physics. For this achievement, Einstein is often regarded as the father of modern physics and one of the most prolific intellects in human history...
in 1905, in order to explain the photoelectric effect
Photoelectric effect
In the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons...
previously reported by Heinrich Hertz in 1887, postulated consistently with Max Planck's quantum hypothesis that light
Light
Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
itself is made of individual quantum particles, which in 1926 came to be called photons by Gilbert N. Lewis. The photoelectric effect was observed upon shining light of particular wavelengths on certain materials, such as metals, which caused electrons to be ejected from those materials only if the light quantum energy was greater than the Fermi level (work function
Work function
In solid-state physics, the work function is the minimum energy needed to remove an electron from a solid to a point immediately outside the solid surface...
) in the metal.


- The phrase "quantum mechanics" was first used in Max BornMax BornMax Born was a German-born physicist and mathematician who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics and supervised the work of a number of notable physicists in the 1920s and 30s...
's 1924 paper "Zur Quantenmechanik". In the years to follow, this theoretical basis slowly began to be applied to chemical structureChemical structureA chemical structure includes molecular geometry, electronic structure and crystal structure of molecules. Molecular geometry refers to the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together. Molecular geometry can range from the very simple, such as...
, reactivity, and bonding.
Overview
In short, Ludwig Eduard Boltzmann was one of the founders of quantum mechanics because he suggested in 1877 that the energy levels of a physical system, such as a molecule, could be discrete. He was also a founder of the Austrian Mathematical SocietyAustrian Mathematical Society
The Austrian Mathematical Society is the national mathematical society of Austria and a member society of the European Mathematical Society.-History:...
together with the mathematicians Gustav von Escherich
Gustav von Escherich
Gustav Ritter von Escherich was an Austrian mathematician.-Biography:Born in Mantua, he studied mathematics and physics at the University of Vienna. From 1876 to 1879 he was professor at the University of Graz...
and Emil Müller
Emil Müller
-Biography:Born in Lanškroun, he studied mathematics and physics at the University of Vienna and Vienna University of Technology. In 1898 he defended his dissertation at the University of Königsberg with Wilhelm Franz Meyer. One year later he received his habilitation at the same university...
. Boltzmann's rationale for the presence of discrete energy levels in molecules such as those of iodine gas had its origins in his statistical thermodynamics and statistical mechanics
Statistical mechanics
Statistical mechanics or statistical thermodynamicsThe terms statistical mechanics and statistical thermodynamics are used interchangeably...
theories, and was backed up by mathematical arguments, as it will also be the case twenty years later with the first quantum theory
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
put forward by Max Planck
Max Planck
Max Karl Ernst Ludwig Planck, ForMemRS, was a German physicist who actualized the quantum physics, initiating a revolution in natural science and philosophy. He is regarded as the founder of the quantum theory, for which he received the Nobel Prize in Physics in 1918.-Life and career:Planck came...
.
Thus, in 1900, the German physicist Max Planck reluctantly introduced the idea that energy is quantized, to derive a formula for the observed frequency dependence of the energy emitted by a black body
Black body
A black body is an idealized physical body that absorbs all incident electromagnetic radiation. Because of this perfect absorptivity at all wavelengths, a black body is also the best possible emitter of thermal radiation, which it radiates incandescently in a characteristic, continuous spectrum...
, called Planck's Law, that included a Boltzmann distribution
Boltzmann distribution
In chemistry, physics, and mathematics, the Boltzmann distribution is a certain distribution function or probability measure for the distribution of the states of a system. It underpins the concept of the canonical ensemble, providing its underlying distribution...
(applicable in the classical limit). Planck's law can be stated as follows:
 where:
where:- I(ν,T) is the energyEnergyIn physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
per unit timeTimeTime is a part of the measuring system used to sequence events, to compare the durations of events and the intervals between them, and to quantify rates of change such as the motions of objects....
(or the powerPower (physics)In physics, power is the rate at which energy is transferred, used, or transformed. For example, the rate at which a light bulb transforms electrical energy into heat and light is measured in watts—the more wattage, the more power, or equivalently the more electrical energy is used per unit...
) radiated per unit area of emitting surface in the normalSurface normalA surface normal, or simply normal, to a flat surface is a vector that is perpendicular to that surface. A normal to a non-flat surface at a point P on the surface is a vector perpendicular to the tangent plane to that surface at P. The word "normal" is also used as an adjective: a line normal to a...
direction per unit solid angleSolid angleThe solid angle, Ω, is the two-dimensional angle in three-dimensional space that an object subtends at a point. It is a measure of how large that object appears to an observer looking from that point...
per unit frequencyFrequencyFrequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
by a black body at temperature T; - h is the Planck constantPlanck constantThe Planck constant , also called Planck's constant, is a physical constant reflecting the sizes of energy quanta in quantum mechanics. It is named after Max Planck, one of the founders of quantum theory, who discovered it in 1899...
; - c is the speed of lightSpeed of lightThe speed of light in vacuum, usually denoted by c, is a physical constant important in many areas of physics. Its value is 299,792,458 metres per second, a figure that is exact since the length of the metre is defined from this constant and the international standard for time...
in a vacuum; - k is the Boltzmann constant;
- ν is the frequencyFrequencyFrequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
of the electromagnetic radiation; and T is the temperatureTemperatureTemperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
of the body in degrees KelvinKelvinThe kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
.
The earlier Wien approximation may be derived from Planck's law by assuming
 .
.Moreover, the application of Planck's quantum theory to the electron allowed Ștefan Procopiu
Stefan Procopiu
-Biography:Procopiu was born in 1890 in Bârlad, Romania. His father, Emanoil Procopiu, was employed at the Bârlad courthouse. His mother, Ecaterina Tașcă was the daughter of Gheorghe I...
in 1911—1913, and subsequently Niels Bohr
Niels Bohr
Niels Henrik David Bohr was a Danish physicist who made foundational contributions to understanding atomic structure and quantum mechanics, for which he received the Nobel Prize in Physics in 1922. Bohr mentored and collaborated with many of the top physicists of the century at his institute in...
in 1913, to calculate the magnetic moment
Magnetic moment
The magnetic moment of a magnet is a quantity that determines the force that the magnet can exert on electric currents and the torque that a magnetic field will exert on it...
of the electron, which was later called the ``magneton"; similar quantum computations, but with numerically quite different values, were subsequently made possible for both the magnetic moments of the proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
and the neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
that are three orders of magnitude smaller than that of the electron.
| Photoelectric effect | |
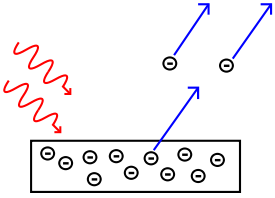 |
|
| The photoelectric effect Photoelectric effect In the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons... reported by Heinrich Hertz in 1887, |
|
| and explained by Albert Einstein Albert Einstein Albert Einstein was a German-born theoretical physicist who developed the theory of general relativity, effecting a revolution in physics. For this achievement, Einstein is often regarded as the father of modern physics and one of the most prolific intellects in human history... in 1905. |
|
| Low-energy phenomena: Photoelectric effect Photoelectric effect In the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons... |
|
| Mid-energy phenomena: Compton scattering Compton scattering In physics, Compton scattering is a type of scattering that X-rays and gamma rays undergo in matter. The inelastic scattering of photons in matter results in a decrease in energy of an X-ray or gamma ray photon, called the Compton effect... |
|
| High-energy phenomena: Pair production Pair production Pair production refers to the creation of an elementary particle and its antiparticle, usually from a photon . For example an electron and its antiparticle, the positron, may be created... |
|
In 1905, Einstein explained the photoelectric effect
Photoelectric effect
In the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons...
by postulating that light, or more generally all electromagnetic radiation
Electromagnetic radiation
Electromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space...
, can be divided into a finite number of "energy quanta" that are localized points in space. From the introduction section of his March 1905 quantum paper, “On a heuristic viewpoint concerning the emission and transformation of light”, Einstein states:
This statement has been called the most revolutionary sentence written by a physicist of the twentieth century. These energy quanta later came to be called "photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
s", a term introduced by Gilbert N. Lewis
Gilbert N. Lewis
Gilbert Newton Lewis was an American physical chemist known for the discovery of the covalent bond , his purification of heavy water, his reformulation of chemical thermodynamics in a mathematically rigorous manner accessible to ordinary chemists, his theory of Lewis acids and...
in 1926. The idea that each photon had to consist of energy in terms of quanta was a remarkable achievement; it effectively solved the problem of black body radiation attaining infinite energy
Ultraviolet catastrophe
The ultraviolet catastrophe, also called the Rayleigh–Jeans catastrophe, was a prediction of late 19th century/early 20th century classical physics that an ideal black body at thermal equilibrium will emit radiation with infinite power....
, which occurred in theory if light were to be explained only in terms of waves. In 1913, Bohr explained the spectral line
Spectral line
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from a deficiency or excess of photons in a narrow frequency range, compared with the nearby frequencies.- Types of line spectra :...
s of the hydrogen atom
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively-charged proton and a single negatively-charged electron bound to the nucleus by the Coulomb force...
, again by using quantization, in his paper of July 1913 On the Constitution of Atoms and Molecules.
These theories, though successful, were strictly phenomenological
Phenomenology (science)
The term phenomenology in science is used to describe a body of knowledge that relates empirical observations of phenomena to each other, in a way that is consistent with fundamental theory, but is not directly derived from theory. For example, we find the following definition in the Concise...
: during this time, there was no rigorous justification for quantization
Quantization (physics)
In physics, quantization is the process of explaining a classical understanding of physical phenomena in terms of a newer understanding known as "quantum mechanics". It is a procedure for constructing a quantum field theory starting from a classical field theory. This is a generalization of the...
, aside, perhaps, from Henri Poincaré
Henri Poincaré
Jules Henri Poincaré was a French mathematician, theoretical physicist, engineer, and a philosopher of science...
's discussion of Planck's theory in his 1912 paper Sur la théorie des quanta. They are collectively known as the old quantum theory.
The phrase "quantum physics" was first used in Johnston's Planck's Universe in Light of Modern Physics (1931).
In 1924, the French physicist Louis de Broglie put forward his theory of matter waves by stating that particles can exhibit wave characteristics and vice versa. This theory was for a single particle and derived from special relativity theory
Special relativity
Special relativity is the physical theory of measurement in an inertial frame of reference proposed in 1905 by Albert Einstein in the paper "On the Electrodynamics of Moving Bodies".It generalizes Galileo's...
. Building on de Broglie's approach, modern quantum mechanics was born in 1925, when the German physicists Werner Heisenberg
Werner Heisenberg
Werner Karl Heisenberg was a German theoretical physicist who made foundational contributions to quantum mechanics and is best known for asserting the uncertainty principle of quantum theory...
and Max Born
Max Born
Max Born was a German-born physicist and mathematician who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics and supervised the work of a number of notable physicists in the 1920s and 30s...
developed matrix mechanics
Matrix mechanics
Matrix mechanics is a formulation of quantum mechanics created by Werner Heisenberg, Max Born, and Pascual Jordan in 1925.Matrix mechanics was the first conceptually autonomous and logically consistent formulation of quantum mechanics. It extended the Bohr Model by describing how the quantum jumps...
and the Austrian physicist Erwin Schrödinger
Erwin Schrödinger
Erwin Rudolf Josef Alexander Schrödinger was an Austrian physicist and theoretical biologist who was one of the fathers of quantum mechanics, and is famed for a number of important contributions to physics, especially the Schrödinger equation, for which he received the Nobel Prize in Physics in 1933...
invented wave mechanics and the non-relativistic Schrödinger equation as an approximation to the generalised case of de Broglie's theory. Schrödinger subsequently showed that the two approaches were equivalent.
Heisenberg formulated his uncertainty principle
Uncertainty principle
In quantum mechanics, the Heisenberg uncertainty principle states a fundamental limit on the accuracy with which certain pairs of physical properties of a particle, such as position and momentum, can be simultaneously known...
in 1927, and the Copenhagen interpretation
Copenhagen interpretation
The Copenhagen interpretation is one of the earliest and most commonly taught interpretations of quantum mechanics. It holds that quantum mechanics does not yield a description of an objective reality but deals only with probabilities of observing, or measuring, various aspects of energy quanta,...
started to take shape at about the same time. Starting around 1927, Paul Dirac
Paul Dirac
Paul Adrien Maurice Dirac, OM, FRS was an English theoretical physicist who made fundamental contributions to the early development of both quantum mechanics and quantum electrodynamics...
began the process of unifying quantum mechanics with special relativity
Special relativity
Special relativity is the physical theory of measurement in an inertial frame of reference proposed in 1905 by Albert Einstein in the paper "On the Electrodynamics of Moving Bodies".It generalizes Galileo's...
by proposing the Dirac equation
Dirac equation
The Dirac equation is a relativistic quantum mechanical wave equation formulated by British physicist Paul Dirac in 1928. It provided a description of elementary spin-½ particles, such as electrons, consistent with both the principles of quantum mechanics and the theory of special relativity, and...
for the electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
. The Dirac equation
Dirac equation
The Dirac equation is a relativistic quantum mechanical wave equation formulated by British physicist Paul Dirac in 1928. It provided a description of elementary spin-½ particles, such as electrons, consistent with both the principles of quantum mechanics and the theory of special relativity, and...
achieves the relativistic description of the wavefunction of an electron that Schrödinger failed to obtain. It predicts electron spin and led Dirac to predict the existence of the positron
Positron
The positron or antielectron is the antiparticle or the antimatter counterpart of the electron. The positron has an electric charge of +1e, a spin of ½, and has the same mass as an electron...
. He also pioneered the use of operator theory, including the influential bra-ket notation
Bra-ket notation
Bra-ket notation is a standard notation for describing quantum states in the theory of quantum mechanics composed of angle brackets and vertical bars. It can also be used to denote abstract vectors and linear functionals in mathematics...
, as described in his famous 1930 textbook. During the same period, Hungarian polymath John von Neumann
John von Neumann
John von Neumann was a Hungarian-American mathematician and polymath who made major contributions to a vast number of fields, including set theory, functional analysis, quantum mechanics, ergodic theory, geometry, fluid dynamics, economics and game theory, computer science, numerical analysis,...
formulated the rigorous mathematical basis for quantum mechanics as the theory of linear operators on Hilbert spaces, as described in his likewise famous 1932 textbook. These, like many other works from the founding period still stand, and remain widely used.
The field of quantum chemistry
Quantum chemistry
Quantum chemistry is a branch of chemistry whose primary focus is the application of quantum mechanics in physical models and experiments of chemical systems...
was pioneered by physicists Walter Heitler
Walter Heitler
Walter Heinrich Heitler was a German physicist who made contributions to quantum electrodynamics and quantum field theory...
and Fritz London
Fritz London
Fritz Wolfgang London was a German theoretical physicist. His fundamental contributions to the theories of chemical bonding and of intermolecular forces are today considered classic and are discussed in standard textbooks of physical chemistry.With his brother Heinz, he made a significant...
, who published a study of the covalent bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
of the hydrogen molecule in 1927. Quantum chemistry was subsequently developed by a large number of workers, including the American theoretical chemist Linus Pauling
Linus Pauling
Linus Carl Pauling was an American chemist, biochemist, peace activist, author, and educator. He was one of the most influential chemists in history and ranks among the most important scientists of the 20th century...
at Caltech, and John C. Slater
John C. Slater
John Clarke Slater was a noted American physicist who made major contributions to the theory of the electronic structure of atoms, molecules and solids. This work is of ongoing importance in chemistry, as well as in many areas of physics. He also made major contributions to microwave electronics....
into various theories such as Molecular Orbital Theory or Valence Theory.
Beginning in 1927, attempts were made to apply quantum mechanics to fields rather than single particles, resulting in what are known as quantum field theories
Quantum field theory
Quantum field theory provides a theoretical framework for constructing quantum mechanical models of systems classically parametrized by an infinite number of dynamical degrees of freedom, that is, fields and many-body systems. It is the natural and quantitative language of particle physics and...
. Early workers in this area included P.A.M. Dirac
Paul Dirac
Paul Adrien Maurice Dirac, OM, FRS was an English theoretical physicist who made fundamental contributions to the early development of both quantum mechanics and quantum electrodynamics...
, W. Pauli
Wolfgang Pauli
Wolfgang Ernst Pauli was an Austrian theoretical physicist and one of the pioneers of quantum physics. In 1945, after being nominated by Albert Einstein, he received the Nobel Prize in Physics for his "decisive contribution through his discovery of a new law of Nature, the exclusion principle or...
, V. Weisskopf, and P. Jordan
Pascual Jordan
-Further reading:...
. This area of research culminated in the formulation of quantum electrodynamics
Quantum electrodynamics
Quantum electrodynamics is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved...
by R.P. Feynman
Richard Feynman
Richard Phillips Feynman was an American physicist known for his work in the path integral formulation of quantum mechanics, the theory of quantum electrodynamics and the physics of the superfluidity of supercooled liquid helium, as well as in particle physics...
, F. Dyson
Freeman Dyson
Freeman John Dyson FRS is a British-born American theoretical physicist and mathematician, famous for his work in quantum field theory, solid-state physics, astronomy and nuclear engineering. Dyson is a member of the Board of Sponsors of the Bulletin of the Atomic Scientists...
, J. Schwinger
Julian Schwinger
Julian Seymour Schwinger was an American theoretical physicist. He is best known for his work on the theory of quantum electrodynamics, in particular for developing a relativistically invariant perturbation theory, and for renormalizing QED to one loop order.Schwinger is recognized as one of the...
, and S.I. Tomonaga
Sin-Itiro Tomonaga
was a Japanese physicist, influential in the development of quantum electrodynamics, work for which he was jointly awarded the Nobel Prize in Physics in 1965 along with Richard Feynman and Julian Schwinger.-Biography:...
during the 1940s. Quantum electrodynamics is a quantum theory of electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s, positron
Positron
The positron or antielectron is the antiparticle or the antimatter counterpart of the electron. The positron has an electric charge of +1e, a spin of ½, and has the same mass as an electron...
s, and the electromagnetic field
Electromagnetic field
An electromagnetic field is a physical field produced by moving electrically charged objects. It affects the behavior of charged objects in the vicinity of the field. The electromagnetic field extends indefinitely throughout space and describes the electromagnetic interaction...
, and served as a role model for subsequent Quantum Field theories
Quantum field theory
Quantum field theory provides a theoretical framework for constructing quantum mechanical models of systems classically parametrized by an infinite number of dynamical degrees of freedom, that is, fields and many-body systems. It is the natural and quantitative language of particle physics and...
.

Quantum chromodynamics
In theoretical physics, quantum chromodynamics is a theory of the strong interaction , a fundamental force describing the interactions of the quarks and gluons making up hadrons . It is the study of the SU Yang–Mills theory of color-charged fermions...
was formulated beginning in the early 1960s. The theory as we know it today was formulated by Politzer, Gross and Wilczek
Frank Wilczek
Frank Anthony Wilczek is a theoretical physicist from the United States and a Nobel laureate. He is currently the Herman Feshbach Professor of Physics at the Massachusetts Institute of Technology ....
in 1975.
Building on pioneering work by Schwinger
Julian Schwinger
Julian Seymour Schwinger was an American theoretical physicist. He is best known for his work on the theory of quantum electrodynamics, in particular for developing a relativistically invariant perturbation theory, and for renormalizing QED to one loop order.Schwinger is recognized as one of the...
, Higgs
Peter Higgs
Peter Ware Higgs, FRS, FRSE, FKC , is an English theoretical physicist and an emeritus professor at the University of Edinburgh....
and Goldstone
Jeffrey Goldstone
Jeffrey Goldstone is a British-born theoretical physicist and an emeritus physics faculty at MIT Center for Theoretical Physics.He worked at the University of Cambridge until 1977....
, the physicists Glashow
Sheldon Lee Glashow
Sheldon Lee Glashow is a Nobel Prize winning American theoretical physicist. He is the Metcalf Professor of Mathematics and Physics at Boston University.-Birth and education:...
, Weinberg
Steven Weinberg
Steven Weinberg is an American theoretical physicist and Nobel laureate in Physics for his contributions with Abdus Salam and Sheldon Glashow to the unification of the weak force and electromagnetic interaction between elementary particles....
and Salam
Abdus Salam
Mohammad Abdus Salam, NI, SPk Mohammad Abdus Salam, NI, SPk Mohammad Abdus Salam, NI, SPk (Urdu: محمد عبد السلام, pronounced , (January 29, 1926– November 21, 1996) was a Pakistani theoretical physicist and Nobel laureate in Physics for his work on the electroweak unification of the...
independently showed how the weak nuclear force and quantum electrodynamics
Quantum electrodynamics
Quantum electrodynamics is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved...
could be merged into a single electroweak force, for which they received the 1979 Nobel Prize in Physics
Nobel Prize in Physics
The Nobel Prize in Physics is awarded once a year by the Royal Swedish Academy of Sciences. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895 and awarded since 1901; the others are the Nobel Prize in Chemistry, Nobel Prize in Literature, Nobel Peace Prize, and...
.
Timeline
The following timeline shows the key steps, precursors and contributors to the development of quantum mechanicsQuantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
, quantum field theories
Quantum field theory
Quantum field theory provides a theoretical framework for constructing quantum mechanical models of systems classically parametrized by an infinite number of dynamical degrees of freedom, that is, fields and many-body systems. It is the natural and quantitative language of particle physics and...
and quantum chemistry
Quantum chemistry
Quantum chemistry is a branch of chemistry whose primary focus is the application of quantum mechanics in physical models and experiments of chemical systems...
:
| Date | Person | Contributions |
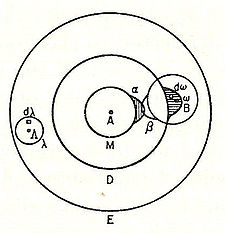
| 1877 | Ludwig Eduard Boltzmann | Suggested that the energy levels of a physical system could be discrete based on statistical mechanics and mathematical arguments; also produced the first circle diagram representation, or atomic model of a molecule (such as an iodine gas molecule) in terms of the overlapping terms α and β, later (in 1928) called molecular orbitals, of the constituting atoms. |
| 1887 | Heinrich Hertz | Discovers the photoelectric effect, shown by Einstein in 1905 to involve quanta of light. |
| 1888 | Johannes Rydberg Johannes Rydberg Johannes Robert Rydberg, , , was a Swedish physicist mainly known for devising the Rydberg formula, in 1888, which is used to predict the wavelengths of photons emitted by changes in the energy level of an electron in a hydrogen atom.The physical constant known as the... |
Modified the Balmer formula to include all spectral series of lines for the hydrogen atom, producing the Rydberg formula which was employed later by Niels Bohr Niels Bohr Niels Henrik David Bohr was a Danish physicist who made foundational contributions to understanding atomic structure and quantum mechanics, for which he received the Nobel Prize in Physics in 1922. Bohr mentored and collaborated with many of the top physicists of the century at his institute in... and others to verify Bohr's first quantum model of the atom. |
| 1895 | Wilhelm Conrad Röntgen Wilhelm Conrad Röntgen Wilhelm Conrad Röntgen was a German physicist, who, on 8 November 1895, produced and detected electromagnetic radiation in a wavelength range today known as X-rays or Röntgen rays, an achievement that earned him the first Nobel Prize in Physics in 1901.... |
Discovered in December 1895 the X-rays in experiments with electron beams in plasma and received the first Nobel prize awarded in 1901; later, in 1922 in experiments involving scattering of X-rays by electrons, Arthur Compton Arthur Compton Arthur Holly Compton was an American physicist and Nobel laureate in physics for his discovery of the Compton effect. He served as Chancellor of Washington University in St. Louis from 1945 to 1953.-Early years:... demonstrated the "particle" aspect of electromagnetic radiation. |
| 1896 | Antoine Henri Becquerel | Discovered accidentally radioactivity while investigating the work of Wilhelm Conrad Röntgen Wilhelm Conrad Röntgen Wilhelm Conrad Röntgen was a German physicist, who, on 8 November 1895, produced and detected electromagnetic radiation in a wavelength range today known as X-rays or Röntgen rays, an achievement that earned him the first Nobel Prize in Physics in 1901.... ; thus, he found that uranium salts emitted radiation that resembled Röntgen's X-rays in their penetrating power. In one experiment, Becquerel wrapped a sample of a phosphorescent substance, potassium uranyl sulfate, in photographic plates surrounded by very thick black paper in preparation for an experiment with bright sunlight; then, to his surprise, prior to actually performing the experiment, Becquerel found that the photographic plates were already exposed, showing a projected image of his sample. |
| 1896 | Pieter Zeeman Pieter Zeeman Pieter Zeeman was a Dutch physicist who shared the 1902 Nobel Prize in Physics with Hendrik Lorentz for his discovery of the Zeeman effect.-Childhood and youth:... |
First observed the Zeeman splitting effect by passing the light emitted by hydrogen through a magnetic field. |
| 1899 to 1903 | Ernest Rutherford Ernest Rutherford Ernest Rutherford, 1st Baron Rutherford of Nelson OM, FRS was a New Zealand-born British chemist and physicist who became known as the father of nuclear physics... , 1st Baron, Lord Rutherford of Nelson, of Cambridge, OM, FRS |
During the investigation of radioactivity he coined the terms alpha Alpha Alpha is the first letter of the Greek alphabet. Alpha or ALPHA may also refer to:-Science:*Alpha , the highest ranking individuals in a community of social animals... and beta Beta Beta is the second letter of the Greek alphabet. Beta or BETA may also refer to:-Biology:*Beta , a genus of flowering plants, mostly referred to as beets*Beta, a rank in a community of social animals... rays in 1899 to describe the two distinct types of radiation emitted by thorium Thorium Thorium is a natural radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 and named after Thor, the Norse god of thunder.... and uranium Uranium Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons... salts. Ernest Rutherford Ernest Rutherford Ernest Rutherford, 1st Baron Rutherford of Nelson OM, FRS was a New Zealand-born British chemist and physicist who became known as the father of nuclear physics... was joined at McGill University in 1900 by Frederick Soddy and together they discovered nuclear transmutation Nuclear transmutation Nuclear transmutation is the conversion of one chemical element or isotope into another. In other words, atoms of one element can be changed into atoms of other element by 'transmutation'... when they found in 1902 that radioactive thorium was converting itself into radium Radium Radium is a chemical element with atomic number 88, represented by the symbol Ra. Radium is an almost pure-white alkaline earth metal, but it readily oxidizes on exposure to air, becoming black in color. All isotopes of radium are highly radioactive, with the most stable isotope being radium-226,... through a process of nuclear decay and a gas (later found to be ); they reported their interpretation of radioactivity in 1903. Sir Ernest Rutherford Ernest Rutherford Ernest Rutherford, 1st Baron Rutherford of Nelson OM, FRS was a New Zealand-born British chemist and physicist who became known as the father of nuclear physics... became known as the ``father of nuclear physics Nuclear physics Nuclear physics is the field of physics that studies the building blocks and interactions of atomic nuclei. The most commonly known applications of nuclear physics are nuclear power generation and nuclear weapons technology, but the research has provided application in many fields, including those... ": with his concept of the nuclear atom model proposed in 1911 he led the exploration of nuclear physics. |
| 1900 | Max Planck Max Planck Max Karl Ernst Ludwig Planck, ForMemRS, was a German physicist who actualized the quantum physics, initiating a revolution in natural science and philosophy. He is regarded as the founder of the quantum theory, for which he received the Nobel Prize in Physics in 1918.-Life and career:Planck came... |
To explain black body radiation (1862), he suggested that electromagnetic energy could only be emitted in quantized form, i.e. the energy could only be a multiple of an elementary unit E = hν, where h is Planck's constant and ν is the frequency of the radiation. |
| 1902 | Gilbert N. Lewis Gilbert N. Lewis Gilbert Newton Lewis was an American physical chemist known for the discovery of the covalent bond , his purification of heavy water, his reformulation of chemical thermodynamics in a mathematically rigorous manner accessible to ordinary chemists, his theory of Lewis acids and... |
To explain the octet rule Octet rule The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low (The octet rule is a chemical rule of thumb that states that atoms of low (... (1893), he developed the “cubical atom Cubical atom The cubical atom was an early atomic model in which electrons were positioned at the eight corners of a cube in a non-polar atom or molecule. This theory was developed in 1902 by Gilbert N. Lewis and published in 1916 in the famous article "The Atom and the Molecule" and used to account for the... ” theory in which electrons in the form of dots were positioned at the corner of a cube and suggested that single, double, or triple “bonds Covalent bond A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding.... ” result when two atoms are held together by multiple pairs of electrons (one pair for each bond) located between the two atoms (1916). |
| 1903 | Antoine Henri Becquerel, Pierre Curie Pierre Curie Pierre Curie was a French physicist, a pioneer in crystallography, magnetism, piezoelectricity and radioactivity, and Nobel laureate. He was the son of Dr. Eugène Curie and Sophie-Claire Depouilly Curie ... and Marie Curie Marie Curie Marie Skłodowska-Curie was a physicist and chemist famous for her pioneering research on radioactivity. She was the first person honored with two Nobel Prizes—in physics and chemistry... , née Skłodowska, Becquerel's doctoral student |
Shared the 1903 Nobel Prize in Physics for their discoveries and study of spontaneous radioactivity; Antoine Henri Becquerel accidentally discovered radioactivity in 1896 while investigating the phosphorescence Phosphorescence Phosphorescence is a specific type of photoluminescence related to fluorescence. Unlike fluorescence, a phosphorescent material does not immediately re-emit the radiation it absorbs. The slower time scales of the re-emission are associated with "forbidden" energy state transitions in quantum... of uranium Uranium Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons... salts. Then, Marie Skłodowska–Curie decided to look into uranium rays as a possible field of research for her doctoral thesis. She used to investigate her uranium salt samples a very sensitive electrometer Electrometer An electrometer is an electrical instrument for measuring electric charge or electrical potential difference. There are many different types, ranging from historical hand-made mechanical instruments to high-precision electronic devices... device that was invented 15 years before by her husband and his brother Jacques Curie to measure electrical charge; using the Curie's electrometer, she discovered that rays emitted by the uranium salt samples caused the air around such samples to conduct electricity, and that the emitted rays' intensity could be quantitated using the Curie electrometer. In April 1898 she found through a systematic search of substances that thorium Thorium Thorium is a natural radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 and named after Thor, the Norse god of thunder.... compounds, like those of uranium, emitted 'Becquerel rays', thus preceding the work of Frederick Soddy Frederick Soddy Frederick Soddy was an English radiochemist who explained, with Ernest Rutherford, that radioactivity is due to the transmutation of elements, now known to involve nuclear reactions. He also proved the existence of isotopes of certain radioactive elements... and Ernest Rutherford Ernest Rutherford Ernest Rutherford, 1st Baron Rutherford of Nelson OM, FRS was a New Zealand-born British chemist and physicist who became known as the father of nuclear physics... on the nuclear decay of thorium to radium Radium Radium is a chemical element with atomic number 88, represented by the symbol Ra. Radium is an almost pure-white alkaline earth metal, but it readily oxidizes on exposure to air, becoming black in color. All isotopes of radium are highly radioactive, with the most stable isotope being radium-226,... by three years. |
| 1904 | Richard Abegg Richard Abegg Richard Wilhelm Heinrich Abegg was a German chemist and pioneer of valence theory. He proposed that the difference of the maximum positive and negative valence of an element tends to be eight. This has come to be called Abegg's rule... |
Noted the pattern that the numerical difference between the maximum positive valence, such as +6 for H2SO4, and the maximum negative valence, such as -2 for H2S, of an element tends to be eight (Abegg's rule Abegg's rule In chemistry, Abegg’s rule states that the difference between the maximum positive and negative valence of an element is frequently eight. The rule used a historic meaning of valence which resembles the modern concept of oxidation state in which an atom is an electron donor or receiver... ). |
| 1905 | Albert Einstein Albert Einstein Albert Einstein was a German-born theoretical physicist who developed the theory of general relativity, effecting a revolution in physics. For this achievement, Einstein is often regarded as the father of modern physics and one of the most prolific intellects in human history... |
Explained the photoelectric effect Photoelectric effect In the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons... (reported in 1887 by Heinrich Hertz), i.e. that shining light on certain materials can function to eject electrons from the material, he postulated, as based on Planck’s quantum hypothesis (1900), that light Light Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz... itself consists of individual quantum particles (photons). |
| 1905 | Albert Einstein Albert Einstein Albert Einstein was a German-born theoretical physicist who developed the theory of general relativity, effecting a revolution in physics. For this achievement, Einstein is often regarded as the father of modern physics and one of the most prolific intellects in human history... |
First to explain the effects of Brownian motion Brownian motion Brownian motion or pedesis is the presumably random drifting of particles suspended in a fluid or the mathematical model used to describe such random movements, which is often called a particle theory.The mathematical model of Brownian motion has several real-world applications... as caused by the kinetic energy Kinetic energy The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes... (i.e., movement) of atoms, which was subsequently, experimentally verified by Jean Baptiste Perrin Jean Baptiste Perrin Jean Baptiste Perrin was a French physicist and Nobel laureate.-Early years:Born in Lille, France, Perrin attended the École Normale Supérieure, the elite grande école in Paris. He became an assistant at the school during the period of 1894-97 when he began the study of cathode rays and X-rays... , thereby settling the century-long dispute about the validity of John Dalton John Dalton John Dalton FRS was an English chemist, meteorologist and physicist. He is best known for his pioneering work in the development of modern atomic theory, and his research into colour blindness .-Early life:John Dalton was born into a Quaker family at Eaglesfield, near Cockermouth, Cumberland,... 's atomic theory Atomic theory In chemistry and physics, atomic theory is a theory of the nature of matter, which states that matter is composed of discrete units called atoms, as opposed to the obsolete notion that matter could be divided into any arbitrarily small quantity... . |
| 1905 | Albert Einstein Albert Einstein Albert Einstein was a German-born theoretical physicist who developed the theory of general relativity, effecting a revolution in physics. For this achievement, Einstein is often regarded as the father of modern physics and one of the most prolific intellects in human history... |
Publishes his Special Theory of Relativity. |
| 1905 | Albert Einstein Albert Einstein Albert Einstein was a German-born theoretical physicist who developed the theory of general relativity, effecting a revolution in physics. For this achievement, Einstein is often regarded as the father of modern physics and one of the most prolific intellects in human history... |
Determines the equivalence of matter and energy. |
| 1907 to 1917 | Ernest Rutherford Ernest Rutherford Ernest Rutherford, 1st Baron Rutherford of Nelson OM, FRS was a New Zealand-born British chemist and physicist who became known as the father of nuclear physics... |
To test his 'plum pudding' model of 1904, later known as the planetary, or Rutherford model Rutherford model The Rutherford model or planetary model is a model of the atom devised by Ernest Rutherford. Rutherford directed the famous Geiger-Marsden experiment in 1909, which suggested on Rutherford's 1911 analysis that the so-called "plum pudding model" of J. J. Thomson of the atom was incorrect... , he sent a beam of positively-charged, alpha particle Alpha particle Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is classically produced in the process of alpha decay, but may be produced also in other ways and given the same name... s onto a gold foil and noticed that some bounced back thus showing that an atom has a small-sized positively charged atomic nucleus Atomic nucleus The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The... at its center. However, he received in 1908 the Nobel Prize in Chemistry "for his investigations into the disintegration of the elements, and the chemistry of radioactive substances", which followed on the work of Marie Curie, not for his planetary model of the atom; he is also widely credited with first "splitting the atom" in 1917. In 1911 Ernest Rutherford explained the Geiger-Marsden experiment Geiger-Marsden experiment The Geiger–Marsden experiment was an experiment to probe the structure of the atom performed by Hans Geiger and Ernest Marsden in 1909, under the direction of Ernest Rutherford at the Physical Laboratories of the University of Manchester... by invoking a nuclear atom model Atomic theory In chemistry and physics, atomic theory is a theory of the nature of matter, which states that matter is composed of discrete units called atoms, as opposed to the obsolete notion that matter could be divided into any arbitrarily small quantity... and derived the Rutherford cross section Cross section (physics) A cross section is the effective area which governs the probability of some scattering or absorption event. Together with particle density and path length, it can be used to predict the total scattering probability via the Beer-Lambert law.... . |
| 1909 | Geoffrey Ingram Taylor Geoffrey Ingram Taylor Sir Geoffrey Ingram Taylor OM was a British physicist, mathematician and expert on fluid dynamics and wave theory. His biographer and one-time student, George Batchelor, described him as "one of the most notable scientists of this century".-Biography:Taylor was born in St. John's Wood, London... |
Demonstrated that interference patterns of light were generated even when the light energy introduced consisted of only one photon. This discovery of the wave-particle duality of matter and energy was fundamental to the later development of quantum field theory Quantum field theory Quantum field theory provides a theoretical framework for constructing quantum mechanical models of systems classically parametrized by an infinite number of dynamical degrees of freedom, that is, fields and many-body systems. It is the natural and quantitative language of particle physics and... . |
| 1909 and 1916 | Albert Einstein Albert Einstein Albert Einstein was a German-born theoretical physicist who developed the theory of general relativity, effecting a revolution in physics. For this achievement, Einstein is often regarded as the father of modern physics and one of the most prolific intellects in human history... |
Showed that, if Planck's law of black-body radiation is accepted, the energy quanta must also carry momentum Momentum In classical mechanics, linear momentum or translational momentum is the product of the mass and velocity of an object... p = h / λ, making them full-fledged particles. |
| 1911 | Lise Meitner Lise Meitner Lise Meitner FRS was an Austrian-born, later Swedish, physicist who worked on radioactivity and nuclear physics. Meitner was part of the team that discovered nuclear fission, an achievement for which her colleague Otto Hahn was awarded the Nobel Prize... and Otto Hahn Otto Hahn Otto Hahn FRS was a German chemist and Nobel laureate, a pioneer in the fields of radioactivity and radiochemistry. He is regarded as "the father of nuclear chemistry". Hahn was a courageous opposer of Jewish persecution by the Nazis and after World War II he became a passionate campaigner... |
Performed an experiment that showed that the energies of electrons emitted by beta decay Beta decay In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a... had a continuous rather than discrete spectrum. This was in apparent contradiction to the law of conservation of energy, as it appeared that energy was lost in the beta decay process. A second problem was that the spin of the Nitrogen-14 atom was 1, in contradiction to the Rutherford prediction of ½. These anomalies were later explained by the discoveries of the neutrino Neutrino A neutrino is an electrically neutral, weakly interacting elementary subatomic particle with a half-integer spin, chirality and a disputed but small non-zero mass. It is able to pass through ordinary matter almost unaffected... and the neutron Neutron The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of... . |
| 1911 | Ștefan Procopiu Stefan Procopiu -Biography:Procopiu was born in 1890 in Bârlad, Romania. His father, Emanoil Procopiu, was employed at the Bârlad courthouse. His mother, Ecaterina Tașcă was the daughter of Gheorghe I... |
Performed experiments in which he determined the correct value of electron's magnetic dipole moment, μB = 9.27×10^(−21) erg·Oe^(−1); (in 1913 he was also able to calculate a theoretical value of the magneton Magneton Magneton may refer to:* Bohr magneton, a physical constant of magnetic moment named after Niels Bohr* Nuclear magneton, a physical constant of magnetic moment* Parson magneton, a hypothetical object in atomic physics suggested by Alfred Lauck Parson in 1915... based on Planck's quantum theory). |
| 1912 | Victor Hess | Discovers the existence of cosmic radiation. |
| 1912 | Henri Poincaré Henri Poincaré Jules Henri Poincaré was a French mathematician, theoretical physicist, engineer, and a philosopher of science... |
Published an influential mathematical argument in support of the essential nature of energy quanta. |
| 1913 | Robert Andrews Millikan | Publishes the results of his "oil drop" experiment, in which he precisely determines the electric charge Electric charge Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two... of the electron. Determination of the fundamental unit of electric charge made it possible to calculate the Avogadro constant (which is the number of atoms or molecules in one mole Mole (unit) The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value... of any substance) and thereby to determine the atomic weight Atomic weight Atomic weight is a dimensionless physical quantity, the ratio of the average mass of atoms of an element to 1/12 of the mass of an atom of carbon-12... of the atoms of each element Chemical element A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements... . |
| 1913 | Ștefan Procopiu Stefan Procopiu -Biography:Procopiu was born in 1890 in Bârlad, Romania. His father, Emanoil Procopiu, was employed at the Bârlad courthouse. His mother, Ecaterina Tașcă was the daughter of Gheorghe I... |
Publishes a theoretical paper with the correct value of the electron's magnetic dipole moment μB: Ştefan Procopiu. 1913. ``Determining the Molecular Magnetic Moment by M. Planck's Quantum Theory". Bulletin scientifique de l’Académie Roumaine de sciences., 1: 151. |
| 1913 | Niels Bohr Niels Bohr Niels Henrik David Bohr was a Danish physicist who made foundational contributions to understanding atomic structure and quantum mechanics, for which he received the Nobel Prize in Physics in 1922. Bohr mentored and collaborated with many of the top physicists of the century at his institute in... |
Obtains theoretically the value of the electron's magnetic dipole moment μB as a consequence of his atom model |
| 1913 | Johannes Stark Johannes Stark Johannes Stark was a German physicist, and Physics Nobel Prize laureate who was closely involved with the Deutsche Physik movement under the Nazi regime.-Early years:... and Antonino Lo Surdo Antonino Lo Surdo Antonino Lo Surdo was a famous Italian physicist. He was appointed as professor of physics at the Istituto di Fisica in Rome in 1919; upon the death of Orso Mario Corbino in 1937, he became the director... |
Independently discovered the shifting and splitting of the spectral lines of atoms and molecules due to the presence of the light source in an external static electric field. |
| 1913 | Niels Bohr Niels Bohr Niels Henrik David Bohr was a Danish physicist who made foundational contributions to understanding atomic structure and quantum mechanics, for which he received the Nobel Prize in Physics in 1922. Bohr mentored and collaborated with many of the top physicists of the century at his institute in... |
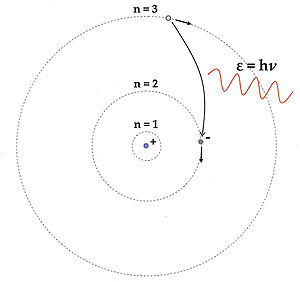
To explain the Rydberg formula Rydberg formula The Rydberg formula is used in atomic physics to describe the wavelengths of spectral lines of many chemical elements. It was formulated by the Swedish physicist Johannes Rydberg, and presented on November 5, 1888.-History:... (1888), which correctly modeled the light emission spectra of atomic hydrogen, Bohr hypothesized that negatively charged electrons revolve around a positively charged nucleus at certain fixed “quantum” distances and that each of these “spherical orbits” has a specific energy associated with it such that electron movements between orbits requires “quantum” emissions or absorptions of energy. |
| 1915 | Albert Einstein Albert Einstein Albert Einstein was a German-born theoretical physicist who developed the theory of general relativity, effecting a revolution in physics. For this achievement, Einstein is often regarded as the father of modern physics and one of the most prolific intellects in human history... |
First presents to the Prussian Academy of Science what are now known as the Einstein field equations Einstein field equations The Einstein field equations or Einstein's equations are a set of ten equations in Albert Einstein's general theory of relativity which describe the fundamental interaction of gravitation as a result of spacetime being curved by matter and energy... . These equations specify how the geometry of space and time is influenced by whatever matter is present, and form the core of Einstein's General Theory of Relativity. Although this theory is not directly applicable to quantum mechanics, theorists of quantum gravity Quantum gravity Quantum gravity is the field of theoretical physics which attempts to develop scientific models that unify quantum mechanics with general relativity... seek to reconcile them. |
| 1916 | Arnold Sommerfeld Arnold Sommerfeld Arnold Johannes Wilhelm Sommerfeld was a German theoretical physicist who pioneered developments in atomic and quantum physics, and also educated and groomed a large number of students for the new era of theoretical physics... |
To account for the Zeeman effect Zeeman effect The Zeeman effect is the splitting of a spectral line into several components in the presence of a static magnetic field. It is analogous to the Stark effect, the splitting of a spectral line into several components in the presence of an electric field... (1896), i.e. that atomic absorption or emission spectral lines change when the light source is subjected to a magnetic field, he suggested there might be “elliptical orbits” in atoms in addition to spherical orbits. |
| 1918 | Sir Ernest Rutherford Ernest Rutherford Ernest Rutherford, 1st Baron Rutherford of Nelson OM, FRS was a New Zealand-born British chemist and physicist who became known as the father of nuclear physics... |
Noticed that, when alpha particles were shot into nitrogen gas, his scintillation detectors showed the signatures of hydrogen Hydrogen Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly... nuclei. Rutherford determined that the only place this hydrogen could have come from was the nitrogen, and therefore nitrogen must contain hydrogen nuclei. He thus suggested that the hydrogen nucleus, which was known to have an atomic number Atomic number In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element... of 1, was an elementary particle Elementary particle In particle physics, an elementary particle or fundamental particle is a particle not known to have substructure; that is, it is not known to be made up of smaller particles. If an elementary particle truly has no substructure, then it is one of the basic building blocks of the universe from which... , which he decided must be the proton Proton The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number.... s hypothesized by Eugen Goldstein Eugen Goldstein Eugen Goldstein was a German physicist. He was an early investigator of discharge tubes, the discoverer of anode rays, and is sometimes credited with the discovery of the proton.- Life :... . |
| 1919 | Irving Langmuir Irving Langmuir Irving Langmuir was an American chemist and physicist. His most noted publication was the famous 1919 article "The Arrangement of Electrons in Atoms and Molecules" in which, building on Gilbert N. Lewis's cubical atom theory and Walther Kossel's chemical bonding theory, he outlined his... |
Building on the work of Lewis (1916), he coined the term "covalence" and postulated that coordinate covalent bond Coordinate covalent bond A dipolar bond, also known as dative covalent bond or coordinate bond is a kind of 2-centre, 2-electron covalent bond in which the two electrons derive from the same atom. Typically, a dipolar bond is formed when a Lewis base donates a pair of electrons to a Lewis acid. This description of bonding... s occur when two electrons of a pair of atoms come from both atoms and are equally shared by them, thus explaining the fundamental nature of chemical bonding and molecular chemistry. |
| 1921 and 1922 | Frederick Soddy Frederick Soddy Frederick Soddy was an English radiochemist who explained, with Ernest Rutherford, that radioactivity is due to the transmutation of elements, now known to involve nuclear reactions. He also proved the existence of isotopes of certain radioactive elements... |
Received the Nobel Prize for 1921 in Chemistry one year later, in 1922, "for his contributions to our knowledge of the chemistry of radioactive substances, and his investigations into the origin and nature of isotopes"; he wrote in his Nobel Lecture of 1922:``The interpretation of radioactivity which was published in 1903 by Sir Ernest Rutherford and myself ascribed the phenomena to the spontaneous disintegration Disintegration Disintegration is the eighth studio album by English alternative rock band The Cure, released on 1 May 1989 by Fiction Records. The record marks a return to the introspective and gloomy gothic rock style the band had established in the early 1980s... of the atoms of the radio-element, whereby a part of the original atom was violently ejected as a radiant particle, and the remainder formed a totally new kind of atom with a distinct chemical and physical character". |
| 1922 | Arthur Compton Arthur Compton Arthur Holly Compton was an American physicist and Nobel laureate in physics for his discovery of the Compton effect. He served as Chancellor of Washington University in St. Louis from 1945 to 1953.-Early years:... |
Found that X-ray wavelengths increase due to scattering of the radiant energy Radiant energy Radiant energy is the energy of electromagnetic waves. The quantity of radiant energy may be calculated by integrating radiant flux with respect to time and, like all forms of energy, its SI unit is the joule. The term is used particularly when radiation is emitted by a source into the... by "free electrons". The scattered quanta Quantum In physics, a quantum is the minimum amount of any physical entity involved in an interaction. Behind this, one finds the fundamental notion that a physical property may be "quantized," referred to as "the hypothesis of quantization". This means that the magnitude can take on only certain discrete... have less energy than the quanta of the original ray. This discovery, known as the "Compton effect," or "Compton scattering Compton scattering In physics, Compton scattering is a type of scattering that X-rays and gamma rays undergo in matter. The inelastic scattering of photons in matter results in a decrease in energy of an X-ray or gamma ray photon, called the Compton effect... " demonstrates the "particle Particle physics Particle physics is a branch of physics that studies the existence and interactions of particles that are the constituents of what is usually referred to as matter or radiation. In current understanding, particles are excitations of quantum fields and interact following their dynamics... " concept of electromagnetic radiation Electromagnetic radiation Electromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space... . |
| 1922 | Otto Stern Otto Stern Otto Stern was a German physicist and Nobel laureate in physics.-Biography:Stern was born in Sohrau, now Żory in the German Empire's Kingdom of Prussia and studied at Breslau, now Wrocław in Lower Silesia.... and Walther Gerlach |
Stern-Gerlach experiment detects discrete values of angular momentum for atoms in the ground state passing through an inhomogeneous magnetic field leading to the discovery of the spin Spin (physics) In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,... of the electron. |
| 1923 | Louis De Broglie Louis, 7th duc de Broglie Louis-Victor-Pierre-Raymond, 7th duc de Broglie, FRS was a French physicist and a Nobel laureate in the year 1929. He was the sixteenth member elected to occupy seat 1 of the Académie française in 1944, and served as Perpetual Secretary of the Académie des sciences, France.-Biography :Louis de... |
Postulated that electrons in motion are associated with waves the lengths of which are given by Planck’s constant h divided by the momentum Momentum In classical mechanics, linear momentum or translational momentum is the product of the mass and velocity of an object... of the mv = p of the electron Electron The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton... : λ = h / mv = h / p. |
| 1924 | Satyendra Nath Bose Satyendra Nath Bose Satyendra Nath Bose FRS was an Indian mathematician and physicist noted for his collaboration with Albert Einstein in developing a theory regarding the gaslike qualities of electromagnetic radiation. He is best known for his work on quantum mechanics in the early 1920s, providing the foundation... |
His work on quantum mechanics Quantum mechanics Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic... provides the foundation for Bose-Einstein statistics, the theory of the Bose-Einstein condensate, and the discovery of the boson Boson In particle physics, bosons are subatomic particles that obey Bose–Einstein statistics. Several bosons can occupy the same quantum state. The word boson derives from the name of Satyendra Nath Bose.... |
| 1925 | George Uhlenbeck and Samuel Goudsmit | Postulated the existence of the electron spin |
| 1925 | Friedrich Hund Friedrich Hund Friedrich Hermann Hund was a German physicist from Karlsruhe known for his work on atoms and molecules.Hund worked at the Universities of Rostock, Leipzig, Jena, Frankfurt am Main, and Göttingen.... |
Outlined the “rule of maximum multiplicity Hund's rule of Maximum Multiplicity Hund's Rule of Maximum Multiplicity is an observational rule which states that a greater total spin state usually makes the resulting atom more stable. Accordingly, it can be taken that if two or more orbitals of equal energy are available, electrons will occupy them singly before filling them in... ” which states that when electrons are added successively to an atom as many levels or orbits are singly occupied as possible before any pairing of electrons with opposite spin occurs and made the distinction that the inner electrons in molecules remained in atomic orbital Atomic orbital An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus... s and only the valence electron Valence electron In chemistry, valence electrons are the electrons of an atom that can participate in the formation of chemical bonds with other atoms. Valence electrons are the "own" electrons, present in the free neutral atom, that combine with valence electrons of other atoms to form chemical bonds. In a single... s needed to be in molecular orbital Molecular orbital In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first... s involving both nuclei. |
| 1925 | Werner Heisenberg Werner Heisenberg Werner Karl Heisenberg was a German theoretical physicist who made foundational contributions to quantum mechanics and is best known for asserting the uncertainty principle of quantum theory... |
Developed the matrix mechanics Matrix mechanics Matrix mechanics is a formulation of quantum mechanics created by Werner Heisenberg, Max Born, and Pascual Jordan in 1925.Matrix mechanics was the first conceptually autonomous and logically consistent formulation of quantum mechanics. It extended the Bohr Model by describing how the quantum jumps... formulation of Quantum Mechanics. |
| 1925 | Wolfgang Pauli Wolfgang Pauli Wolfgang Ernst Pauli was an Austrian theoretical physicist and one of the pioneers of quantum physics. In 1945, after being nominated by Albert Einstein, he received the Nobel Prize in Physics for his "decisive contribution through his discovery of a new law of Nature, the exclusion principle or... |
Outlined the “Pauli exclusion principle Pauli exclusion principle The Pauli exclusion principle is the quantum mechanical principle that no two identical fermions may occupy the same quantum state simultaneously. A more rigorous statement is that the total wave function for two identical fermions is anti-symmetric with respect to exchange of the particles... ” which states that no two identical fermion Fermion In particle physics, a fermion is any particle which obeys the Fermi–Dirac statistics . Fermions contrast with bosons which obey Bose–Einstein statistics.... s may occupy the same quantum state simultaneously. |
| 1926 | Gilbert Lewis Gilbert Lewis Gilbert Lewis is an American actor who is best known for playing The King of Cartoons in the first season of the 1986 children's show, Pee-wee's Playhouse. Lewis played the King of Cartoons in thirteen episodes before being replaced by actor, William Marshall... |
Coined the term photon Photon In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force... , which he derived from the Greek word for light, φως (transliterated phôs). |
| 1926 | Oskar Klein Oskar Klein Oskar Benjamin Klein was a Swedish theoretical physicist.Klein was born in Danderyd outside Stockholm, son of the chief rabbi of Stockholm, Dr. Gottlieb Klein from Homonna in Hungary and Antonie Levy... and Walter Gordon (physicist) Walter Gordon (physicist) Walter Gordon was a German theoretical physicist.-Life:Walter Gordon was the son of businessman Arnold Gordon and his wife Bianca Gordon . The family moved to Switzerland in his early years. In 1900 he attended school in St. Gallen and in 1915 he began his studies of mathematics and physics at... |
Stated their relativistic quantum wave equation, now called the Klein-Gordon equation Klein-Gordon equation The Klein–Gordon equation is a relativistic version of the Schrödinger equation.... |
| 1926 | Enrico Fermi Enrico Fermi Enrico Fermi was an Italian-born, naturalized American physicist particularly known for his work on the development of the first nuclear reactor, Chicago Pile-1, and for his contributions to the development of quantum theory, nuclear and particle physics, and statistical mechanics... |
Discovered the spin-statistics theorem Spin-statistics theorem In quantum mechanics, the spin-statistics theorem relates the spin of a particle to the particle statistics it obeys. The spin of a particle is its intrinsic angular momentum... connection |
| 1926 | Paul Dirac Paul Dirac Paul Adrien Maurice Dirac, OM, FRS was an English theoretical physicist who made fundamental contributions to the early development of both quantum mechanics and quantum electrodynamics... |
Introduced Fermi-Dirac statistics Fermi-Dirac statistics Fermi–Dirac statistics is a part of the science of physics that describes the energies of single particles in a system comprising many identical particles that obey the Pauli Exclusion Principle... |
| 1926 | Erwin Schrödinger Erwin Schrödinger Erwin Rudolf Josef Alexander Schrödinger was an Austrian physicist and theoretical biologist who was one of the fathers of quantum mechanics, and is famed for a number of important contributions to physics, especially the Schrödinger equation, for which he received the Nobel Prize in Physics in 1933... |
Used De Broglie’s electron wave postulate (1924) to develop a “wave equation Schrödinger equation The Schrödinger equation was formulated in 1926 by Austrian physicist Erwin Schrödinger. Used in physics , it is an equation that describes how the quantum state of a physical system changes in time.... ” that represents mathematically the distribution of a charge of an electron distributed through space, being spherically symmetric or prominent in certain directions, i.e. directed valence bonds Valence bond theory In chemistry, valence bond theory is one of two basic theories, along with molecular orbital theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds... , which gave the correct values for spectral lines of the hydrogen atom; also introduced the Hamiltonian operator in quantum mechanics. |
| 1926 to 1932 | John von Neumann John von Neumann John von Neumann was a Hungarian-American mathematician and polymath who made major contributions to a vast number of fields, including set theory, functional analysis, quantum mechanics, ergodic theory, geometry, fluid dynamics, economics and game theory, computer science, numerical analysis,... |
Laid the mathematical foundations of Quantum Mechanics Quantum mechanics Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic... in terms of Hermitian operators on Hilbert space Hilbert space The mathematical concept of a Hilbert space, named after David Hilbert, generalizes the notion of Euclidean space. It extends the methods of vector algebra and calculus from the two-dimensional Euclidean plane and three-dimensional space to spaces with any finite or infinite number of dimensions... s, subsequently published in 1932 as a basic textbook of quantum mechanics. |
| 1927 | Werner Heisenberg Werner Heisenberg Werner Karl Heisenberg was a German theoretical physicist who made foundational contributions to quantum mechanics and is best known for asserting the uncertainty principle of quantum theory... |
Formulates the quantum uncertainty principle Uncertainty principle In quantum mechanics, the Heisenberg uncertainty principle states a fundamental limit on the accuracy with which certain pairs of physical properties of a particle, such as position and momentum, can be simultaneously known... |
| 1927 | Max Born Max Born Max Born was a German-born physicist and mathematician who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics and supervised the work of a number of notable physicists in the 1920s and 30s... |
interpreted the probabilistic nature Copenhagen interpretation The Copenhagen interpretation is one of the earliest and most commonly taught interpretations of quantum mechanics. It holds that quantum mechanics does not yield a description of an objective reality but deals only with probabilities of observing, or measuring, various aspects of energy quanta,... of wavefunctions |
| 1927 | Walter Heitler Walter Heitler Walter Heinrich Heitler was a German physicist who made contributions to quantum electrodynamics and quantum field theory... and Fritz London Fritz London Fritz Wolfgang London was a German theoretical physicist. His fundamental contributions to the theories of chemical bonding and of intermolecular forces are today considered classic and are discussed in standard textbooks of physical chemistry.With his brother Heinz, he made a significant... |
Introduced the concepts of valence bond theory Valence bond theory In chemistry, valence bond theory is one of two basic theories, along with molecular orbital theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds... and applied it to the hydrogen Hydrogen Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly... molecule. |
| 1927 | Thomas and Fermi Enrico Fermi Enrico Fermi was an Italian-born, naturalized American physicist particularly known for his work on the development of the first nuclear reactor, Chicago Pile-1, and for his contributions to the development of quantum theory, nuclear and particle physics, and statistical mechanics... |
developed the Thomas-Fermi model Gas in a box In quantum mechanics, the results of the quantum particle in a box can be used to look at the equilibrium situation for a quantum ideal gas in a box which is a box containing a large number of molecules which do not interact with each other except for instantaneous thermalizing collisions... |
| 1927 | Chandrasekhara Raman | Studied optical photon scattering by electrons |
| 1927 | Paul Dirac Paul Dirac Paul Adrien Maurice Dirac, OM, FRS was an English theoretical physicist who made fundamental contributions to the early development of both quantum mechanics and quantum electrodynamics... |
Stated his relativistic electron quantum wave equation Dirac equation The Dirac equation is a relativistic quantum mechanical wave equation formulated by British physicist Paul Dirac in 1928. It provided a description of elementary spin-½ particles, such as electrons, consistent with both the principles of quantum mechanics and the theory of special relativity, and... |
| 1927 | Charles G. Darwin and Walter Gordon Walter Gordon (physicist) Walter Gordon was a German theoretical physicist.-Life:Walter Gordon was the son of businessman Arnold Gordon and his wife Bianca Gordon . The family moved to Switzerland in his early years. In 1900 he attended school in St. Gallen and in 1915 he began his studies of mathematics and physics at... |
Solved the Dirac equation Dirac equation The Dirac equation is a relativistic quantum mechanical wave equation formulated by British physicist Paul Dirac in 1928. It provided a description of elementary spin-½ particles, such as electrons, consistent with both the principles of quantum mechanics and the theory of special relativity, and... for a Coulomb potential |
| 1927 | Charles Drummond Ellis Charles Drummond Ellis Sir Charles Drummond Ellis was a physicist and scientific administrator. His work on the magnetic spectrum of the beta-rays helped to develop a better understanding of nuclear structure.... (along with James Chadwick James Chadwick Sir James Chadwick CH FRS was an English Nobel laureate in physics awarded for his discovery of the neutron.... and colleagues) |
Finally established clearly that the beta decay spectrum is in fact continuous and not discrete, posing a problem that would later by solved by theorizing (and later discovering) the existence of the neutrino Neutrino A neutrino is an electrically neutral, weakly interacting elementary subatomic particle with a half-integer spin, chirality and a disputed but small non-zero mass. It is able to pass through ordinary matter almost unaffected... . |
| 1927 | Walter Heitler Walter Heitler Walter Heinrich Heitler was a German physicist who made contributions to quantum electrodynamics and quantum field theory... |
Used Schrödinger’s wave equation (1926) to show how two hydrogen atom wavefunction Wavefunction Not to be confused with the related concept of the Wave equationA wave function or wavefunction is a probability amplitude in quantum mechanics describing the quantum state of a particle and how it behaves. Typically, its values are complex numbers and, for a single particle, it is a function of... s join together, with plus, minus, and exchange terms, to form a covalent bond Covalent bond A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding.... . |
| 1927 | Robert Mulliken | In 1927 Mulliken worked, in coordination with Hund, to develop a molecular orbital theory where electrons are assigned to states that extend over an entire molecule and, in 1932, introduced many new molecular orbital terminologies, such as σ bond, π bond, and δ bond. |
| 1927 | Hermann Klaus Hugo Weyl Hermann Weyl Hermann Klaus Hugo Weyl was a German mathematician and theoretical physicist. Although much of his working life was spent in Zürich, Switzerland and then Princeton, he is associated with the University of Göttingen tradition of mathematics, represented by David Hilbert and Hermann Minkowski.His... |
Proved in collaboration with his student Fritz Peter Fritz Peter Fritz Peter was a German mathematician who helped prove the Peter–Weyl theorem. He was a student of Hermann Weyl, and later became headmaster of a secondary school .... a fundamental theorem in harmonic analysis—the Peter-Weyl theorem-- relevant to group representation Group representation In the mathematical field of representation theory, group representations describe abstract groups in terms of linear transformations of vector spaces; in particular, they can be used to represent group elements as matrices so that the group operation can be represented by matrix multiplication... s in quantum theory (including the complete reducibility of unitary representation Unitary representation In mathematics, a unitary representation of a group G is a linear representation π of G on a complex Hilbert space V such that π is a unitary operator for every g ∈ G... s of a compact Compact Compact as used in politics may refer broadly to a pact or treaty; in more specific cases it may refer to:* Interstate compact* Compact government, a type of colonial rule utilized in British North America... topological group Topological group In mathematics, a topological group is a group G together with a topology on G such that the group's binary operation and the group's inverse function are continuous functions with respect to the topology. A topological group is a mathematical object with both an algebraic structure and a... ); introduced the Weyl quantization Weyl quantization In mathematics and physics, in the area of quantum mechanics, Weyl quantization is a method for systematically associating a "quantum mechanical" Hermitian operator with a "classical" kernel function in phase space invertibly... , and earlier, in 1918, introduced the concept of gauge and a gauge theory Gauge theory In physics, gauge invariance is the property of a field theory in which different configurations of the underlying fundamental but unobservable fields result in identical observable quantities. A theory with such a property is called a gauge theory... ; later in 1935 he introduced and characterized with Richard Bauer the concept of spinor in n-dimensions Spinor In mathematics and physics, in particular in the theory of the orthogonal groups , spinors are elements of a complex vector space introduced to expand the notion of spatial vector. Unlike tensors, the space of spinors cannot be built up in a unique and natural way from spatial vectors... . |
| 1928 | Paul Dirac Paul Dirac Paul Adrien Maurice Dirac, OM, FRS was an English theoretical physicist who made fundamental contributions to the early development of both quantum mechanics and quantum electrodynamics... |
In the Dirac equations, Paul Dirac integrated the principle of special relativity with quantum electrodynamics and hypothesized the existence of the positron. |
| 1928 | Linus Pauling Linus Pauling Linus Carl Pauling was an American chemist, biochemist, peace activist, author, and educator. He was one of the most influential chemists in history and ranks among the most important scientists of the 20th century... |
Outlined the nature of the chemical bond Chemical bond A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction... in which he used Heitler’s quantum mechanical covalent bond model (1927) to outline the quantum mechanical Quantum mechanics Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic... basis for all types of molecular structure and bonding and suggested that different types of bonds in molecules can become equalized by rapid shifting of electrons, a process called “resonance Resonance (chemistry) In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula... ” (1931), such that resonance hybrids contain contributions from the different possible electronic configurations. |
| 1928 | Friedrich Hund Friedrich Hund Friedrich Hermann Hund was a German physicist from Karlsruhe known for his work on atoms and molecules.Hund worked at the Universities of Rostock, Leipzig, Jena, Frankfurt am Main, and Göttingen.... and Robert S. Mulliken Robert S. Mulliken Robert Sanderson Mulliken was an American physicist and chemist, primarily responsible for the early development of molecular orbital theory, i.e. the elaboration of the molecular orbital method of computing the structure of molecules. Dr. Mulliken received the Nobel Prize for chemistry in 1966... |
Introduce the concept of molecular orbital Molecular orbital In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first... |
| 1929 | Oskar Klein Oskar Klein Oskar Benjamin Klein was a Swedish theoretical physicist.Klein was born in Danderyd outside Stockholm, son of the chief rabbi of Stockholm, Dr. Gottlieb Klein from Homonna in Hungary and Antonie Levy... |
Discovers the Klein paradox Klein paradox In 1929, physicist Oskar Klein obtained a surprising result by applying the Dirac equation to the familiar problem of electron scattering from a potential barrier. In nonrelativistic quantum mechanics, electron tunneling into a barrier is observed, with exponential damping... |
| 1929 | Oskar Klein Oskar Klein Oskar Benjamin Klein was a Swedish theoretical physicist.Klein was born in Danderyd outside Stockholm, son of the chief rabbi of Stockholm, Dr. Gottlieb Klein from Homonna in Hungary and Antonie Levy... and Yoshio Nishina Yoshio Nishina was the founding father of modern physics research in Japan. He co-authored the well-known Klein–Nishina formula. He was a principal investigator of RIKEN and mentored generations... |
Derive the Klein-Nishina cross section for high energy photon scattering by electrons |
| 1929 | Sir Nevill Mott | Derives the Mott cross section Mott scattering Mott scattering, also referred to as spin-coupling inelastic Coulomb scattering, is the separation of the two spin states of an electron beam by scattering the beam off the Coulomb field of heavy atoms... for the Coulomb scattering of relativistic electrons |
| 1929 | John Lennard-Jones John Lennard-Jones Sir John Edward Lennard-Jones KBE, FRS was a mathematician who was a professor of theoretical physics at Bristol University, and then of theoretical science at Cambridge University... |
Introduced the linear combination of atomic orbitals approximation for the calculation of molecular orbital Molecular orbital In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first... s. |
| 1930 | Paul Dirac Paul Dirac Paul Adrien Maurice Dirac, OM, FRS was an English theoretical physicist who made fundamental contributions to the early development of both quantum mechanics and quantum electrodynamics... |
Introduces electron hole theory |
| 1930 | Fritz London Fritz London Fritz Wolfgang London was a German theoretical physicist. His fundamental contributions to the theories of chemical bonding and of intermolecular forces are today considered classic and are discussed in standard textbooks of physical chemistry.With his brother Heinz, he made a significant... |
Explains van der Waals force Van der Waals force In physical chemistry, the van der Waals force , named after Dutch scientist Johannes Diderik van der Waals, is the sum of the attractive or repulsive forces between molecules other than those due to covalent bonds or to the electrostatic interaction of ions with one another or with neutral... s as due to the interacting fluctuating dipole moments Bond dipole moment The bond dipole moment uses the idea of electric dipole moment to measure the polarity of a chemical bond within a molecule. The bond dipole μ is given by:\mu = \delta \, d.... between molecules |
| 1930 | Wolfgang Pauli Wolfgang Pauli Wolfgang Ernst Pauli was an Austrian theoretical physicist and one of the pioneers of quantum physics. In 1945, after being nominated by Albert Einstein, he received the Nobel Prize in Physics for his "decisive contribution through his discovery of a new law of Nature, the exclusion principle or... |
In a famous letter, Pauli suggested that, in addition to electrons and protons, atoms also contained an extremely light neutral particle which he called the "neutron." He suggested that this "neutron" was also emitted during beta decay and had simply not yet been observed. Later it was determined that this particle was actually the almost massless neutrino Neutrino A neutrino is an electrically neutral, weakly interacting elementary subatomic particle with a half-integer spin, chirality and a disputed but small non-zero mass. It is able to pass through ordinary matter almost unaffected... |
| 1931 | John Lennard-Jones John Lennard-Jones Sir John Edward Lennard-Jones KBE, FRS was a mathematician who was a professor of theoretical physics at Bristol University, and then of theoretical science at Cambridge University... |
Proposes the Lennard-Jones interatomic potential Lennard-Jones potential The Lennard-Jones potential is a mathematically simple model that approximates the interaction between a pair of neutral atoms or molecules. A form of the potential was first proposed in 1924 by John Lennard-Jones... |
| 1931 | Walther Bothe Walther Bothe Walther Wilhelm Georg Bothe was a German nuclear physicist, who shared the Nobel Prize in Physics in 1954 with Max Born.... and Herbert Becker |
Found that if the very energetic alpha particles emitted from polonium Polonium Polonium is a chemical element with the symbol Po and atomic number 84, discovered in 1898 by Marie Skłodowska-Curie and Pierre Curie. A rare and highly radioactive element, polonium is chemically similar to bismuth and tellurium, and it occurs in uranium ores. Polonium has been studied for... fell on certain light elements, specifically beryllium Beryllium Beryllium is the chemical element with the symbol Be and atomic number 4. It is a divalent element which occurs naturally only in combination with other elements in minerals. Notable gemstones which contain beryllium include beryl and chrysoberyl... , boron Boron Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the... , or lithium Lithium Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly... , an unusually penetrating radiation was produced. At first this radiation was thought to be gamma radiation, although it was more penetrating than any gamma rays known, and the details of experimental results were very difficult to interpret on this basis. Some scientists began to hypothesize the possible existence of another fundamental, atomic particle. |
| 1931 | Enrico Fermi Enrico Fermi Enrico Fermi was an Italian-born, naturalized American physicist particularly known for his work on the development of the first nuclear reactor, Chicago Pile-1, and for his contributions to the development of quantum theory, nuclear and particle physics, and statistical mechanics... |
Renamed Pauli's "neutron" to neutrino Neutrino A neutrino is an electrically neutral, weakly interacting elementary subatomic particle with a half-integer spin, chirality and a disputed but small non-zero mass. It is able to pass through ordinary matter almost unaffected... to distinguish it from the then-hypothetical possibility of a much more massive neutron Neutron The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of... . |
| 1932 | Irène Joliot-Curie Irène Joliot-Curie Irène Joliot-Curie was a French scientist, the daughter of Marie Skłodowska-Curie and Pierre Curie and the wife of Frédéric Joliot-Curie. Jointly with her husband, Joliot-Curie was awarded the Nobel Prize for chemistry in 1935 for their discovery of artificial radioactivity. This made the Curies... and Frédéric Joliot |
Showed that if the unknown radiation generated by alpha particles fell on paraffin or any other hydrogen-containing compound, it ejected protons of very high energy. This was not in itself inconsistent with the proposed gamma ray Gamma ray Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency . Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei... nature of the new radiation, but detailed quantitative analysis of the data became increasingly difficult to reconcile with such a hypothesis. |
| 1932 | James Chadwick James Chadwick Sir James Chadwick CH FRS was an English Nobel laureate in physics awarded for his discovery of the neutron.... |
Performed a series of experiments showing that the gamma ray hypothesis for the unknown radiation produced by alpha particles was untenable, and that the new particles must be the neutrons hypothesized by Enrico Fermi Enrico Fermi Enrico Fermi was an Italian-born, naturalized American physicist particularly known for his work on the development of the first nuclear reactor, Chicago Pile-1, and for his contributions to the development of quantum theory, nuclear and particle physics, and statistical mechanics... . Chadwick suggested that, in fact, the new radiation consisted of uncharged particles of approximately the same mass as the proton, and he performed a series of experiments verifying his suggestion. |
| 1932 | Werner Heisenberg Werner Heisenberg Werner Karl Heisenberg was a German theoretical physicist who made foundational contributions to quantum mechanics and is best known for asserting the uncertainty principle of quantum theory... |
Applied perturbation theory Perturbation theory Perturbation theory comprises mathematical methods that are used to find an approximate solution to a problem which cannot be solved exactly, by starting from the exact solution of a related problem... to the two-electron problem and showed how resonance Resonance (chemistry) In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula... arising from electron exchange could explain exchange forces. |
| 1932 | Mark Oliphant Mark Oliphant Sir Marcus 'Mark' Laurence Elwin Oliphant, AC, KBE, FRS was an Australian physicist and humanitarian who played a fundamental role in the first experimental demonstration of nuclear fusion and also the development of the atomic bomb.During his retirement, Oliphant was appointed as the Governor of... |
Building upon the nuclear transmutation experiments of Ernest Rutherford Ernest Rutherford Ernest Rutherford, 1st Baron Rutherford of Nelson OM, FRS was a New Zealand-born British chemist and physicist who became known as the father of nuclear physics... done a few years earlier, fusion of light nuclei (hydrogen isotopes) was first observed by Oliphant in 1932. The steps of the main cycle of nuclear fusion in stars were subsequently worked out by Hans Bethe throughout the remainder of that decade. |
| 1932 | Carl D. Anderson | Experimentally proves the existence of the positron. |
| 1933 | Leó Szilárd Leó Szilárd Leó Szilárd was an Austro-Hungarian physicist and inventor who conceived the nuclear chain reaction in 1933, patented the idea of a nuclear reactor with Enrico Fermi, and in late 1939 wrote the letter for Albert Einstein's signature that resulted in the Manhattan Project that built the atomic bomb... |
First theorized the concept of a nuclear chain reaction. He filed a patent for his idea of a simple nuclear reactor the following year. |
| 1934 | Enrico Fermi Enrico Fermi Enrico Fermi was an Italian-born, naturalized American physicist particularly known for his work on the development of the first nuclear reactor, Chicago Pile-1, and for his contributions to the development of quantum theory, nuclear and particle physics, and statistical mechanics... |
Published a very successful model of beta decay Fermi's interaction In particle physics, Fermi's interaction also known as Fermi coupling, is an old explanation of the weak force, proposed by Enrico Fermi, in which four fermions directly interact with one another at one vertex... in which neutrinos were produced. |
| 1934 | Enrico Fermi Enrico Fermi Enrico Fermi was an Italian-born, naturalized American physicist particularly known for his work on the development of the first nuclear reactor, Chicago Pile-1, and for his contributions to the development of quantum theory, nuclear and particle physics, and statistical mechanics... |
Studies the effects of bombarding uranium Uranium Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons... isotopes with neutrons. |
| 1934 | N.N.Semyonov | Develops the total quantitative chain chemical reaction theory. The idea of the chain reaction, developed by Semyonov, is the basis of various high technologies using the incineration of gas mixtures. The idea was also used for the description of the nuclear reaction. |
| 1934 | Irène Joliot-Curie Irène Joliot-Curie Irène Joliot-Curie was a French scientist, the daughter of Marie Skłodowska-Curie and Pierre Curie and the wife of Frédéric Joliot-Curie. Jointly with her husband, Joliot-Curie was awarded the Nobel Prize for chemistry in 1935 for their discovery of artificial radioactivity. This made the Curies... and Frédéric Joliot-Curie |
Discovered artificial radioactivity and were jointly awarded the 1935 Novel Prize in Chemistry |
| 1935 | Hideki Yukawa Hideki Yukawa né , was a Japanese theoretical physicist and the first Japanese Nobel laureate.-Biography:Yukawa was born in Tokyo and grew up in Kyoto. In 1929, after receiving his degree from Kyoto Imperial University, he stayed on as a lecturer for four years. After graduation, he was interested in... |
Formulated his hypothesis of the Yukawa potential and predicted the existence of the pion Pion In particle physics, a pion is any of three subatomic particles: , , and . Pions are the lightest mesons and they play an important role in explaining the low-energy properties of the strong nuclear force.... , stating that such a potential arises from the exchange of a massive scalar field Scalar field In mathematics and physics, a scalar field associates a scalar value to every point in a space. The scalar may either be a mathematical number, or a physical quantity. Scalar fields are required to be coordinate-independent, meaning that any two observers using the same units will agree on the... , as it would be found in the field of the pion. Prior to Yukawa's paper, it was believed that the scalar fields of the fundamental forces necessitated massless particles. |
| 1936 | Alexandru Proca Alexandru Proca Alexandru Proca was a Romanian physicist who studied and worked in France. He developed the vector meson theory of nuclear forces and the relativistic quantum field equations that bear his name for the massive, vector spin-1 mesons... |
Published prior to Hideki Yukawa Hideki Yukawa né , was a Japanese theoretical physicist and the first Japanese Nobel laureate.-Biography:Yukawa was born in Tokyo and grew up in Kyoto. In 1929, after receiving his degree from Kyoto Imperial University, he stayed on as a lecturer for four years. After graduation, he was interested in... his relativistic quantum field equations for a massive vector meson Vector meson In high energy physics, a vector meson is a meson with total spin 1 and odd parity . Compare to a pseudovector meson, which has a total spin 1 and even parity.... of spin Spin (physics) In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,... -1 as a basis for nuclear force Nuclear force The nuclear force is the force between two or more nucleons. It is responsible for binding of protons and neutrons into atomic nuclei. The energy released causes the masses of nuclei to be less than the total mass of the protons and neutrons which form them... s. |
| 1936 | Garrett Birkhoff Garrett Birkhoff Garrett Birkhoff was an American mathematician. He is best known for his work in lattice theory.The mathematician George Birkhoff was his father.... and John von Neumann John von Neumann John von Neumann was a Hungarian-American mathematician and polymath who made major contributions to a vast number of fields, including set theory, functional analysis, quantum mechanics, ergodic theory, geometry, fluid dynamics, economics and game theory, computer science, numerical analysis,... |
Introduced Quantum Logic Quantum logic In quantum mechanics, quantum logic is a set of rules for reasoning about propositions which takes the principles of quantum theory into account... in an attempt to reconcile the apparent inconsistency of classical, Boolean logic with the Heisenberg Uncertainty Principle Uncertainty principle In quantum mechanics, the Heisenberg uncertainty principle states a fundamental limit on the accuracy with which certain pairs of physical properties of a particle, such as position and momentum, can be simultaneously known... of quantum mechanics as applied, for example, to the measurement of complementary (noncommuting) observable Observable In physics, particularly in quantum physics, a system observable is a property of the system state that can be determined by some sequence of physical operations. For example, these operations might involve submitting the system to various electromagnetic fields and eventually reading a value off... s in quantum mechanics, such as position Position Position may refer to:* Position , a player role within a team* Position , the orientation of a baby prior to birth* Position , a mathematical identification of relative location... and momentum Momentum In classical mechanics, linear momentum or translational momentum is the product of the mass and velocity of an object... ; current approaches to quantum logic involve noncommutative and non-associative many-valued logic. |
| 1936 | Carl D. Anderson | Discovered muons while he studied cosmic radiation. |
| 1937 | Carl Anderson Carl David Anderson Carl David Anderson was an American physicist. He is best known for his discovery of the positron in 1932, an achievement for which he received the 1936 Nobel Prize in Physics, and of the muon in 1936.-Biography:... |
Experimentally proves the existence of the pion Pion In particle physics, a pion is any of three subatomic particles: , , and . Pions are the lightest mesons and they play an important role in explaining the low-energy properties of the strong nuclear force.... . |
| 1937 | Hermann Arthur Jahn and Edward Teller Edward Teller Edward Teller was a Hungarian-American theoretical physicist, known colloquially as "the father of the hydrogen bomb," even though he did not care for the title. Teller made numerous contributions to nuclear and molecular physics, spectroscopy , and surface physics... |
Proved, using group theory Group theory In mathematics and abstract algebra, group theory studies the algebraic structures known as groups.The concept of a group is central to abstract algebra: other well-known algebraic structures, such as rings, fields, and vector spaces can all be seen as groups endowed with additional operations and... , that non-linear degenerate molecules are unstable. The Jahn-Teller theorem essentially states that any non-linear molecule with a degenerate Degenerate energy level In physics, two or more different quantum states are said to be degenerate if they are all at the same energy level. Statistically this means that they are all equally probable of being filled, and in Quantum Mechanics it is represented mathematically by the Hamiltonian for the system having more... electronic ground state will undergo a geometrical distortion that removes that degeneracy, because the distortion lowers the overall energy of the complex. The latter process is called the Jahn-Teller effect; this effect was recently considered also in relation to the superconductivity mechanism in YBCO and other high temperature superconductors. The details of the Jahn-Teller effect are presented with several examples and EPR data in the basic textbook by Abragam and Bleaney (1970). |
| 1938 | Charles Coulson Charles Coulson Charles Alfred Coulson FRS was an applied mathematician, theoretical chemist and religious author.His major scientific work was as a pioneer of the application of the quantum theory of valency to problems of molecular structure, dynamics and reactivity... |
Made the first accurate calculation of a molecular orbital Molecular orbital In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first... wavefunction Wavefunction Not to be confused with the related concept of the Wave equationA wave function or wavefunction is a probability amplitude in quantum mechanics describing the quantum state of a particle and how it behaves. Typically, its values are complex numbers and, for a single particle, it is a function of... with the hydrogen molecule. |
| 1938 | Otto Hahn Otto Hahn Otto Hahn FRS was a German chemist and Nobel laureate, a pioneer in the fields of radioactivity and radiochemistry. He is regarded as "the father of nuclear chemistry". Hahn was a courageous opposer of Jewish persecution by the Nazis and after World War II he became a passionate campaigner... , Fritz Strassmann Fritz Strassmann Friedrich Wilhelm "Fritz" Strassmann was a German chemist who, with Otto Hahn in 1938, identified barium in the residue after bombarding uranium with neutrons, which led to the interpretation of their results as being from nuclear fission... , Lise Meitner Lise Meitner Lise Meitner FRS was an Austrian-born, later Swedish, physicist who worked on radioactivity and nuclear physics. Meitner was part of the team that discovered nuclear fission, an achievement for which her colleague Otto Hahn was awarded the Nobel Prize... , and Otto Robert Frisch Otto Robert Frisch Otto Robert Frisch , Austrian-British physicist. With his collaborator Rudolf Peierls he designed the first theoretical mechanism for the detonation of an atomic bomb in 1940.- Overview :... |
Hahn and Strassmann sent a manuscript to Naturwissenschaften reporting they had detected the element barium after bombarding uranium with neutrons. Simultaneously, they communicated these results to Meitner. Meitner, and her nephew Frisch, correctly interpreted these results as being nuclear fission. Frisch confirmed this experimentally on 13 January 1939. |
| 1939 | Leó Szilárd Leó Szilárd Leó Szilárd was an Austro-Hungarian physicist and inventor who conceived the nuclear chain reaction in 1933, patented the idea of a nuclear reactor with Enrico Fermi, and in late 1939 wrote the letter for Albert Einstein's signature that resulted in the Manhattan Project that built the atomic bomb... and Enrico Fermi Enrico Fermi Enrico Fermi was an Italian-born, naturalized American physicist particularly known for his work on the development of the first nuclear reactor, Chicago Pile-1, and for his contributions to the development of quantum theory, nuclear and particle physics, and statistical mechanics... |
Discovered neutron multiplication in uranium, proving that a chain reaction was indeed possible. |
| 1942 | Kan-Chang Wang Kan-Chang Wang Wang Ganchang was a nuclear physicist from China. He was one of the initiators of research in China in nuclear physics, cosmic rays and particle physics. Wang figured among the top leaders, pioneers and scientists of the Chinese nuclear deterrent program... |
First proposed the use of beta capture to experimentally detect neutrinos. |
| 1942 | Enrico Fermi Enrico Fermi Enrico Fermi was an Italian-born, naturalized American physicist particularly known for his work on the development of the first nuclear reactor, Chicago Pile-1, and for his contributions to the development of quantum theory, nuclear and particle physics, and statistical mechanics... |
Created the first artificial self-sustaining nuclear chain reaction, called Chicago Pile-1 (CP-1), in a racquets court below the bleachers of Stagg Field at the University of Chicago on December 2, 1942. |
| 1945 | Julius Robert Oppenheimer | Lead successfully the Manhattan Project Manhattan Project The Manhattan Project was a research and development program, led by the United States with participation from the United Kingdom and Canada, that produced the first atomic bomb during World War II. From 1942 to 1946, the project was under the direction of Major General Leslie Groves of the US Army... , predicted quantum tunneling and proposed the Oppenheimer–Phillips process in nuclear fusion Nuclear fusion Nuclear fusion is the process by which two or more atomic nuclei join together, or "fuse", to form a single heavier nucleus. This is usually accompanied by the release or absorption of large quantities of energy... |
| 1945 | Manhattan Project Manhattan Project The Manhattan Project was a research and development program, led by the United States with participation from the United Kingdom and Canada, that produced the first atomic bomb during World War II. From 1942 to 1946, the project was under the direction of Major General Leslie Groves of the US Army... |
First nuclear fission explosion on July 16, 1945 in the Trinity test Trinity test Trinity was the code name of the first test of a nuclear weapon. This test was conducted by the United States Army on July 16, 1945, in the Jornada del Muerto desert about 35 miles southeast of Socorro, New Mexico, at the new White Sands Proving Ground, which incorporated the Alamogordo Bombing... in New Mexico. |
| 1946 | Theodor V. Ionescu Theodor V. Ionescu Theodor V. Ionescu, Prof. Dr. Doc. was a Romanian physicist and inventor who made remarkable discoveries in plasma physics, ionosphere physics, ion coupling electrons in dense plasmas, masers, magnetron amplifiers, and Zeeman effects related to controlled nuclear fusion and quantum emission... and Vasile Mihu |
Reported the construction of the first hydrogen maser Hydrogen maser A Hydrogen maser, also known as hydrogen frequency standard, is a specific type of maser that uses the intrinsic properties of the hydrogen atom to serve as a precision frequency reference.... by coherent stimulation of radiation in molecular hydrogen. |
| 1947 | G. D. Rochester and C. C. Butler | Published two cloud chamber Cloud chamber The cloud chamber, also known as the Wilson chamber, is a particle detector used for detecting ionizing radiation. In its most basic form, a cloud chamber is a sealed environment containing a supersaturated vapor of water or alcohol. When a charged particle interacts with the mixture, it ionizes it... photographs of cosmic ray-induced events, one showing what appeared to be a neutral particle decaying into two charged pions, and one which appeared to be a charged particle decaying into a charged pion and something neutral. The estimated mass of the new particles was very rough, about half a proton's mass. More examples of these "V-particles" were slow in coming, and they were soon given the name kaons. |
| 1948 | Sin-Itiro Tomonaga Sin-Itiro Tomonaga was a Japanese physicist, influential in the development of quantum electrodynamics, work for which he was jointly awarded the Nobel Prize in Physics in 1965 along with Richard Feynman and Julian Schwinger.-Biography:... and Julian Schwinger Julian Schwinger Julian Seymour Schwinger was an American theoretical physicist. He is best known for his work on the theory of quantum electrodynamics, in particular for developing a relativistically invariant perturbation theory, and for renormalizing QED to one loop order.Schwinger is recognized as one of the... |
Independently introduced perturbative renormalization Renormalization In quantum field theory, the statistical mechanics of fields, and the theory of self-similar geometric structures, renormalization is any of a collection of techniques used to treat infinities arising in calculated quantities.... as a method of correcting the original Lagrangian Lagrangian The Lagrangian, L, of a dynamical system is a function that summarizes the dynamics of the system. It is named after Joseph Louis Lagrange. The concept of a Lagrangian was originally introduced in a reformulation of classical mechanics by Irish mathematician William Rowan Hamilton known as... of a quantum field theory Quantum field theory Quantum field theory provides a theoretical framework for constructing quantum mechanical models of systems classically parametrized by an infinite number of dynamical degrees of freedom, that is, fields and many-body systems. It is the natural and quantitative language of particle physics and... so as to eliminate an infinite series of counterterms that would otherwise result. |
| 1948 | Richard Feynman Richard Feynman Richard Phillips Feynman was an American physicist known for his work in the path integral formulation of quantum mechanics, the theory of quantum electrodynamics and the physics of the superfluidity of supercooled liquid helium, as well as in particle physics... |
Stated the path integral formulation Path integral formulation The path integral formulation of quantum mechanics is a description of quantum theory which generalizes the action principle of classical mechanics... of quantum mechanics. |
| 1949 | Freeman Dyson Freeman Dyson Freeman John Dyson FRS is a British-born American theoretical physicist and mathematician, famous for his work in quantum field theory, solid-state physics, astronomy and nuclear engineering. Dyson is a member of the Board of Sponsors of the Bulletin of the Atomic Scientists... |
Determined the equivalence of the formulations of quantum electrodynamics Quantum electrodynamics Quantum electrodynamics is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved... that existed by that time — Richard Feynman Richard Feynman Richard Phillips Feynman was an American physicist known for his work in the path integral formulation of quantum mechanics, the theory of quantum electrodynamics and the physics of the superfluidity of supercooled liquid helium, as well as in particle physics... 's diagrammatic path integral formulation Path integral formulation The path integral formulation of quantum mechanics is a description of quantum theory which generalizes the action principle of classical mechanics... and the operator method developed by Julian Schwinger Julian Schwinger Julian Seymour Schwinger was an American theoretical physicist. He is best known for his work on the theory of quantum electrodynamics, in particular for developing a relativistically invariant perturbation theory, and for renormalizing QED to one loop order.Schwinger is recognized as one of the... and Sin-Itiro Tomonaga Sin-Itiro Tomonaga was a Japanese physicist, influential in the development of quantum electrodynamics, work for which he was jointly awarded the Nobel Prize in Physics in 1965 along with Richard Feynman and Julian Schwinger.-Biography:... . A by-product of that demonstration was the invention of the Dyson series Dyson series In scattering theory, the Dyson series, formulated by British-born American physicist Freeman Dyson, is a perturbative series, and each term is represented by Feynman diagrams. This series diverges asymptotically, but in quantum electrodynamics at the second order the difference from... . |
| 1951 | Clemens C. J. Roothaan Clemens C. J. Roothaan Clemens C.J. Roothaan was born in 1918 in Nijmegen, the Netherlands. He enrolled TU Delft in 1935 to study electrical engineering. During World War II he was first detained as a prisoner of war camp, later he was sent for a year to a concentration camp in Vught... and George G. Hall George G. Hall George Garfield Hall , is an applied mathematician and scientist of distinction, known for original work and contributions to the field of Quantum chemistry.... |
Derived the Roothaan-Hall equations, putting rigorous molecular orbital methods on a firm basis. |
| 1951 | Edward Teller Edward Teller Edward Teller was a Hungarian-American theoretical physicist, known colloquially as "the father of the hydrogen bomb," even though he did not care for the title. Teller made numerous contributions to nuclear and molecular physics, spectroscopy , and surface physics... --'Father of the Hydrogen bomb', physicist and Stanisław Ulam, mathematician |
Were reported to have written jointly in March 1951 a classified report on “Hydrodynamic Lenses and Radiation Mirrors” that resulted in the next step in the Manhattan Project Manhattan Project The Manhattan Project was a research and development program, led by the United States with participation from the United Kingdom and Canada, that produced the first atomic bomb during World War II. From 1942 to 1946, the project was under the direction of Major General Leslie Groves of the US Army... . In 1999, Edward Teller told a Scientific American Scientific American Scientific American is a popular science magazine. It is notable for its long history of presenting science monthly to an educated but not necessarily scientific public, through its careful attention to the clarity of its text as well as the quality of its specially commissioned color graphics... reporter: "I contributed; Ulam did not. I'm sorry I had to answer it in this abrupt way. Ulam was rightly dissatisfied with an old approach. He came to me with a part of an idea which I already had worked out and (had) difficulty getting people to listen to. He was willing to sign a paper. When it then came to defending that paper and really putting work into it, he refused. He said: ``I don't believe in it". |
| 1951 and 1952 | Manhattan Project Manhattan Project The Manhattan Project was a research and development program, led by the United States with participation from the United Kingdom and Canada, that produced the first atomic bomb during World War II. From 1942 to 1946, the project was under the direction of Major General Leslie Groves of the US Army... |
First planned fusion thermonuclear reaction experiment was carried out successfully in the Spring of 1951 at Eniwetok, based only on the work of Edward Teller and Dr. Hans A. Bethe who wrote in 1952:``the results of the calculations of Ulam and Fermi in 1950 (which were logical steps in the program) would have led nearly every scientist to give up the thermonuclear program altogether. Only Teller's persistent belief in the practicality of thermonuclear reactions led to our present, completely novel concepts in this field.". The Los Alamos Laboratory proposed a date in November 1952 for a Hydrogen bomb, full-scale test that was apparently kept. |
| 1951 | Felix Bloch Felix Bloch Felix Bloch was a Swiss physicist, working mainly in the U.S.-Life and work:Bloch was born in Zürich, Switzerland to Jewish parents Gustav and Agnes Bloch. He was educated there and at the Eidgenössische Technische Hochschule, also in Zürich. Initially studying engineering he soon changed to physics... and Edward Mills Purcell Edward Mills Purcell Edward Mills Purcell was an American physicist who shared the 1952 Nobel Prize for Physics for his independent discovery of nuclear magnetic resonance in liquids and in solids. Nuclear magnetic resonance has become widely used to study the molecular structure of pure materials and the... |
Received a shared Nobel Prize in Physics for their first observations of the quantum phenomenon of nuclear magnetic resonance Nuclear magnetic resonance Nuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation... reported in 1949 ("for their development of new methods for nuclear magnetic precision measurements and discoveries in connection therewith"); Felix Bloch reported his NMR NMR NMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR... discovery as "the Principle of Nuclear Induction" (in collaboration with W. W. Hansen, and M. Packard); Purcell reported his contribution as ``Research in Nuclear Magnetism", and gave credit to his coworkers such as Herbert S. Gutowsky Herbert S. Gutowsky Herbert S. Gutowsky was an American chemist who was a Professor of Chemistry at the University of Illinois at Urbana-Champaign. His pioneering work made nuclear magnetic resonance spectroscopy one of the most effective tools in chemical and medical research.- Birth and education :Herbert S... for their NMR contributions, as well as theoretical researchers of nuclear magnetism such as Professor Van Vleck. |
| 1952 | Albert W. Overhauser | Formulated a theory of theory of the dynamic nuclear polarization, also known as the Overhauser Effect; other contenders are the subsequent theory of Ionel Solomon reported in 1955 that includes the Solomon equations for dipolar coupled spin dynamics, and that of R. Kaiser in 1963; Overhauser was elected to the US National Academy of Sciences in 1974 and received the National Medal of Science in 1994. The general Overhauser effect was first demonstrated experimentally by T. R. Carver and Charles P. Slichter in 1953 |
| 1953 | Charles H. Townes,(collaborating with J. P. Gordon, and H. J. Zeiger) | Built and reported the first ammonia maser Maser A maser is a device that produces coherent electromagnetic waves through amplification by stimulated emission. Historically, “maser” derives from the original, upper-case acronym MASER, which stands for "Microwave Amplification by Stimulated Emission of Radiation"... ; received a Nobel prize in 1964 for his experimental success in producing coherent radiation by atoms and molecules. |
| 1954 | Chen Ning Yang and Robert Mills Robert Mills (physicist) Robert L. Mills was a physicist, specializing in quantum field theory, the theory of alloys, and many-body theory. While sharing an office at Brookhaven National Laboratory, in 1954, Chen Ning Yang and Mills proposed a tensor equation for what are now called Yang-Mills fields... |
Derived a gauge theory Gauge theory In physics, gauge invariance is the property of a field theory in which different configurations of the underlying fundamental but unobservable fields result in identical observable quantities. A theory with such a property is called a gauge theory... for nonabelian group Nonabelian group In mathematics, a non-abelian group, also sometimes called a non-commutative group, is a group in which there are at least two elements a and b of G such that a * b ≠ b * a... s, leading to the successful formulation of both electroweak unification Electroweak interaction In particle physics, the electroweak interaction is the unified description of two of the four known fundamental interactions of nature: electromagnetism and the weak interaction. Although these two forces appear very different at everyday low energies, the theory models them as two different... and quantum chromodynamics Quantum chromodynamics In theoretical physics, quantum chromodynamics is a theory of the strong interaction , a fundamental force describing the interactions of the quarks and gluons making up hadrons . It is the study of the SU Yang–Mills theory of color-charged fermions... . |
| 1955 | Ionel Solomon | First nuclear magnetic resonance Nuclear magnetic resonance Nuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation... theory of magnetic dipole Magnetic dipole A magnetic dipole is the limit of either a closed loop of electric current or a pair of poles as the dimensions of the source are reduced to zero while keeping the magnetic moment constant. It is a magnetic analogue of the electric dipole, but the analogy is not complete. In particular, a magnetic... coupled nuclear spins and of the Nuclear Overhauser Effect Nuclear Overhauser effect The Nuclear Overhauser Effect is the transfer of nuclear spin polarization from one nuclear spin population to another via cross-relaxation. It is a common phenomenon observed by nuclear magnetic resonance spectroscopy. The theoretical basis for the NOE was described and experimentally verified... (NOE). |
| 1955 and 1956 | Murray Gell-Mann Murray Gell-Mann Murray Gell-Mann is an American physicist and linguist who received the 1969 Nobel Prize in physics for his work on the theory of elementary particles... and Kazuhiko Nishijima Kazuhiko Nishijima -Awards:*Nishina Memorial Prize*Japan Academy Prize*Order of Culture of Japan*Guggenheim Fellowship-Further reading:... |
Independently derived the Gell-Mann–Nishijima formula Gell-Mann–Nishijima formula The Gell-Mann–Nishijima formula relates the baryon number B, the strangeness S, the isospin I3 of hadrons to the charge Q. It was originally given by Kazuhiko Nishijima and Tadao Nakano in 1953, and lead to the proposal of strangeness as a concept, which Nishijima originally called "eta-charge"... , which relates the baryon number B, the strangeness Strangeness In particle physics, strangeness S is a property of particles, expressed as a quantum number, for describing decay of particles in strong and electromagnetic reactions, which occur in a short period of time... S, and the isospin Isospin In physics, and specifically, particle physics, isospin is a quantum number related to the strong interaction. This term was derived from isotopic spin, but the term is confusing as two isotopes of a nucleus have different numbers of nucleons; in contrast, rotations of isospin maintain the number... Iz of hadron Hadron In particle physics, a hadron is a composite particle made of quarks held together by the strong force... s to the charge Q, eventually leading to the systematic categorization of hadrons and, ultimately, the Quark Model Quark model In physics, the quark model is a classification scheme for hadrons in terms of their valence quarks—the quarks and antiquarks which give rise to the quantum numbers of the hadrons.... of hadron composition. |
| 1956 | P. Kuroda | Predicted that self-sustaining nuclear chain reactions should occur in natural uranium deposits. |
| 1956 | Clyde L. Cowan and Frederick Reines Frederick Reines Frederick Reines was an American physicist. He was awarded the 1995 Nobel Prize in Physics for his co-detection of the neutrino with Clyde Cowan in the neutrino experiment, and may be the only scientist in history "so intimately associated with the discovery of an elementary particle and the... |
Experimentally proved the existence of the neutrino. |
| 1957 | John Bardeen John Bardeen John Bardeen was an American physicist and electrical engineer, the only person to have won the Nobel Prize in Physics twice: first in 1956 with William Shockley and Walter Brattain for the invention of the transistor; and again in 1972 with Leon Neil Cooper and John Robert Schrieffer for a... , Leon Cooper Leon Cooper Leon N Cooper is an American physicist and Nobel Prize laureate, who with John Bardeen and John Robert Schrieffer, developed the BCS theory of superconductivity... and John Robert Schrieffer John Robert Schrieffer John Robert Schrieffer is an American physicist and, with John Bardeen and Leon N Cooper, recipient of the 1972 Nobel Prize for Physics for developing the BCS theory, the first successful microscopic theory of superconductivity.-Biography:... |
Proposed their quantum BCS theory BCS theory BCS theory — proposed by Bardeen, Cooper, and Schrieffer in 1957 — is the first microscopic theory of superconductivity since its discovery in 1911. The theory describes superconductivity as a microscopic effect caused by a "condensation" of pairs of electrons into a boson-like state... of low temperature superconductivity Superconductivity Superconductivity is a phenomenon of exactly zero electrical resistance occurring in certain materials below a characteristic temperature. It was discovered by Heike Kamerlingh Onnes on April 8, 1911 in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum... as a macroscopic quantum coherence phenomenon involving phonon Phonon In physics, a phonon is a collective excitation in a periodic, elastic arrangement of atoms or molecules in condensed matter, such as solids and some liquids... coupled electron pairs with opposite spin, for which their received a Nobel prize in 1972. |
| 1957 | William Alfred Fowler William Alfred Fowler William Alfred "Willy" Fowler was an American astrophysicist and winner of the Nobel Prize for Physics in 1983. He should not be confused with the British astronomer Alfred Fowler.... , Margaret Burbidge Margaret Burbidge Eleanor Margaret Burbidge, née Peachey, FRS is a British-born American astrophysicist, noted for original research and holding many administrative posts, including director of the Royal Greenwich Observatory.... , Geoffrey Burbidge Geoffrey Burbidge Geoffrey Ronald Burbidge FRS was an English astronomy professor, most recently at the University of California, San Diego. He was married to astrophysicist Dr. Margaret Burbidge.-Education:... , and Fred Hoyle Fred Hoyle Sir Fred Hoyle FRS was an English astronomer and mathematician noted primarily for his contribution to the theory of stellar nucleosynthesis and his often controversial stance on other cosmological and scientific matters—in particular his rejection of the "Big Bang" theory, a term originally... |
In their 1957 paper Synthesis of the Elements in Stars, they explained how the abundances of essentially all but the lightest chemical elements could be explained by the process of nucleosynthesis Nucleosynthesis Nucleosynthesis is the process of creating new atomic nuclei from pre-existing nucleons . It is thought that the primordial nucleons themselves were formed from the quark–gluon plasma from the Big Bang as it cooled below two trillion degrees... in stars. |
| 1958 and 1976 | Edward Raymond Andrew, FRS | Made critical field measurements on superconducting tin foils in 1949 for his PhD; then in 1958 he discovered the magic angle spinning Magic angle spinning In nuclear magnetic resonance, magic angle spinning is a technique often used to perform experiments in solid-state NMR spectroscopy.By spinning the sample at the magic angle θm In nuclear magnetic resonance, magic angle spinning (MAS) is a technique often used to perform experiments in... (MAS) technique for obtaining resolved chemical shifts in solids, including Knight shift Knight shift The Knight shift is a shift in the nuclear magnetic resonance frequency of a paramagneticsubstance first published in 1949 by the American physicist Walter David Knight.The Knight shift is due to the conduction electrons in metals... s in metals, and subsequently in 1964 carried out pioneering experiments with nuclear magnetic resonance imaging (NMRI) also in solids; for his important discoveries he was elected Fellow of the Royal Society in 1984; together with R.G. Eades he published an important theoretical paper on the separation of intramolecular and intermolecular contributions to the Van Vleck second moment of the NMR NMR NMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR... spectrum |
| 1961 | Clauss Jönsson | Performed Young's Thomas Young (scientist) Thomas Young was an English polymath. He is famous for having partly deciphered Egyptian hieroglyphics before Jean-François Champollion eventually expanded on his work... double-slit experiment Double-slit experiment The double-slit experiment, sometimes called Young's experiment, is a demonstration that matter and energy can display characteristics of both waves and particles... (1909) for the first time with particles other than photons by using electrons and with similar results, confirming that massive particles also behaved according to the wave-particle duality that is a fundamental principle of quantum field theory Quantum field theory Quantum field theory provides a theoretical framework for constructing quantum mechanical models of systems classically parametrized by an infinite number of dynamical degrees of freedom, that is, fields and many-body systems. It is the natural and quantitative language of particle physics and... . |
| 1961 | Anatole Abragam Anatole Abragam Anatole Abragam was a French physicist who wrote The Principles of Nuclear Magnetism and has made significant contributions to the field of nuclear magnetic resonance. Originally from Russia, Abragam and his family emigrated to France in 1925.After being educated at the University of Paris, , he... |
Published in 1961 the fundamental textbook on the quantum theory of Nuclear Magnetic Resonance Nuclear magnetic resonance Nuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation... entitled ``The Principles of Nuclear Magnetism". Clarendon Press: Oxford. pp. 599. OCLC 242700 (1961); it surpasses by far both the earlier textbook by E.R. Andrew published in 1955, and the Magnetic Resonance textbook published two years later by Professor Charles P. Slichter |
| 1961 | Sheldon Lee Glashow Sheldon Lee Glashow Sheldon Lee Glashow is a Nobel Prize winning American theoretical physicist. He is the Metcalf Professor of Mathematics and Physics at Boston University.-Birth and education:... |
Extended the electroweak unification models developed by Julian Schwinger Julian Schwinger Julian Seymour Schwinger was an American theoretical physicist. He is best known for his work on the theory of quantum electrodynamics, in particular for developing a relativistically invariant perturbation theory, and for renormalizing QED to one loop order.Schwinger is recognized as one of the... by including a short range neutral current Neutral current Weak neutral current interactions are one of the ways in which subatomic particles can interact by means of the weak force. These interactions are mediated by the boson... , the Z_o. The resulting symmetry structure that Glashow proposed, SU(2) X U(1), formed the basis of the accepted theory of the electroweak interaction Electroweak interaction In particle physics, the electroweak interaction is the unified description of two of the four known fundamental interactions of nature: electromagnetism and the weak interaction. Although these two forces appear very different at everyday low energies, the theory models them as two different... s. |
| 1962 | Leon M. Lederman Leon M. Lederman Leon Max Lederman is an American experimental physicist and Nobel Prize in Physics laureate for his work with neutrinos. He is Director Emeritus of Fermi National Accelerator Laboratory in Batavia, Illinois, USA... , Melvin Schwartz Melvin Schwartz Melvin Schwartz was an American physicist. He shared the 1988 Nobel Prize in Physics with Leon M. Lederman and Jack Steinberger for their development of the neutrino beam method and their demonstration of the doublet structure of the leptons through the discovery of the muon neutrino.He grew up in... and Jack Steinberger Jack Steinberger Jack Steinberger is a German-American physicist currently residing near Geneva, Switzerland. He co-discovered the muon neutrino, along with Leon Lederman and Melvin Schwartz, for which they were given the 1988 Nobel Prize in Physics.-Life:... |
Showed that more than one type of neutrino Neutrino A neutrino is an electrically neutral, weakly interacting elementary subatomic particle with a half-integer spin, chirality and a disputed but small non-zero mass. It is able to pass through ordinary matter almost unaffected... exists by detecting interactions of the muon Muon The muon |mu]] used to represent it) is an elementary particle similar to the electron, with a unitary negative electric charge and a spin of ½. Together with the electron, the tau, and the three neutrinos, it is classified as a lepton... neutrino (already hypothesised with the name "neutretto") |
| 1962 | Murray Gell-Mann Murray Gell-Mann Murray Gell-Mann is an American physicist and linguist who received the 1969 Nobel Prize in physics for his work on the theory of elementary particles... and Yuval Ne'eman Yuval Ne'eman Yuval Ne'eman , was a renowned Israeli theoretical physicist, military scientist, and politician. He was a minister in the Israeli government in the 1980s and early 1990s.-Biography:... |
Independently classified the hadrons according to a system that Gell-Mann called the "Eightfold Way Eightfold way (physics) In physics, the Eightfold Way is a term coined by American physicist Murray Gell-Mann for a theory organizing subatomic baryons and mesons into octets... ," and which ultimately led to the quark model Quark model In physics, the quark model is a classification scheme for hadrons in terms of their valence quarks—the quarks and antiquarks which give rise to the quantum numbers of the hadrons.... (1964) of hadron Hadron In particle physics, a hadron is a composite particle made of quarks held together by the strong force... composition. |
| 1962 | Jeffrey Goldstone Jeffrey Goldstone Jeffrey Goldstone is a British-born theoretical physicist and an emeritus physics faculty at MIT Center for Theoretical Physics.He worked at the University of Cambridge until 1977.... , Yoichiro Nambu Yoichiro Nambu is a Japanese-born American physicist, currently a professor at the University of Chicago. Known for his contributions to the field of theoretical physics, he was awarded a one-half share of the Nobel Prize in Physics in 2008 for the discovery of the mechanism of spontaneous broken symmetry in... , Abdus Salam Abdus Salam Mohammad Abdus Salam, NI, SPk Mohammad Abdus Salam, NI, SPk Mohammad Abdus Salam, NI, SPk (Urdu: محمد عبد السلام, pronounced , (January 29, 1926– November 21, 1996) was a Pakistani theoretical physicist and Nobel laureate in Physics for his work on the electroweak unification of the... , and Steven Weinberg Steven Weinberg Steven Weinberg is an American theoretical physicist and Nobel laureate in Physics for his contributions with Abdus Salam and Sheldon Glashow to the unification of the weak force and electromagnetic interaction between elementary particles.... |
Developed what is now known as Goldstone's Theorem Goldstone boson In particle and condensed matter physics, Goldstone bosons or Nambu–Goldstone bosons are bosons that appear necessarily in models exhibiting spontaneous breakdown of continuous symmetries... , in which it was proved that, if there is continuous symmetry transformation under which the Lagrangian is invariant, then either the vacuum state is also invariant under the transformation, or there must exist spinless particles of zero mass, thereafter called Nambu-Goldstone bosons Goldstone boson In particle and condensed matter physics, Goldstone bosons or Nambu–Goldstone bosons are bosons that appear necessarily in models exhibiting spontaneous breakdown of continuous symmetries... . Subsequently, in 2004 Steven Weinberg explained in his 3-volume book on "Quantum Field Theory" that low temperature superconductivity could not be explained by the BCS model alone without the appearance of Goldstone bosons upon symmetry breaking. One notes however that the importance of symmetry breaking for superconductivity was already pointed out in 1973 by Brian David Josephson in his Nobel lecture. |
| 1962 to 1973 | Brian David Josephson Brian David Josephson Brian David Josephson, FRS is a Welsh physicist. He became a Nobel Prize laureate in 1973 for the prediction of the eponymous Josephson effect.... , FRS |
Predicted correctly the quantum tunnelling effect involving supercurrents while he was a PhD student under the supervision of Professor Brian Pippard at the Royal Society Mond Laboratory in Cambridge, UK; subsequently, in 1964, he applied his theory to coupled superconductors. The Josephson, tunnelling supercurrent effect was later demonstrated experimentally at Bell Labs in the USA. For his important quantum discovery he was awarded the Nobel Prize in Physics in 1973. |
| 1963 | Eugene P. Wigner | Laid the foundation for the theory of symmetries in quantum mechanics as well as for basic research into the structure of the atomic nucleus; made important "contributions to the theory of the atomic nucleus and the elementary particles, particularly through the discovery and application of fundamental symmetry principles"; he shared half of his Nobel prize in Physics with Maria Goeppert-Mayer and J. Hans D. Jensen J. Hans D. Jensen Johannes Hans Daniel Jensen was a German nuclear physicist. During World War II, he worked on the German nuclear energy project, known as the Uranium Club, in which he made contributions to the separation of uranium isotopes. After the war Jensen was a professor at the University of Heidelberg... . |
| 1963 | Maria Goeppert Mayer and J. Hans D. Jensen J. Hans D. Jensen Johannes Hans Daniel Jensen was a German nuclear physicist. During World War II, he worked on the German nuclear energy project, known as the Uranium Club, in which he made contributions to the separation of uranium isotopes. After the war Jensen was a professor at the University of Heidelberg... |
Shared with Eugene P. Wigner one half of the Nobel Prize in Physics in 1963 "for their discoveries concerning nuclear shell structure theory". |
| 1963 | Nicola Cabibbo Nicola Cabibbo Nicola Cabibbo was an Italian physicist, best known for his work on the weak interaction. He was also the president of the Italian National Institute of Nuclear Physics from 1983 to 1992, and from 1993 until his death he was the president of the Pontifical Academy of Sciences... |
Developed the mathematical matrix by which the first two (and ultimately three) generations of quarks could be predicted. |
| 1964 | Murray Gell-Mann Murray Gell-Mann Murray Gell-Mann is an American physicist and linguist who received the 1969 Nobel Prize in physics for his work on the theory of elementary particles... and George Zweig George Zweig George Zweig was originally trained as a particle physicist under Richard Feynman and later turned his attention to neurobiology... |
Independently proposed the quark model Quark A quark is an elementary particle and a fundamental constituent of matter. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. Due to a phenomenon known as color confinement, quarks are never directly... of hadrons, predicting the arbitrarily named up Up quark The up quark or u quark is the lightest of all quarks, a type of elementary particle, and a major constituent of matter. It, along with the down quark, forms the neutrons and protons of atomic nuclei... , down Down quark The down quark or d quark is the second-lightest of all quarks, a type of elementary particle, and a major constituent of matter. It, along with the up quark, forms the neutrons and protons of atomic nuclei... , and strange Strange quark The strange quark or s quark is the third-lightest of all quarks, a type of elementary particle. Strange quarks are found in hadrons, which are subatomic particles. Example of hadrons containing strange quarks include kaons , strange D mesons , Sigma baryons , and other strange particles... quarks. Gell-Mann is credited with coining the term "quark," which he found in James Joyce James Joyce James Augustine Aloysius Joyce was an Irish novelist and poet, considered to be one of the most influential writers in the modernist avant-garde of the early 20th century... 's book Finnegans Wake Finnegans Wake Finnegans Wake is a novel by Irish author James Joyce, significant for its experimental style and resulting reputation as one of the most difficult works of fiction in the English language. Written in Paris over a period of seventeen years, and published in 1939, two years before the author's... . |
| 1964 | François Englert François Englert François Englert is a Belgian theoretical physicist. He was awarded the 2010 J. J. Sakurai Prize for Theoretical Particle Physics , the Wolf Prize in Physics in 2004 and the High Energy and Particle Prize of the European Physical Society François Englert (born 6 November 1932) is a... , Robert Brout Robert Brout Robert Brout was an American-Belgian theoretical physicist who has made significant contributions in elementary particle physics... , Peter Higgs Peter Higgs Peter Ware Higgs, FRS, FRSE, FKC , is an English theoretical physicist and an emeritus professor at the University of Edinburgh.... , Gerald Guralnik Gerald Guralnik Gerald Stanford Guralnik is the Chancellor’s Professor of Physics at Brown University. He is most famous for his co-discovery of the Higgs mechanism and Higgs Boson with C. R. Hagen and Tom Kibble... , C. R. Hagen C. R. Hagen Carl Richard Hagen is a professor of particle physics at the University of Rochester. He is most noted for his contributions to the Standard Model and Symmetry breaking as well as the co-discovery of the Higgs mechanism and Higgs boson with Gerald Guralnik and Tom Kibble... , and Tom Kibble |
Postulated that a fundamental quantum field, now called the Higgs field, permeates space and, by way of the Higgs mechanism Higgs mechanism In particle physics, the Higgs mechanism is the process in which gauge bosons in a gauge theory can acquire non-vanishing masses through absorption of Nambu-Goldstone bosons arising in spontaneous symmetry breaking.... , provides mass to all the elementary subatomic particles that interact with it. While the Higgs field is postulated to confer mass on quarks and leptons, it represents only a tiny portion of the masses of other subatomic particles, such as protons and neutrons. In these, gluons that bind quarks together confer most of the particle mass. The Higgs mechanism, which gives mass to vector bosons, such as Proca's vector spin-1 mesons, was theorized in 1964 by François Englert and Robert Brout. In October of the same year, Peter Higgs, working from the ideas of Philip Anderson reached the same conclusions; and, independently, by Gerald Guralnik, C. R. Hagen, and Tom Kibble, who worked out the results by the spring of 1963. |
| 1964 | Sheldon Lee Glashow Sheldon Lee Glashow Sheldon Lee Glashow is a Nobel Prize winning American theoretical physicist. He is the Metcalf Professor of Mathematics and Physics at Boston University.-Birth and education:... and James Bjorken James Bjorken James Daniel "BJ" Bjorken is one of the world's foremost theoretical physicists. He was a Putnam Fellow in 1954, received a BS in physics from MIT in 1956, and obtained his PhD from Stanford University in 1959... |
Predicted the existence of the charm quark Charm quark The charm quark or c quark is the third most massive of all quarks, a type of elementary particle. Charm quarks are found in hadrons, which are subatomic particles made of quarks... . The addition was proposed because it allowed for a better description of the weak interaction Weak interaction Weak interaction , is one of the four fundamental forces of nature, alongside the strong nuclear force, electromagnetism, and gravity. It is responsible for the radioactive decay of subatomic particles and initiates the process known as hydrogen fusion in stars... (the mechanism that allows quarks and other particles to decay), equalized the number of known quarks with the number of known leptons, and implied a mass formula that correctly reproduced the masses of the known mesons. |
| 1964 | Nikolai G. Basov and Aleksandr M. Prokhorov | Shared the Nobel Prize in Physics in 1964 for, respectively, semiconductor lasers and Quantum Electronics; they also shared the prize with Charles H. Townes, the inventor of the ammonium maser Maser A maser is a device that produces coherent electromagnetic waves through amplification by stimulated emission. Historically, “maser” derives from the original, upper-case acronym MASER, which stands for "Microwave Amplification by Stimulated Emission of Radiation"... . |
| 1967 | Steven Weinberg Steven Weinberg Steven Weinberg is an American theoretical physicist and Nobel laureate in Physics for his contributions with Abdus Salam and Sheldon Glashow to the unification of the weak force and electromagnetic interaction between elementary particles.... and Abdus Salam Abdus Salam Mohammad Abdus Salam, NI, SPk Mohammad Abdus Salam, NI, SPk Mohammad Abdus Salam, NI, SPk (Urdu: محمد عبد السلام, pronounced , (January 29, 1926– November 21, 1996) was a Pakistani theoretical physicist and Nobel laureate in Physics for his work on the electroweak unification of the... |
Published a paper in which he described Yang-Mills Theory using the SU(2) X U(1) supersymmetry Supersymmetry In particle physics, supersymmetry is a symmetry that relates elementary particles of one spin to other particles that differ by half a unit of spin and are known as superpartners... group, thereby yielding a mass for the W particle of the Weak Interaction Weak interaction Weak interaction , is one of the four fundamental forces of nature, alongside the strong nuclear force, electromagnetism, and gravity. It is responsible for the radioactive decay of subatomic particles and initiates the process known as hydrogen fusion in stars... via spontaneous symmetry breaking Spontaneous symmetry breaking Spontaneous symmetry breaking is the process by which a system described in a theoretically symmetrical way ends up in an apparently asymmetric state.... . |
| 1968 | Stanford University Stanford University The Leland Stanford Junior University, commonly referred to as Stanford University or Stanford, is a private research university on an campus located near Palo Alto, California. It is situated in the northwestern Santa Clara Valley on the San Francisco Peninsula, approximately northwest of San... |
Deep inelastic scattering Deep Inelastic Scattering Deep inelastic scattering is the name given to a process used to probe the insides of hadrons , using electrons, muons and neutrinos. It provided the first convincing evidence of the reality of quarks, which up until that point had been considered by many to be a purely mathematical phenomenon... experiments at the Stanford Linear Accelerator Center Stanford Linear Accelerator Center The SLAC National Accelerator Laboratory, originally named Stanford Linear Accelerator Center, is a United States Department of Energy National Laboratory operated by Stanford University under the programmatic direction of the U.S... (SLAC) showed that the proton Proton The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number.... contained much smaller, point-like objects and was therefore not an elementary particle. Physicists at the time were reluctant to identify these objects with quarks, instead calling them "partons" — a term coined by Richard Feynman. The objects that were observed at SLAC would later be identified as up Up quark The up quark or u quark is the lightest of all quarks, a type of elementary particle, and a major constituent of matter. It, along with the down quark, forms the neutrons and protons of atomic nuclei... and down Down quark The down quark or d quark is the second-lightest of all quarks, a type of elementary particle, and a major constituent of matter. It, along with the up quark, forms the neutrons and protons of atomic nuclei... quarks. Nevertheless, "parton" remains in use as a collective term for the constituents of hadrons (quarks, antiquarks, and gluons). The strange quark's Strange quark The strange quark or s quark is the third-lightest of all quarks, a type of elementary particle. Strange quarks are found in hadrons, which are subatomic particles. Example of hadrons containing strange quarks include kaons , strange D mesons , Sigma baryons , and other strange particles... existence was indirectly validated by the SLAC's scattering experiments: not only was it a necessary component of Gell-Mann and Zweig's three-quark model, but it provided an explanation for the kaon Kaon In particle physics, a kaon is any one of a group of four mesons distinguished by the fact that they carry a quantum number called strangeness... (K) and pion Pion In particle physics, a pion is any of three subatomic particles: , , and . Pions are the lightest mesons and they play an important role in explaining the low-energy properties of the strong nuclear force.... (π) hadrons discovered in cosmic rays in 1947. |
| 1969 to 1977 | Sir Neville Mott and Philip Warren Anderson Philip Warren Anderson Philip Warren Anderson is an American physicist and Nobel laureate. Anderson has made contributions to the theories of localization, antiferromagnetism and high-temperature superconductivity.- Biography :... |
Published quantum theories for electrons in non-crystalline solids, such as glasses and amorphous semiconductors; received in 1977 a Nobel prize in Physics for their investigations into the electronic structure of magnetic and disordered systems,which allowed for the development of electronic switching and memory devices in computers; shared with John Hasbrouck Van Vleck John Hasbrouck van Vleck John Hasbrouck Van Vleck was an American physicist and mathematician, co-awarded the 1977 Nobel Prize in Physics, for his contributions to the understanding of the behavior of electrons in magnetic solids.... for his contributions to the understanding of the behavior of electrons in magnetic solids; he established the fundamentals of the quantum mechanical theory of magnetism and the crystal field theory (chemical bonding in metal complexes) and is regarded as the Father of modern Magnetism. |
| 1969 and 1970 | Theodor V. Ionescu Theodor V. Ionescu Theodor V. Ionescu, Prof. Dr. Doc. was a Romanian physicist and inventor who made remarkable discoveries in plasma physics, ionosphere physics, ion coupling electrons in dense plasmas, masers, magnetron amplifiers, and Zeeman effects related to controlled nuclear fusion and quantum emission... , Radu Pârvan and I.C. Baianu |
Observed and reported quantum amplified stimulation of electromagnetic radiation in hot deuterium plasmas in a longitudinal magnetic field; published a quantum theory of the amplified coherent emission of radiowaves and microwaves by focused electron beams coupled to ions in hot plasmas. |
| 1970 | Sheldon Lee Glashow Sheldon Lee Glashow Sheldon Lee Glashow is a Nobel Prize winning American theoretical physicist. He is the Metcalf Professor of Mathematics and Physics at Boston University.-Birth and education:... , John Iliopoulos John Iliopoulos John Iliopoulos is a Greek physicist and the first person to present the Standard Model of particle physics in a single report. He is best known for his prediction of the charm quark with Sheldon Lee Glashow and Luciano Maiani... and Luciano Maiani Luciano Maiani Luciano Maiani is a San Marino citizen physicist best known for his prediction of the charm quark with Sheldon Lee Glashow and John Iliopoulos .-Academic history:... |
Predicted the charmed quark that was subsequently found experimentally and shared a Nobel prize for their theoretical prediction. |
| 1970 | Anatole Abragam Anatole Abragam Anatole Abragam was a French physicist who wrote The Principles of Nuclear Magnetism and has made significant contributions to the field of nuclear magnetic resonance. Originally from Russia, Abragam and his family emigrated to France in 1925.After being educated at the University of Paris, , he... and B. Bleaney |
Presented an extensive quantum theory of Electron Paramagnetic Resonance Electron paramagnetic resonance Electron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion... of transition ions with thoroughly worked out examples in an encyclopedic style that remains todate a key, enormous reference book; significantly, this unsurpassed quantum textbook, which is widely appreciated in the quantum mechanics community, was dedicated to J. H. Van Vleck. |
| 1971 | Martinus J. G. Veltman Martinus J. G. Veltman Martinus Justinus Godefriedus Veltman is a Dutch theoretical physicist. He shared the 1999 Nobel Prize in physics with his former student Gerardus 't Hooft for their work on particle theory.-Biography:... and Gerardus 't Hooft Gerardus 't Hooft Gerardus 't Hooft is a Dutch theoretical physicist and professor at Utrecht University, the Netherlands. He shared the 1999 Nobel Prize in Physics with his thesis advisor Martinus J. G... |
Showed that, if the symmetries of Yang-Mills Theory were to be broken according to the method suggested by Peter Higgs Peter Higgs Peter Ware Higgs, FRS, FRSE, FKC , is an English theoretical physicist and an emeritus professor at the University of Edinburgh.... , then Yang-Mills theory can be renormalized. The renormalization of Yang-Mills Theory predicted the existence of a massless particle, called the gluon Gluon Gluons are elementary particles which act as the exchange particles for the color force between quarks, analogous to the exchange of photons in the electromagnetic force between two charged particles.... , which could explain the nuclear Strong Force. It also explained how the particles of the Weak Interaction Weak interaction Weak interaction , is one of the four fundamental forces of nature, alongside the strong nuclear force, electromagnetism, and gravity. It is responsible for the radioactive decay of subatomic particles and initiates the process known as hydrogen fusion in stars... , the W and Z bosons W and Z bosons The W and Z bosons are the elementary particles that mediate the weak interaction; their symbols are , and . The W bosons have a positive and negative electric charge of 1 elementary charge respectively and are each other's antiparticle. The Z boson is electrically neutral and its own... , obtained their mass via spontaneous symmetry breaking Spontaneous symmetry breaking Spontaneous symmetry breaking is the process by which a system described in a theoretically symmetrical way ends up in an apparently asymmetric state.... and the Yukawa interaction Yukawa interaction In particle physics, Yukawa's interaction, named after Hideki Yukawa, is an interaction between a scalar field \phi and a Dirac field \Psi of the typeV \approx g\bar\Psi \phi \Psi or g \bar \Psi \gamma^5 \phi \Psi .... . |
| 1971 | Jean Jeener Jean Jeener Jean Louis Charles Jeener is a Belgian physical chemist and physicist, well known for his experimental and theoretical contributions to spin thermodynamics in solids and for his invention of Two-dimensional nuclear magnetic resonance spectroscopy. He was born in Brussels in 1931, son of Raymond... , solid-state NMR physicist, professor |
Introduced two-dimensional FT-NMR Spectroscopy at the Ampere Summer School in Basko Polje, Yugoslavia, in September 1971; his unpublished lecture notes for this presentation were later published in “NMR and More in Honour of Anatole Abragam”, Eds. M. Goldman and M. Porneuf, Les editions de physique, Avenue du Hoggar, Zone Industrielle de Courtaboeuf, BP 112, F-91944 Les Ulis cedex A, France (1994); ``it has shown an unprecedented impact on the development of state-of-the-art NMR spectroscopy. In principle, any multiple-dimensional NMR experiment introduced so far relies on the method proposed by Jean Jeener. Countless examples can be found in both liquid-state and solid-state NMR, as well as in NMR imaging applications in medicine, biology and material science". |
| 1972 | Francis Perrin Francis Perrin Francis Perrin was a French physicist,the son of Nobel prize-winning physicist Jean Perrin.- Physicist :Francis Perrin was born in Paris and attended École Normale Supérieure in Paris.In 1928 he obtained a doctorate in mathematical sciences from the faculté des sciences of Paris, based upon a... |
Discovered the existence of "natural nuclear fission reactors" in uranium deposits in Oklo Oklo Oklo is a region near the town of Franceville, in the Haut-Ogooué province of the Central African state of Gabon. Several natural nuclear fission reactors were discovered in the uranium mines in the region in 1972.-History:... , Gabon Gabon Gabon , officially the Gabonese Republic is a state in west central Africa sharing borders with Equatorial Guinea to the northwest, Cameroon to the north, and with the Republic of the Congo curving around the east and south. The Gulf of Guinea, an arm of the Atlantic Ocean is to the west... , where analysis of isotope ratios demonstrated that self-sustaining, nuclear chain reactions had occurred. The conditions under which a natural nuclear reactor could exist were predicted in 1956 by P. Kuroda. |
| 1973 | Frank Anthony Wilczek Frank Wilczek Frank Anthony Wilczek is a theoretical physicist from the United States and a Nobel laureate. He is currently the Herman Feshbach Professor of Physics at the Massachusetts Institute of Technology .... |
Discovered the quark asymptotic freedom in the theory of strong interactions; received the Lorentz Medal Lorentz Medal Lorentz Medal is a prize awarded every four years by the Royal Netherlands Academy of Arts and Sciences. It was established in 1925 on the occasion of the 50th anniversary of the doctorate of Hendrik Lorentz. This solid gold medal is given for important contributions to theoretical physics, though... in 2002, and the Nobel Prize in Physics in 2004 for his discovery and his subsequent contributions to Quantum Chromodynamics Quantum chromodynamics In theoretical physics, quantum chromodynamics is a theory of the strong interaction , a fundamental force describing the interactions of the quarks and gluons making up hadrons . It is the study of the SU Yang–Mills theory of color-charged fermions... . |
| 1973 | Makoto Kobayashi Makoto Kobayashi (physicist) is a Japanese physicist known for his work on CP-violation who was awarded one quarter of the 2008 Nobel Prize in Physics "for the discovery of the origin of the broken symmetry which predicts the existence of at least three families of quarks in nature."- Biography :After completing his PhD at... and Toshihide Maskawa Toshihide Maskawa is a Japanese theoretical physicist known for his work on CP-violation who was awarded one quarter of the 2008 Nobel Prize in Physics "for the discovery of the origin of the broken symmetry which predicts the existence of at least three families of quarks in nature."-Biography:A native of Aichi... |
Noted that the experimental observation of CP violation CP violation In particle physics, CP violation is a violation of the postulated CP-symmetry: the combination of C-symmetry and P-symmetry . CP-symmetry states that the laws of physics should be the same if a particle were interchanged with its antiparticle , and left and right were swapped... could be explained if an additional pair of quarks existed. The two new quarks were eventually named top Top quark The top quark, also known as the t quark or truth quark, is an elementary particle and a fundamental constituent of matter. Like all quarks, the top quark is an elementary fermion with spin-, and experiences all four fundamental interactions: gravitation, electromagnetism, weak interactions, and... and bottom Bottom quark The bottom quark, also known as the beauty quark, is a third-generation quark with a charge of − e. Although all quarks are described in a similar way by the quantum chromodynamics, the bottom quark's large bare mass , combined with low values of the CKM matrix elements Vub and Vcb, gives it a... . |
| 1973 | Peter Mansfield Peter Mansfield Sir Peter Mansfield, FRS, , is a British physicist who was awarded the 2003 Nobel Prize in Physiology or Medicine for his discoveries concerning magnetic resonance imaging . The Nobel Prize was shared with Paul Lauterbur, who also contributed to the development of MRI... |
Formulated the physical theory of Nuclear Magnetic Resonance Imaging (NMRI) |
| 1974 | Pier Giorgio Merli | Performed Young's Thomas Young (scientist) Thomas Young was an English polymath. He is famous for having partly deciphered Egyptian hieroglyphics before Jean-François Champollion eventually expanded on his work... double-slit experiment Double-slit experiment The double-slit experiment, sometimes called Young's experiment, is a demonstration that matter and energy can display characteristics of both waves and particles... (1909) using a single electron with similar results, confirming the existence of quantum fields for massive particles. |
| 1974 | Burton Richter Burton Richter Burton Richter is a Nobel Prize-winning American physicist. He led the Stanford Linear Accelerator Center team which co-discovered the J/ψ meson in 1974, alongside the Brookhaven National Laboratory team led by Samuel Ting. This discovery was part of the so-called November Revolution of particle... and Samuel Ting |
Charm quark Charm quark The charm quark or c quark is the third most massive of all quarks, a type of elementary particle. Charm quarks are found in hadrons, which are subatomic particles made of quarks... s were produced almost simultaneously by two teams in November 1974 (see November Revolution) — one at SLAC under Burton Richter, and one at Brookhaven National Laboratory Brookhaven National Laboratory Brookhaven National Laboratory , is a United States national laboratory located in Upton, New York on Long Island, and was formally established in 1947 at the site of Camp Upton, a former U.S. Army base... under Samuel Ting. The charm quarks were observed bound with charm antiquarks in mesons. The two discovering parties had independently assigned the discovered meson two different symbols, J and ψ; thus, it became formally known as the J/ψ meson. The discovery finally convinced the physics community of the quark model's validity. |
| 1975 | Martin Lewis Perl Martin Lewis Perl Martin Lewis Perl is an American physicist, who won the Nobel Prize in Physics in 1995 for his discovery of the tau lepton.His parents were Jewish emigrants to the US from the Polish area of Russia.... |
With his colleagues at the SLAC–LBL Lawrence Berkeley National Laboratory The Lawrence Berkeley National Laboratory , is a U.S. Department of Energy national laboratory conducting unclassified scientific research. It is located on the grounds of the University of California, Berkeley, in the Berkeley Hills above the central campus... group, he detected the tau in a series of experiments between 1974 and 1977. |
| 1977 | Leon Lederman | Observed the bottom quark Bottom quark The bottom quark, also known as the beauty quark, is a third-generation quark with a charge of − e. Although all quarks are described in a similar way by the quantum chromodynamics, the bottom quark's large bare mass , combined with low values of the CKM matrix elements Vub and Vcb, gives it a... with his team at Fermilab Fermilab Fermi National Accelerator Laboratory , located just outside Batavia, Illinois, near Chicago, is a US Department of Energy national laboratory specializing in high-energy particle physics... . This discovery was a strong indicator of the top quark Top quark The top quark, also known as the t quark or truth quark, is an elementary particle and a fundamental constituent of matter. Like all quarks, the top quark is an elementary fermion with spin-, and experiences all four fundamental interactions: gravitation, electromagnetism, weak interactions, and... 's existence: without the top quark, the bottom quark would have been without a partner that was required by the mathematics of the theory. |
| 1977 | Ilya Prigogine Ilya Prigogine Ilya, Viscount Prigogine was a Russian-born naturalized Belgian physical chemist and Nobel Laureate noted for his work on dissipative structures, complex systems, and irreversibility.-Biography :... |
Developed non-equilibrium, irreversible thermodynamics Thermodynamics Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation... and quantum operator theory, especially the time superoperator theory; he was awarded the Nobel Prize in Chemistry in 1977 "for his contributions to non-equilibrium thermodynamics, particularly the theory of dissipative structures". |
| 1978 | Pyotr Kapitsa Pyotr Kapitsa Pyotr Leonidovich Kapitsa was a prominent Soviet/Russian physicist and Nobel laureate.-Biography:Kapitsa was born in the city of Kronstadt and graduated from the Petrograd Polytechnical Institute in 1918. He worked for over ten years with Ernest Rutherford in the Cavendish Laboratory in Cambridge... |
Observed new phenomena in hot deuterium plasmas excited by very high power microwaves in attempts to obtain controlled thermonuclear fusion reactions in such plasmas placed in longitudinal magnetic fields, using a novel and low-cost design of thermonuclear reactor, similar in concept to that reported by Theodor V. Ionescu Theodor V. Ionescu Theodor V. Ionescu, Prof. Dr. Doc. was a Romanian physicist and inventor who made remarkable discoveries in plasma physics, ionosphere physics, ion coupling electrons in dense plasmas, masers, magnetron amplifiers, and Zeeman effects related to controlled nuclear fusion and quantum emission... et al. in 1969; received a Nobel prize for early low temperature physics experiments on helium superfluidity carried out in 1937 at the Cavendish Laboratory in Cambridge, UK, and discussed his 1977 thermonuclear reactor results in his Nobel lecture on December 8, 1978. |
| 1979 | Kenneth A. Rubinson and coworkers | Observed at the Cavendish Laboratory Cavendish Laboratory The Cavendish Laboratory is the Department of Physics at the University of Cambridge, and is part of the university's School of Physical Sciences. It was opened in 1874 as a teaching laboratory.... ferromagnetic spin wave Spin wave Spin waves are propagating disturbances in the ordering of magnetic materials. These low-lying collective excitations occur in magnetic lattices with continuous symmetry. From the equivalent quasiparticle point of view, spin waves are known as magnons, which are boson modes of the spin lattice... resonant excitations (FSWR) in locally anisotropic, FENiPB metallic glasses and interpreted the experimental results in terms of two-magnon Magnon A magnon is a collective excitation of the electrons' spin structure in a crystal lattice. In contrast, a phonon is a collective excitation of the crystal lattice atoms or ions. In the equivalent wave picture of quantum mechanics, a magnon can be viewed as a quantized spin wave. As a... dispersion and a spin exchange Spin-exchange interaction A spin-exchange interaction preserves total angular momentum of the system but may allow other aspects of the system to change. When two spin-polarized atoms in their ground state experience a spin-exchange collision, the total spin of the atoms is preserved yet the orientation of the individual... Hamiltonian Hamiltonian Hamiltonian may refer toIn mathematics :* Hamiltonian system* Hamiltonian path, in graph theory** Hamiltonian cycle, a special case of a Hamiltonian path* Hamiltonian group, in group theory* Hamiltonian... , similar in form to that of a Heisenberg ferromagnet. |
| 1980 to 1982 | Alain Aspect Alain Aspect Alain Aspect is a French physicist noted for his experimental work on quantum entanglement.... |
Verified experimentally the quantum entanglement Quantum entanglement Quantum entanglement occurs when electrons, molecules even as large as "buckyballs", photons, etc., interact physically and then become separated; the type of interaction is such that each resulting member of a pair is properly described by the same quantum mechanical description , which is... hypothesis; his ``Bell test" experiments provided strong evidence that a quantum event at one location can affect an event at another location without any obvious mechanism for communication between the two locations. |
| 1982 to 1997 | Tokamak Fusion Test Reactor Tokamak Fusion Test Reactor The Tokamak Fusion Test Reactor was an experimental tokamak built at Princeton Plasma Physics Laboratory circa 1980. Following on from the PDX and PLT devices, it was hoped that TFTR would finally achieve fusion energy break-even. Unfortunately, the TFTR never achieved this goal... (TFTR) at PPPL, Princeton, USA |
Operated since 1982, produced 10.7MW of controlled fusion power for only 0.21s in 1994 by using T-D nuclear fusion in a tokamak reactor with ``a toroidal 6T magnetic field for plasma confinement, a 3MA plasma current and an electron density of 1.0 x 10**20 m-3 of 13.5keV" |
| 1983 | Carlo Rubbia Carlo Rubbia Carlo Rubbia Knight Grand Cross is an Italian particle physicist and inventor who shared the Nobel Prize in Physics in 1984 with Simon van der Meer for work leading to the discovery of the W and Z particles at CERN.-Biography:... and Simon van der Meer Simon van der Meer Simon van der Meer was a Dutch particle accelerator physicist who shared the Nobel Prize in Physics in 1984 with Carlo Rubbia for contributions to the CERN project which led to the discovery of the W and Z particles, two of the most fundamental constituents of matter.-Biography:One of four... |
Unambiguous signals of W particles were seen in January 1983 during a series of experiments conducted by Carlo Rubbia and Simon van der Meer at the Super Proton Synchrotron Super Proton Synchrotron The Super Proton Synchrotron is a particle accelerator of the synchrotron type at CERN. It is housed in a circular tunnel, in circumference, straddling the border of France and Switzerland near Geneva, Switzerland. The SPS was designed by a team led by John Adams, director-general of what was... . The actual experiments were called UA1 (led by Rubbia) and UA2 (led by Peter Jenni), and were the collaborative effort of many people. Simon van der Meer Simon van der Meer Simon van der Meer was a Dutch particle accelerator physicist who shared the Nobel Prize in Physics in 1984 with Carlo Rubbia for contributions to the CERN project which led to the discovery of the W and Z particles, two of the most fundamental constituents of matter.-Biography:One of four... was the driving force on the use of the accelerator. UA1 and UA2 found the Z particle a few months later, in May 1983. |
| 1983 to 2011 | JET Joint European Torus JET, the Joint European Torus, is the largest magnetic confinement plasma physics experiment worldwide currently in operation. Its main purpose is to open the way to future nuclear fusion experimental tokamak reactors such as ITER and :DEMO.... |
Began operation of the largest and most powerful, experimental nuclear fusion tokamak reactor in the world at Culham Facility in UK; operates with T-D plasma pulses and had a reported gain factor Q of 0.7 in 2009, with an input of 40MW for plasma heating, and a 2800 ton iron magnet for confinement; in 1997 in a tritium-deuterium experiment JET produced 16 MW of fusion power, a total of 22 MJ of fusion, energy and a steady fusion power of 4 MW which was maintained for 4 seconds. |
| 1985 to 2010 | JT-60 (Japan Torus) JT-60 JT-60 is the flagship of Japan's magnetic fusion program, previously run by the Japan Atomic Energy Research Institute and currently run by the Japan Atomic Energy Agency's Naka Fusion Institute in Ibaraki Prefecture, Japan... |
Began operation in 1985 with an experimental D-D nuclear fusion tokamak similar to JET Joint European Torus JET, the Joint European Torus, is the largest magnetic confinement plasma physics experiment worldwide currently in operation. Its main purpose is to open the way to future nuclear fusion experimental tokamak reactors such as ITER and :DEMO.... , currently run by the Japan Atomic Energy Agency Japan Atomic Energy Agency The was formed October 1, 2005 by a merger of two previous semi-governmental organizations. While it inherited the activities of both PNC and JAERI, it also inherited the nickname of JAERI, "Genken" 原研, an abbreviated word for "nuclear research".... 's (JAEA) Naka Fusion Institute in the Ibaraki Prefecture; in 2010 JT-60 held the record for the highest value of the fusion triple product achieved: = .; JT-60 claimed an equivalent energy gain factor, Q of 1.25 if it would have been operated with a T-D plasma instead of the D-D plasma, and on May 9, 2006 attained a fusion hold time of 28.6 s in full operation; moreover, a high-power microwave gyrotron Gyrotron Gyrotrons are high powered vacuum tubes which emit millimeter-wave beams by bunching electrons with cyclotron motion in a strong magnetic field. Output frequencies range from about 20 to 250 GHz, covering wavelengths from microwave to the edge of the terahertz gap. Typical output powers range from... construction was completed which is capable of 1.5MW output for 1s, thus meeting the conditions for the planned ITER ITER ITER is an international nuclear fusion research and engineering project, which is currently building the world's largest and most advanced experimental tokamak nuclear fusion reactor at Cadarache in the south of France... , large-scale nuclear fusion reactor;; JT-60 was disassembled in 2010 in order to be upgraded to a more powerful nuclear fusion reactor—the JT-60SA—by using niobium-titanium superconducting coils for the magnet confining the ultra-hot D-D plasma. |
| 1986 | Johannes Georg Bednorz Johannes Georg Bednorz Johannes Georg Bednorz is a physicist at the IBM Zürich Research Laboratory. He is best known for his role in the discovery of high-temperature superconductivity, for which he shared the 1987 Nobel Prize in Physics.-Life and work:... and Karl Alexander Müller Karl Alexander Müller Karl Alexander Müller is a Swiss physicist and Nobel laureate. He received the Nobel Prize in Physics in 1987 with Johannes Georg Bednorz for their work in superconductivity in ceramic materials.-Biography:... |
Produced unambiguous experimental proof of high temperature superconductivity involving Jahn-Teller polaron Polaron A polaron is a quasiparticle composed of a charge and its accompanying polarization field. A slow moving electron in a dielectric crystal, interacting with lattice ions through long-range forces will permanently be surrounded by a region of lattice polarization and deformation caused by the moving... s in orthorhombic La_2CuO_4, YBCO and other perovskite-type oxides; promptly received a Nobel prize in 1987 and delivered their Nobel lecture on December 8, 1987. |
| 1986 | Vladimir Gershonovich Drinfel'd | Introduced the concept of 'quantum group Quantum group In mathematics and theoretical physics, the term quantum group denotes various kinds of noncommutative algebra with additional structure. In general, a quantum group is some kind of Hopf algebra... s' as Hopf algebra Hopf algebra In mathematics, a Hopf algebra, named after Heinz Hopf, is a structure that is simultaneously an algebra and a coalgebra, with these structures' compatibility making it a bialgebra, and that moreover is equipped with an antiautomorphism satisfying a certain property.Hopf algebras occur naturally... s in his seminal address on quantum theory at the International Congress of Mathematicians International Congress of Mathematicians The International Congress of Mathematicians is the largest conference for the topic of mathematics. It meets once every four years, hosted by the International Mathematical Union .... , and also connected them to the study of the Yang–Baxter equation, which is a necessary condition for the solvability of statistical mechanics models; he also generalized Hopf algebras to quasi-Hopf algebras, and introduced the study of Drinfeld twists, which can be used to factorize the R-matrix R-matrix The term R-matrix has several meanings, depending on the field of study.The term R-matrix is used in connection with the Yang–Baxter equation. This is an equation which was first introduced in the field of statistical mechanics, taking its name from independent work of C. N. Yang and R. J.... corresponding to the solution of the Yang–Baxter equation associated with a quasitriangular Hopf algebra. |
| 1988 to 1998 | Mihai Gavrilă Mihai Gavrilă Mihai Gavrilă is a Romanian quantum physicist, member of the Romanian Academy since 1974. He made fundamental contributions to quantum theories of electromagnetic interactions with atoms. His parents were Ion and Florica Gavrilă... |
Discovered in 1988 the new quantum phenomenon of Atomic Dichotomy in hydrogen and subsequently published a book on the atomic structure and decay in high-frequency fields of hydrogen atoms placed in ultra-intense laser fields;. |
| 1991 | Richard R. Ernst Richard R. Ernst Richard Robert Ernst is a Swiss physical chemist and Nobel Laureate.Born in Winterthur, Switzerland, Ernst was awarded the Nobel Prize in Chemistry in 1991 for his contributions towards the development of Fourier Transform nuclear magnetic resonance spectroscopy while at Varian Associates, Palo... |
Developed Two-Dimensional Nuclear Magnetic Resonance Spectroscopy (2D-FT NMRS) for small molecules in solution and was awarded the Nobel Prize in Chemistry in 1991 "for his contributions to the development of the methodology of high resolution nuclear magnetic resonance (NMR) spectroscopy". |
| 1977 to 1995 | Fermilab Fermilab Fermi National Accelerator Laboratory , located just outside Batavia, Illinois, near Chicago, is a US Department of Energy national laboratory specializing in high-energy particle physics... |
The top quark Top quark The top quark, also known as the t quark or truth quark, is an elementary particle and a fundamental constituent of matter. Like all quarks, the top quark is an elementary fermion with spin-, and experiences all four fundamental interactions: gravitation, electromagnetism, weak interactions, and... was finally observed by a team at Fermilab after an 18-year search. It had a mass much greater than had been previously expected — almost as great as a gold atom. |
| 1995 | Eric Cornell, Carl Wieman Carl Wieman Carl Edwin Wieman is an American physicist at the University of British Columbia and recipient of the Nobel Prize in Physics for the production, in 1995 with Eric Allin Cornell, of the first true Bose–Einstein condensate.-Biography:... and Wolfgang Ketterle Wolfgang Ketterle Wolfgang Ketterle is a German physicist and professor of physics at the Massachusetts Institute of Technology . His research has focused on experiments that trap and cool atoms to temperatures close to absolute zero, and he led one of the first groups to realize Bose-Einstein condensation in these... |
The first "pure" Bose–Einstein condensate was created by Eric Cornell, Carl Wieman, and co-workers at JILA. They did this by cooling a dilute vapor consisting of approximately two thousand rubidium-87 atoms to below 170 nK using a combination of laser cooling and magnetic evaporative cooling. About four months later, an independent effort led by Wolfgang Ketterle at MIT created a condensate made of sodium-23. Ketterle's condensate had about a hundred times more atoms, allowing him to obtain several important results such as the observation of quantum mechanical interference between two different condensates. |
| 1998 | Super-Kamiokande Super-Kamiokande Super-Kamiokande is a neutrino observatory which is under Mount Kamioka near the city of Hida, Gifu Prefecture, Japan... (Japan) detector facility |
Reported experimental evidence for neutrino oscillation Neutrino oscillation Neutrino oscillation is a quantum mechanical phenomenon predicted by Bruno Pontecorvowhereby a neutrino created with a specific lepton flavor can later be measured to have a different flavor. The probability of measuring a particular flavor for a neutrino varies periodically as it propagates... s, implying that at least one neutrino has mass. |
| 1999 to 2013 | NSTX—The National Spherical Torus Experiment National Spherical Torus Experiment The National Spherical Torus Experiment is an innovative magnetic fusion device based on the spherical tokamak concept that was constructed by the Princeton Plasma Physics Laboratory in collaboration with the Oak Ridge National Laboratory, Columbia University, and the University of Washington at... at PPPL, Princeton, USA |
PPPL launched a nuclear fusion project on February 12, 1999 for ``an innovative magnetic fusion device that was constructed by the Princeton Plasma Physics Laboratory (PPPL) in collaboration with the Oak Ridge National Laboratory, Columbia University, and the University of Washington at Seattle"; NSTX is being used to study the physics principles of spherically shaped plasmas. |
| 2000 | CERN CERN The European Organization for Nuclear Research , known as CERN , is an international organization whose purpose is to operate the world's largest particle physics laboratory, which is situated in the northwest suburbs of Geneva on the Franco–Swiss border... |
CERN scientists publish experimental results in which they claim to have observed indirect evidence of the existence of a quark-gluon plasma Quark-gluon plasma A quark–gluon plasma or quark soup is a phase of quantum chromodynamics which exists at extremely high temperature and/or density. This phase consists of asymptotically free quarks and gluons, which are several of the basic building blocks of matter... , which they call a "new state of matter." |
| 2001 | The Sudbury Neutrino Observatory Sudbury Neutrino Observatory The Sudbury Neutrino Observatory is a neutrino observatory located 6,800 feet underground in Vale Inco's Creighton Mine in Sudbury, Ontario, Canada. The detector was designed to detect solar neutrinos through their interactions with a large tank of heavy water. The detector turned on in May 1999,... (Canada) |
Confirmed the existence of neutrino oscillations. |
| 2002 | Leonid Vainerman | Organized at Strasbourg a meeting of theoretical physicists and mathematicians focused on quantum group and quantum groupoid applications in quantum theories; the proceedings of the meeting were published in 2003 in a book edited by the meeting organizer |
| 2003 | Sir Anthony James Leggett Anthony James Leggett Sir Anthony James Leggett, KBE, FRS , aka Tony Leggett, has been a Professor of Physics at the University of Illinois at Urbana-Champaign since 1983.... , KBE, FRS |
Received the 2003 Nobel Prize in Physics for pioneering contributions to the quantum theory of superconductors, and superfluid Superfluid Superfluidity is a state of matter in which the matter behaves like a fluid without viscosity and with extremely high thermal conductivity. The substance, which appears to be a normal liquid, will flow without friction past any surface, which allows it to continue to circulate over obstructions and... s such as Helium-3 Helium-3 Helium-3 is a light, non-radioactive isotope of helium with two protons and one neutron. It is rare on Earth, and is sought for use in nuclear fusion research... , shared with V. L. Ginzburg and A. A. Abrikosov. |
| 2005 | The RHIC accelerator of Brookhaven National Laboratory Brookhaven National Laboratory Brookhaven National Laboratory , is a United States national laboratory located in Upton, New York on Long Island, and was formally established in 1947 at the site of Camp Upton, a former U.S. Army base... |
Generated a quark-gluon fluid, perhaps the quark-gluon plasma Quark-gluon plasma A quark–gluon plasma or quark soup is a phase of quantum chromodynamics which exists at extremely high temperature and/or density. This phase consists of asymptotically free quarks and gluons, which are several of the basic building blocks of matter... |
| 2007 to 2010 | Charles Pence Slichter Charles Pence Slichter Charles Pence Slichter is an American physicist, best known for his work on nuclear magnetic resonance and superconductivity.... |
Was awarded the National Medal of Science National Medal of Science The National Medal of Science is an honor bestowed by the President of the United States to individuals in science and engineering who have made important contributions to the advancement of knowledge in the fields of behavioral and social sciences, biology, chemistry, engineering, mathematics and... in 2007 for his studies of Nuclear Magnetic Resonance in Solids, and especially his NMR Studies of High-Temperature Superconductors. |
| 2008 to 2010 | Lithium Tokamak experiment (LTX) | Started in September 2008—based on the Andrei Zakharov theory—using a very thin lithium Lithium Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly... metal layer (<40 microns) on the inside surface of a 'small' tokamak reactor—facing the ultra-hot plasma; it was however planned to achieve only 400kA plasma currents in 100 ms pulses in the Spring of 2009, but was expected to achieve higher plasma ignition temperatures than in other tokamaks that do not utilize the liquid lithium—plasma interface so that the lithium would "soak up the particles at the plasma edge", thus avoiding plasma cooling by hot plasma particles reflected at the walls, as shown in the earlier experiments with the CDX-U toroidal lithium tray where a 50% recycling coefficient was measured, that is 35% lower than in the TFTR; in CDX-U the measured thickness of the coating lithium layer was on the order of 10 nm; shut down for upgrades in 2010, including a neutral beam injector, and then to be re-started during 2011. |
| 2007 to 2010 | Alain Aspect Alain Aspect Alain Aspect is a French physicist noted for his experimental work on quantum entanglement.... , Anton Zeilinger Anton Zeilinger Anton Zeilinger is an Austrian quantum physicist. He is currently professor of physics at the University of Vienna, previously University of Innsbruck. He is also the director of the Vienna branch of the Institute for Quantum Optics and Quantum Information IQOQI at the Austrian Academy of Sciences... and John Clauser John Clauser John Francis Clauser is an American theoretical and experimental physicist known for contributions to the foundations of quantum mechanics, in particular the Clauser-Horne-Shimony-Holt inequality.... |
Presented progess with the resolution of the non-locality aspect of quantum theory and was awarded in 2010 the Wolf Prize in Physics, together with Anton Zeilinger Anton Zeilinger Anton Zeilinger is an Austrian quantum physicist. He is currently professor of physics at the University of Vienna, previously University of Innsbruck. He is also the director of the Vienna branch of the Institute for Quantum Optics and Quantum Information IQOQI at the Austrian Academy of Sciences... and John Clauser John Clauser John Francis Clauser is an American theoretical and experimental physicist known for contributions to the foundations of quantum mechanics, in particular the Clauser-Horne-Shimony-Holt inequality.... |
| 2010 | Andre Geim Andre Geim Andre Konstantin Geim, FRS is a Dutch-Russian-British physicist working at the University of Manchester. Geim was awarded the 2010 Nobel Prize in Physics jointly with Konstantin Novoselov for his work on graphene... and Konstantin Novoselov Konstantin Novoselov Konstantin Sergeevich Novoselov FRS is a Russo-British physicist, most notably known for his works on graphene together with Andre Geim, which earned them the Nobel Prize in Physics in 2010. Novoselov is currently a member of the mesoscopic physics research group at the University of Manchester as... |
Received the Nobel Prize in Physics ``for groundbreaking experiments regarding the two-dimensional material graphene" |
Founding experiments
- Thomas YoungThomas Young (scientist)Thomas Young was an English polymath. He is famous for having partly deciphered Egyptian hieroglyphics before Jean-François Champollion eventually expanded on his work...
's double-slit experimentDouble-slit experimentThe double-slit experiment, sometimes called Young's experiment, is a demonstration that matter and energy can display characteristics of both waves and particles...
demonstrating the wave nature of light (c1805) - Henri BecquerelHenri BecquerelAntoine Henri Becquerel was a French physicist, Nobel laureate, and the discoverer of radioactivity along with Marie Curie and Pierre Curie, for which all three won the 1903 Nobel Prize in Physics.-Early life:...
discovers radioactivity (1896) - J. J. ThomsonJ. J. ThomsonSir Joseph John "J. J." Thomson, OM, FRS was a British physicist and Nobel laureate. He is credited for the discovery of the electron and of isotopes, and the invention of the mass spectrometer...
's cathode ray tube experiments (discovers the electronElectronThe electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
and its negative charge) (1897) - The study of black body radiation between 1850 and 1900, which could not be explained without quantum concepts.
- The photoelectric effectPhotoelectric effectIn the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons...
: EinsteinAlbert EinsteinAlbert Einstein was a German-born theoretical physicist who developed the theory of general relativity, effecting a revolution in physics. For this achievement, Einstein is often regarded as the father of modern physics and one of the most prolific intellects in human history...
explained this in 1905 (and later received a Nobel prize for it) using the concept of photons, particles of light with quantized energy - Robert MillikanRobert MillikanRobert A. Millikan was an American experimental physicist, and Nobel laureate in physics for his measurement of the charge on the electron and for his work on the photoelectric effect. He served as president of Caltech from 1921 to 1945...
's oil-drop experimentOil-drop experimentThe oil drop experiment was an experiment performed by Robert Millikan and Harvey Fletcher in 1909 to measure the elementary electric charge ....
, which showed that electric chargeElectric chargeElectric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
occurs as quantaQuantumIn physics, a quantum is the minimum amount of any physical entity involved in an interaction. Behind this, one finds the fundamental notion that a physical property may be "quantized," referred to as "the hypothesis of quantization". This means that the magnitude can take on only certain discrete...
(whole units), (1909) - Ernest RutherfordErnest RutherfordErnest Rutherford, 1st Baron Rutherford of Nelson OM, FRS was a New Zealand-born British chemist and physicist who became known as the father of nuclear physics...
's gold foil experimentGeiger-Marsden experimentThe Geiger–Marsden experiment was an experiment to probe the structure of the atom performed by Hans Geiger and Ernest Marsden in 1909, under the direction of Ernest Rutherford at the Physical Laboratories of the University of Manchester...
disproved the plum pudding model of the atomAtomThe atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
which suggested that the mass and positive charge of the atom are almost uniformly distributed. (1911) - Otto SternOtto SternOtto Stern was a German physicist and Nobel laureate in physics.-Biography:Stern was born in Sohrau, now Żory in the German Empire's Kingdom of Prussia and studied at Breslau, now Wrocław in Lower Silesia....
and Walther Gerlach conduct the Stern-Gerlach experiment, which demonstrates the quantized nature of particle spinSpin (physics)In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
(1920) - Clinton DavissonClinton DavissonClinton Joseph Davisson , was an American physicist who won the 1937 Nobel Prize in Physics for his discovery of electron diffraction. Davisson shared the Nobel Prize with George Paget Thomson, who independently discovered electron diffraction at about the same time as Davisson.-Early...
and Lester GermerLester GermerLester Halbert Germer was an American physicist. With Clinton Davisson, he proved the wave-particle duality of matter in the Davisson–Germer experiment, which was important to the development of the electron microscope. These studies supported the theoretical work of De Broglie. He also studied...
demonstrate the wave nature of the electronElectronThe electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
in the Electron diffractionElectron diffractionElectron diffraction refers to the wave nature of electrons. However, from a technical or practical point of view, it may be regarded as a technique used to study matter by firing electrons at a sample and observing the resulting interference pattern...
experiment (1927) - Clyde L. Cowan and Frederick ReinesFrederick ReinesFrederick Reines was an American physicist. He was awarded the 1995 Nobel Prize in Physics for his co-detection of the neutrino with Clyde Cowan in the neutrino experiment, and may be the only scientist in history "so intimately associated with the discovery of an elementary particle and the...
confirm the existence of the neutrinoNeutrinoA neutrino is an electrically neutral, weakly interacting elementary subatomic particle with a half-integer spin, chirality and a disputed but small non-zero mass. It is able to pass through ordinary matter almost unaffected...
in the neutrino experimentNeutrino experimentThe neutrino experiment, also called the Cowan and Reines neutrino experiment, was performed by Clyde L. Cowan and Frederick Reines in 1956...
(1955) - Claus Jönsson`s double-slit experimentDouble-slit experimentThe double-slit experiment, sometimes called Young's experiment, is a demonstration that matter and energy can display characteristics of both waves and particles...
with electrons (1961) - The Quantum Hall effect, discovered in 1980 by Klaus von KlitzingKlaus von KlitzingKlaus von Klitzing is a German physicist known for discovery of the integer quantum Hall Effect, for which he was awarded the 1985 Nobel Prize in Physics....
. The quantized version of the Hall effectHall effectThe Hall effect is the production of a voltage difference across an electrical conductor, transverse to an electric current in the conductor and a magnetic field perpendicular to the current...
has allowed for the definition of a new practical standard for electrical resistanceElectrical resistanceThe electrical resistance of an electrical element is the opposition to the passage of an electric current through that element; the inverse quantity is electrical conductance, the ease at which an electric current passes. Electrical resistance shares some conceptual parallels with the mechanical...
and for an extremely precise independent determination of the fine structure constant. - The experimental verificationBell test experimentsThe Bell test experiments serve to investigate the validity of the entanglement effect in quantum mechanics by using some kind of Bell inequality...
of quantum entanglementQuantum entanglementQuantum entanglement occurs when electrons, molecules even as large as "buckyballs", photons, etc., interact physically and then become separated; the type of interaction is such that each resulting member of a pair is properly described by the same quantum mechanical description , which is...
by Alain AspectAlain AspectAlain Aspect is a French physicist noted for his experimental work on quantum entanglement....
in 1982.
See also
- QuantumQuantumIn physics, a quantum is the minimum amount of any physical entity involved in an interaction. Behind this, one finds the fundamental notion that a physical property may be "quantized," referred to as "the hypothesis of quantization". This means that the magnitude can take on only certain discrete...
- History of quantum field theoryHistory of quantum field theoryThe history of quantum field theory starts with its creation by Paul Dirac, when he attempted to quantize the electromagnetic field in the late 1920s. Major advances in the theory were made in the 1950s, and led to the introduction of quantum electrodynamics . QED was so successful and "natural"...
- Timeline of atomic and subatomic physics
- History of the moleculeHistory of the moleculeIn chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms....
- History of thermodynamicsHistory of thermodynamicsThe history of thermodynamics is a fundamental strand in the history of physics, the history of chemistry, and the history of science in general...
- History of chemistryHistory of chemistryBy 1000 BC, ancient civilizations used technologies that would eventually form the basis of the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, making pigments for cosmetics and painting, extracting chemicals from...
- Golden age of physicsGolden Age of physicsA Golden Age of physics appears to have been delineated for certain periods of progress in the physics sciences, and this includes the previous and current developments of cosmology, and astronomy. Each "golden age" introduces significant advancements in theoretical and experimental methods...
Further reading
- F. Bayen, M. Flato, C. Fronsdal, A. Lichnerowicz and D. Sternheimer, Deformation theory and quantization I,and II, Ann. Phys. (N.Y.), 111 (1978) pp. 61–110, 111-151.
- D. Cohen, An Introduction to Hilbert Space and Quantum Logic, Springer-Verlag, 1989. This is a thorough and well-illustrated introduction.
- A. Gleason. Measures on the Closed Subspaces of a Hilbert Space, Journal of Mathematics and Mechanics, 1957.
- R. Kadison. Isometries of Operator Algebras, Annals of Mathematics, Vol. 54, pp. 325–338, 1951
- G. Ludwig. Foundations of Quantum Mechanics, Springer-Verlag, 1983.
- G. Mackey. Mathematical Foundations of Quantum Mechanics, W. A. Benjamin, 1963 (paperback reprint by Dover 2004).
- R. Omnès. Understanding Quantum Mechanics, Princeton University Press, 1999. (Discusses logical and philosophical issues of quantum mechanics, with careful attention to the history of the subject).
- N. Papanikolaou. Reasoning Formally About Quantum Systems: An Overview, ACM SIGACT News, 36(3), pp. 51–66, 2005.
- C. Piron. Foundations of Quantum Physics, W. A. Benjamin, 1976.
- Hermann Weyl. The Theory of Groups and Quantum Mechanics, Dover Publications, 1950.

