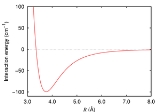
Van der Waals force
Overview
Physical chemistry
Physical chemistry is the study of macroscopic, atomic, subatomic, and particulate phenomena in chemical systems in terms of physical laws and concepts...
, the van der Waals force (or van der Waals interaction), named after Dutch
Netherlands
The Netherlands is a constituent country of the Kingdom of the Netherlands, located mainly in North-West Europe and with several islands in the Caribbean. Mainland Netherlands borders the North Sea to the north and west, Belgium to the south, and Germany to the east, and shares maritime borders...
scientist
Scientist
A scientist in a broad sense is one engaging in a systematic activity to acquire knowledge. In a more restricted sense, a scientist is an individual who uses the scientific method. The person may be an expert in one or more areas of science. This article focuses on the more restricted use of the word...
Johannes Diderik van der Waals
Johannes Diderik van der Waals
Johannes Diderik van der Waals was a Dutch theoretical physicist and thermodynamicist famous for his work on an equation of state for gases and liquids....
, is the sum of the attractive or repulsive forces between molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s (or between parts of the same molecule) other than those due to covalent bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
s or to the electrostatic interaction of ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s with one another or with neutral molecules. The term includes:
- force between two permanent dipoles (Keesom force)
- force between a permanent dipole and a corresponding induced dipole (Debye force)
- force between two instantaneously induced dipoles (London dispersion forceLondon dispersion forceLondon dispersion forces is a type of force acting between atoms and molecules. They are part of the van der Waals forces...
)
It is also sometimes used loosely as a synonym
Synonym
Synonyms are different words with almost identical or similar meanings. Words that are synonyms are said to be synonymous, and the state of being a synonym is called synonymy. The word comes from Ancient Greek syn and onoma . The words car and automobile are synonyms...
for the totality of intermolecular forces.
Discussions

