
Gamma ray
Encyclopedia

Gamma
Gamma is the third letter of the Greek alphabet. In the system of Greek numerals it has a value of 3. It was derived from the Phoenician letter Gimel . Letters that arose from Gamma include the Roman C and G and the Cyrillic letters Ge Г and Ghe Ґ.-Greek:In Ancient Greek, gamma represented a...
, is electromagnetic radiation
Electromagnetic radiation
Electromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space...
of high frequency (very short wavelength). Gamma rays are usually naturally produced on Earth by decay of high energy states in atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
ic nuclei (gamma decay). Important natural sources are also high-energy sub-atomic
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
particle interactions resulting from cosmic rays. Such high-energy reactions are also the common artificial source of gamma rays. Other man-made mechanisms include electron-positron annihilation
Electron-positron annihilation
Electron–positron annihilation occurs when an electron and a positron collide. The result of the collision is the annihilation of the electron and positron, and the creation of gamma ray photons or, at higher energies, other particles:...
, neutral pion decay
Pion
In particle physics, a pion is any of three subatomic particles: , , and . Pions are the lightest mesons and they play an important role in explaining the low-energy properties of the strong nuclear force....
, fusion
Nuclear fusion
Nuclear fusion is the process by which two or more atomic nuclei join together, or "fuse", to form a single heavier nucleus. This is usually accompanied by the release or absorption of large quantities of energy...
, and induced fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
. Some rare natural sources are lightning strike
Lightning strike
Lightning strikes are electrical discharges caused by lightning, typically during thunderstorms.Humans can be hit by lightning directly when outdoors. Contrary to popular notion, there is no 'safe' location outdoors. People have been struck in sheds and makeshift shelters...
s and terrestrial gamma-ray flash
Terrestrial gamma-ray flash
Terrestrial gamma-ray flashes are bursts of gamma rays in the Earth's atmosphere. TGFs have been recorded to last 0.2 to 3.5 milliseconds, and have energies of up to 20 MeV. They are probably caused by electric fields produced above thunderstorms...
es, which produce high energy particles from natural high-energy voltages. Gamma rays are also produced by astronomical processes in which very high-energy electrons are produced. Such electrons produce secondary gamma rays by the mechanisms of bremsstrahlung
Bremsstrahlung
Bremsstrahlung is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into a photon because energy is conserved. The term is...
, inverse Compton scattering
Compton scattering
In physics, Compton scattering is a type of scattering that X-rays and gamma rays undergo in matter. The inelastic scattering of photons in matter results in a decrease in energy of an X-ray or gamma ray photon, called the Compton effect...
and synchrotron radiation
Synchrotron radiation
The electromagnetic radiation emitted when charged particles are accelerated radially is called synchrotron radiation. It is produced in synchrotrons using bending magnets, undulators and/or wigglers...
. Gamma rays are ionizing radiation
Ionizing radiation
Ionizing radiation is radiation composed of particles that individually have sufficient energy to remove an electron from an atom or molecule. This ionization produces free radicals, which are atoms or molecules containing unpaired electrons...
and are thus biologically hazardous.
A classical gamma ray source, and the first to be discovered historically, is a type of radioactive decay
Radioactive decay
Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
called gamma decay. In this type of decay, an excited nucleus emits a gamma ray almost immediately on formation, although isomeric transition
Isomeric transition
An isomeric transition is a radioactive decay process that involves emission of a gamma ray from an atom where the nucleus is in an excited metastable state, referred to in its excited state, as a nuclear isomer....
can produce inhibited gamma decay with a measurable and much longer half-life. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900, while studying radiation emitted from radium
Radium
Radium is a chemical element with atomic number 88, represented by the symbol Ra. Radium is an almost pure-white alkaline earth metal, but it readily oxidizes on exposure to air, becoming black in color. All isotopes of radium are highly radioactive, with the most stable isotope being radium-226,...
. Villard's radiation was named "gamma rays" by Ernest Rutherford
Ernest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson OM, FRS was a New Zealand-born British chemist and physicist who became known as the father of nuclear physics...
in 1903.
Gamma rays typically have frequencies above 10 exahertz
Hertz
The hertz is the SI unit of frequency defined as the number of cycles per second of a periodic phenomenon. One of its most common uses is the description of the sine wave, particularly those used in radio and audio applications....
(or >1019 Hz), and therefore have energies above 100 keV
Electronvolt
In physics, the electron volt is a unit of energy equal to approximately joule . By definition, it is equal to the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt...
and wavelength less than 10 picometers, less than the diameter of an atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
. However, this is not a hard and fast definition but rather only a rule-of-thumb description for natural processes. Gamma rays from radioactive decay
Radioactive decay
Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
commonly have energies of a few hundred keV
Kev
Kev can refer to:*Kev Hawkins, a fictional character.*Kevin, a given name occasionally shortened to "Kev".*Kiloelectronvolt, a unit of energy who symbol is "KeV".* Krefelder Eislauf-VereinKEV can refer to:...
, and almost always less than 10 MeV
MEV
MeV and meV are multiples and submultiples of the electron volt unit referring to 1,000,000 eV and 0.001 eV, respectively.Mev or MEV may refer to:In entertainment:* Musica Elettronica Viva, an Italian musical group...
. On the other side of the decay energy range, there is effectively no lower limit to gamma energy derived from radioactive decay. By contrast, energies from astronomical sources can be much higher, ranging over 10 TeV (this is far too large to result from radioactive decay).
The distinction between X-ray
X-ray
X-radiation is a form of electromagnetic radiation. X-rays have a wavelength in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz and energies in the range 120 eV to 120 keV. They are shorter in wavelength than UV rays and longer than gamma...
s and gamma rays has changed in recent decades. Originally, the electromagnetic radiation emitted by X-ray tube
X-ray tube
An X-ray tube is a vacuum tube that produces X-rays. They are used in X-ray machines. X-rays are part of the electromagnetic spectrum, an ionizing radiation with wavelengths shorter than ultraviolet light...
s almost invariably had a longer wavelength
Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
than the radiation emitted by radioactive nuclei
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
(gamma rays). Older literature distinguished between X- and gamma radiation on the basis of wavelength, with radiation shorter than some arbitrary wavelength, such as 10−11 m, defined as gamma rays.
However, with artificial sources now able to duplicate any electromagnetic radiation that originates in the nucleus, as well as far higher energies, the wavelengths characteristic of radioactive gamma ray sources vs. other types, now completely overlaps. Thus, gamma rays are now usually distinguished by their origin: X-ray
X-ray
X-radiation is a form of electromagnetic radiation. X-rays have a wavelength in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz and energies in the range 120 eV to 120 keV. They are shorter in wavelength than UV rays and longer than gamma...
s are emitted by definition by electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s outside the nucleus, while gamma rays are emitted by the nucleus. Exceptions to this convention occur in astronomy, where high energy processes known to involve other than radioactive decay are still named as sources of gamma radiation. A notable example is extremely powerful bursts of high-energy radiation normally referred to as long duration gamma-ray bursts, which produce gamma rays by a mechanism not compatible with radioactive decay. These bursts of gamma rays, thought to be due to collapse of stars called hypernova
Hypernova
Hypernova , also known as a type 1c Supernova, refers to an incredibly large star that collapses at the end of its lifespan...
s, are the most powerful single events so far discovered in the cosmos
Cosmos
In the general sense, a cosmos is an orderly or harmonious system. It originates from the Greek term κόσμος , meaning "order" or "ornament" and is antithetical to the concept of chaos. Today, the word is generally used as a synonym of the word Universe . The word cosmos originates from the same root...
.
Naming conventions and overlap in terminology

Nuclear medicine
In nuclear medicine procedures, elemental radionuclides are combined with other elements to form chemical compounds, or else combined with existing pharmaceutical compounds, to form radiopharmaceuticals. These radiopharmaceuticals, once administered to the patient, can localize to specific organs...
, technetium-99m
Technetium-99m
Technetium-99m is a metastable nuclear isomer of technetium-99, symbolized as 99mTc. The "m" indicates that this is a metastable nuclear isomer, i.e., that its half-life of 6 hours is considerably longer than most nuclear isomers that undergo gamma decay...
, produces gamma radiation of about the same energy (140 keV) as produced by a diagnostic X-ray machine, and significantly lower energy than therapeutic photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
s from linacs.
Because of this broad overlap in energy ranges, the two types of electromagnetic radiation are now usually defined by their origin: X-rays are emitted by electrons (either in orbitals outside of the nucleus, or while being accelerated to produce Bremsstrahlung
Bremsstrahlung
Bremsstrahlung is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into a photon because energy is conserved. The term is...
-type radiation), while gamma rays are emitted by the nucleus or from other particle decay
Particle decay
Particle decay is the spontaneous process of one elementary particle transforming into other elementary particles. During this process, an elementary particle becomes a different particle with less mass and an intermediate particle such as W boson in muon decay. The intermediate particle then...
s or annihilation events. There is no lower limit to the energy of photons produced by nuclear reactions, and thus ultraviolet
Ultraviolet
Ultraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
and even lower energy photons produced by these processes would also be defined as "gamma rays".
In astronomy, higher energy gamma and X-rays are defined by energy, since the processes which produce them may be uncertain and photon energy, not origin, determines the required astronomical detectors needed. Occasionally, high energy photons in nature which are known not to be produced by nuclear decay, are nevertheless referred to as gamma radiation. An example is "gamma rays" from lightning discharges at 10 to 20 MeV, which are known to be produced by the Bremsstrahlung mechanism.
Another example is gamma ray burst
Gamma ray burst
Gamma-ray bursts are flashes of gamma rays associated with extremely energetic explosions that have been observed in distant galaxies. They are the most luminous electromagnetic events known to occur in the universe. Bursts can last from ten milliseconds to several minutes, although a typical...
s, which are named historically, and now known to be produced from processes too powerful to involve simple collections of atoms undergoing radioactive decay. A few gamma rays known to be explicitly from nuclear origin are known in astronomy, with a classic example being that of supernova SN 1987A
SN 1987A
SN 1987A was a supernova in the outskirts of the Tarantula Nebula in the Large Magellanic Cloud, a nearby dwarf galaxy. It occurred approximately 51.4 kiloparsecs from Earth, approximately 168,000 light-years, close enough that it was visible to the naked eye. It could be seen from the Southern...
emitting an "afterglow" of gamma-ray photons from the decay of newly-made radioactive cobalt-56 ejected into space in a cloud, by the explosion. However, many gamma rays produced in astronomical processes are produced not in radioactive decay or particle annihilation, but rather in much the same manner as the production of X-rays, but simply using electrons with higher energies. Astronomical literature tends to write "gamma-ray" with a hyphen, by analogy to X-rays, rather than in a way analogous to alpha rays and beta rays. This notation tends to subtley stress the non-nuclear source of many astronomical gamma rays.
Units of measure and exposure
The measure of gamma rays' ionizingIonization
Ionization is the process of converting an atom or molecule into an ion by adding or removing charged particles such as electrons or other ions. This is often confused with dissociation. A substance may dissociate without necessarily producing ions. As an example, the molecules of table sugar...
ability is called the exposure:
- The coulomb per kilogram (C/kg) is the SI unit of ionizing radiationIonizing radiationIonizing radiation is radiation composed of particles that individually have sufficient energy to remove an electron from an atom or molecule. This ionization produces free radicals, which are atoms or molecules containing unpaired electrons...
exposure, and is the amount of radiation required to create 1 coulomb of charge of each polarity in 1 kilogram of matter. - The röntgenRöntgenThe roentgen is a unit of measurement for exposure to ionizing radiation , and is named after the German physicist Wilhelm Röntgen...
(R) is an obsolete traditional unit of exposure, which represented the amount of radiation required to create 1 esu of charge of each polarity in 1 cubic centimeter of dry air. 1 röntgen = 2.58×10−4 C/kg
However, the effect of gamma and other ionizing radiation on living tissue is more closely related to the amount of energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
deposited rather than the charge
Electric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
. This is called the absorbed dose
Absorbed dose
Absorbed dose is a measure of the energy deposited in a medium by ionizing radiation per unit mass...
:
- The grayGray (unit)The gray is the SI unit of absorbed radiation dose of ionizing radiation , and is defined as the absorption of one joule of ionizing radiation by one kilogram of matter ....
(Gy), which has units of (J/kg), is the SI unit of absorbed doseAbsorbed doseAbsorbed dose is a measure of the energy deposited in a medium by ionizing radiation per unit mass...
, and is the amount of radiation required to deposit 1 jouleJouleThe joule ; symbol J) is a derived unit of energy or work in the International System of Units. It is equal to the energy expended in applying a force of one newton through a distance of one metre , or in passing an electric current of one ampere through a resistance of one ohm for one second...
of energy in 1 kilogram of any kind of matter. - The radRad (unit)The rad is a unit of absorbed radiation dose. The rad was first proposed in 1918 as "that quantity of X rays which when absorbed will cause the destruction of the malignant mammalian cells in question..." It was defined in CGS units in 1953 as the dose causing 100 ergs of energy to be absorbed by...
is the (obsolete) corresponding traditional unit, equal to 0.01 J deposited per kg. 100 rad = 1 Gy.
The equivalent dose
Equivalent dose
The equivalent absorbed radiation dose, usually shortened to equivalent dose, is a computed average measure of the radiation absorbed by a fixed mass of biological tissue, that attempts to account for the different biological damage potential of different types of ionizing radiation...
is the measure of the biological effect of radiation on human tissue. For gamma rays it is equal to the absorbed dose
Absorbed dose
Absorbed dose is a measure of the energy deposited in a medium by ionizing radiation per unit mass...
.
- The sievertSievertThe sievert is the International System of Units SI derived unit of dose equivalent radiation. It attempts to quantitatively evaluate the biological effects of ionizing radiation as opposed to just the absorbed dose of radiation energy, which is measured in gray...
(Sv) is the SI unit of equivalent dose, which for gamma rays is numerically equal to the grayGray (unit)The gray is the SI unit of absorbed radiation dose of ionizing radiation , and is defined as the absorption of one joule of ionizing radiation by one kilogram of matter ....
(Gy). - The remRöntgen equivalent manNamed after Wilhelm Röntgen , the roentgen equivalent in man or rem is a unit of radiation dose equivalent...
is the traditional unit of equivalent dose. For gamma rays it is equal to the radRADRad may mean:* Rad , a villainous character in AC Comics's "Femforce"* Rad , a 1986 release about a young BMX rider* Rad , several fictional characters in the Transformers toy line...
or 0.01 J of energy deposited per kg. 1 Sv = 100 rem.
Shielding
Shielding from gamma rays requires large amounts of mass, in contrast to alpha particles which can be blocked by paper or skin, and beta particles which can be shielded by foil. They are better absorbed by materials with high atomic numbers and high density, although neither effect is important compared to the total mass per area in the path of the gamma ray. For this reason, a lead shield is only modestly better (20–30%) as a gamma shield than an equal mass of another shielding material such as aluminium, concrete, water or soil; lead's major advantage is its density. Protective clothing, goggles and respirators can protect from internal contact with or ingestion of alpha or beta particles, but provide no protection from gamma radiation.The higher the energy of the gamma rays, the thicker the shielding required. Materials for shielding gamma rays are typically measured by the thickness required to reduce the intensity of the gamma rays by one half (the half value layer or HVL). For example gamma rays that require (0.4″) of lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
to reduce their intensity by 50% will also have their intensity reduced in half by of granite
Granite
Granite is a common and widely occurring type of intrusive, felsic, igneous rock. Granite usually has a medium- to coarse-grained texture. Occasionally some individual crystals are larger than the groundmass, in which case the texture is known as porphyritic. A granitic rock with a porphyritic...
rock, 6 cm (2½″) of concrete
Concrete
Concrete is a composite construction material, composed of cement and other cementitious materials such as fly ash and slag cement, aggregate , water and chemical admixtures.The word concrete comes from the Latin word...
, or 9 cm (3½″) of packed soil
Soil
Soil is a natural body consisting of layers of mineral constituents of variable thicknesses, which differ from the parent materials in their morphological, physical, chemical, and mineralogical characteristics...
. However, the mass of this much concrete or soil is only 20–30% larger than that of lead with the same absorption capability. Depleted uranium
Depleted uranium
Depleted uranium is uranium with a lower content of the fissile isotope U-235 than natural uranium . Uses of DU take advantage of its very high density of 19.1 g/cm3...
is used for shielding in portable gamma ray sources, but again the savings in weight over lead is modest, and the main effect is to reduce shielding bulk. In a nuclear power plant, shielding can be provided by steel and concrete in the pressure vessel and containment, while water also provides a shielding material for fuel rods in storage or transport into the reactor core. A loss of water or removal of a "hot" spent fuel assembly into the air would result in much higher radiation levels than under water.
Matter interaction
When a gamma ray passes through matter, the probability for absorption in a thin layer is proportional to the thickness of that layer. This leads to an exponential decrease of intensity with thickness. The exponential absorption holds only for a narrow beam of gamma rays. If a wide beam of gamma rays passes through a thick slab of concrete the scattering from the sides reduces the absorption to
where μ = nσ is the absorption coefficient, measured in cm−1, n the number of atoms per cm3 in the material, σ the absorption cross section
Cross section (physics)
A cross section is the effective area which governs the probability of some scattering or absorption event. Together with particle density and path length, it can be used to predict the total scattering probability via the Beer-Lambert law....
in cm2 and d the thickness of material in cm.
In passing through matter, gamma radiation ionizes via three main processes: the photoelectric effect
Photoelectric effect
In the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons...
, Compton scattering
Compton scattering
In physics, Compton scattering is a type of scattering that X-rays and gamma rays undergo in matter. The inelastic scattering of photons in matter results in a decrease in energy of an X-ray or gamma ray photon, called the Compton effect...
, and pair production
Pair production
Pair production refers to the creation of an elementary particle and its antiparticle, usually from a photon . For example an electron and its antiparticle, the positron, may be created...
.
- Photoelectric effect: This describes the case in which a gamma photonPhotonIn physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
interacts with and transfers its energy to an atomic electron, ejecting that electron from the atom. The kinetic energy of the resulting photoelectron is equal to the energy of the incident gamma photon minus the binding energy of the electron. The photoelectric effect is the dominant energy transfer mechanism for X-ray and gamma ray photons with energies below 50 keV (thousand electron voltsElectronvoltIn physics, the electron volt is a unit of energy equal to approximately joule . By definition, it is equal to the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt...
), but it is much less important at higher energies. - Compton scattering: This is an interaction in which an incident gamma photon loses enough energy to an atomic electron to cause its ejection, with the remainder of the original photon's energy being emitted as a new, lower energy gamma photon with an emission direction different from that of the incident gamma photon. The probability of Compton scatter decreases with increasing photon energy. Compton scattering is thought to be the principal absorption mechanism for gamma rays in the intermediate energy range 100 keVElectronvoltIn physics, the electron volt is a unit of energy equal to approximately joule . By definition, it is equal to the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt...
to 10 MeV. Compton scattering is relatively independent of the atomic numberAtomic numberIn chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
of the absorbing material, which is why very dense metals like lead are only modestly better shields, on a per weight basis, than are less dense materials. - Pair production: This becomes possible with gamma energies exceeding 1.02 MeV, and becomes important as an absorption mechanism at energies over about 5 MeV (see illustration at right, for lead). By interaction with the electric fieldElectric fieldIn physics, an electric field surrounds electrically charged particles and time-varying magnetic fields. The electric field depicts the force exerted on other electrically charged objects by the electrically charged particle the field is surrounding...
of a nucleus, the energy of the incident photon is converted into the mass of an electron-positronPositronThe positron or antielectron is the antiparticle or the antimatter counterpart of the electron. The positron has an electric charge of +1e, a spin of ½, and has the same mass as an electron...
pair. Any gamma energy in excess of the equivalent rest mass of the two particles (1.02 MeV) appears as the kinetic energy of the pair and the recoil nucleus. At the end of the positron's rangeRange (particle radiation)In passing through matter, charged particles ionize and thus lose energy in many steps, until their energy is zero. The distance to this point is called the range of the particle...
, it combines with a free electron. The entire mass of these two particles is then converted into two gamma photons of at least 0.51 MeV energy each (or higher according to the kinetic energy of the annihilated particles).
The secondary electrons (and/or positrons) produced in any of these three processes frequently have enough energy to produce much ionization
Ionization
Ionization is the process of converting an atom or molecule into an ion by adding or removing charged particles such as electrons or other ions. This is often confused with dissociation. A substance may dissociate without necessarily producing ions. As an example, the molecules of table sugar...
themselves.
Light interaction
High-energy (from 80 to 500 GeVElectronvolt
In physics, the electron volt is a unit of energy equal to approximately joule . By definition, it is equal to the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt...
) gamma rays arriving from far far-distant quasars are used to estimate the extragalactic background light
Extragalactic background light
The Extragalactic Background Light or simply the "extragalactic background" is the faint diffuse light of the night sky, consisting of the combined flux of all extragalactic sources...
in the universe: The highest-energy rays interact more readily with the background light photons and thus their density may be estimated by analyzing the incoming gamma ray spectrums.
Radioactive decay (gamma decay)
Gamma rays from radioactive gamma decay are produced alongside other forms of radiation such as alpha
Alpha decay
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less...
or beta
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
, and are produced after the other types of decay occur. The mechanism is that when a nucleus emits an or particle, the daughter nucleus is usually left in an excited state. It can then move to a lower energy state by emitting a gamma ray, in much the same way that an atomic electron can jump to a lower energy state by emitting infrared
Infrared
Infrared light is electromagnetic radiation with a wavelength longer than that of visible light, measured from the nominal edge of visible red light at 0.74 micrometres , and extending conventionally to 300 µm...
, visible, or ultraviolet
Ultraviolet
Ultraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
light. Emission of a gamma ray from an excited nuclear state typically requires only 10−12 seconds, and is thus nearly instantaneous, following types of radioactive decay that produce other radioactive particles. Gamma decay from excited states may also happen rapidly following nuclear reaction
Nuclear reaction
In nuclear physics and nuclear chemistry, a nuclear reaction is semantically considered to be the process in which two nuclei, or else a nucleus of an atom and a subatomic particle from outside the atom, collide to produce products different from the initial particles...
s such as neutron capture
Neutron capture
Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled...
, nuclear fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
, or nuclear fusion
Nuclear fusion
Nuclear fusion is the process by which two or more atomic nuclei join together, or "fuse", to form a single heavier nucleus. This is usually accompanied by the release or absorption of large quantities of energy...
.
In certain cases, the excited nuclear state following the emission of a beta particle may be more stable than average, and is termed a metastable excited state, if its decay is 100 to 1000 times longer than the average 10−12 seconds. Such nuclei have half-lives
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
that are easily measurable, and are termed nuclear isomer
Nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus caused by the excitation of one or more of its nucleons . "Metastable" refers to the fact that these excited states have half-lives more than 100 to 1000 times the half-lives of the other possible excited nuclear states...
s. Some nuclear isomers are able to stay in their excited state for minutes, hours, days, or occasionally far longer, before emitting a gamma ray. Isomeric transition
Isomeric transition
An isomeric transition is a radioactive decay process that involves emission of a gamma ray from an atom where the nucleus is in an excited metastable state, referred to in its excited state, as a nuclear isomer....
is the name given to a gamma decay from such a state. The process of isomeric transition is therefore similar to any gamma emission, but differs in that it involves metastable excited states of the nuclei.
An emitted gamma ray from any type of excited state may transfer its energy directly to one of the most tightly bound electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s causing it to be ejected from the atom, a process termed the photoelectric effect
Photoelectric effect
In the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons...
(it should not be confused with the internal conversion
Internal conversion
Internal conversion is a radioactive decay process where an excited nucleus interacts with an electron in one of the lower atomic orbitals, causing the electron to be emitted from the atom. Thus, in an internal conversion process, a high-energy electron is emitted from the radioactive atom, but...
process, in which no real gamma ray photon is produced as an intermediate particle).
Light
Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
, and radio waves are all forms of electromagnetic radiation
Electromagnetic radiation
Electromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space...
. The only difference is the frequency
Frequency
Frequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
and hence the energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
of the photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
s. Gamma rays are generally the most energetic of these, although broad overlap with X-ray
X-ray
X-radiation is a form of electromagnetic radiation. X-rays have a wavelength in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz and energies in the range 120 eV to 120 keV. They are shorter in wavelength than UV rays and longer than gamma...
energies occurs. An example of gamma ray production follows:
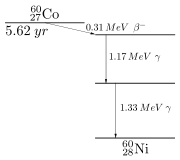
First decays to excited
Excited state
Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
by beta decay. Then the drops down to the ground state (see nuclear shell model) by emitting two gamma rays in succession (1.17 MeV then 1.33 MeV):
- {| border="0"
|- style="height:2em;"
| ||→ || ||+ || ||+ || ||+ || ||+ ||1.17 MeV
|- style="height:2em;"
| ||→ || || || || || ||+ || ||+ ||1.33 MeV
|}
Another example is the alpha decay of to form ; this alpha decay is accompanied by gamma
Gamma
Gamma is the third letter of the Greek alphabet. In the system of Greek numerals it has a value of 3. It was derived from the Phoenician letter Gimel . Letters that arose from Gamma include the Roman C and G and the Cyrillic letters Ge Г and Ghe Ґ.-Greek:In Ancient Greek, gamma represented a...
emission. In some cases, the gamma emission spectrum for a nucleus (daughter nucleus) is quite simple, (e.g. /) while in other cases, such as with (/ and /), the gamma emission spectrum is complex, revealing that a series of nuclear energy levels can exist. The fact that an alpha spectrum can have a series of different peaks with different energies reinforces the idea that several nuclear energy levels are possible.
Because a beta decay is accompanied by the emission of a neutrino
Neutrino
A neutrino is an electrically neutral, weakly interacting elementary subatomic particle with a half-integer spin, chirality and a disputed but small non-zero mass. It is able to pass through ordinary matter almost unaffected...
which also carries energy away, the beta spectrum does not have sharp lines, but instead is a broad peak. Hence from beta decay alone it is not possible to probe the different energy levels found in the nucleus.
In optical spectroscopy, it is well known that an entity which emits light can also absorb light at the same wavelength
Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
(photon energy). For instance, a sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
flame can emit yellow light as well as absorb the yellow light from a sodium vapor lamp. In the case of gamma rays, this can be seen in Mössbauer spectroscopy. Here, a correction for the energy lost by the recoil of the nucleus is made and the exact conditions for gamma ray absorption through resonance can be attained.
This is similar to the Franck Condon
Franck-Condon principle
The Franck–Condon principle is a rule in spectroscopy and quantum chemistry that explains the intensity of vibronic transitions. Vibronic transitions are the simultaneous changes in electronic and vibrational energy levels of a molecule due to the absorption or emission of a photon of the...
effects seen in optical spectroscopy.
Gamma rays from sources other than radioactive decay
Gamma radiation, like X-radiation, can be produced by a variety of phenomena. For example, when high-energy gamma rays, electrons, or protons bombard materials, the excited atoms within emit characteristic "secondary" (or fluorescent) gamma rays, which are products of temporary creation of excited nuclear states in the bombarded atoms (such transitions form a topic in nuclear spectroscopy). Such gamma rays are produced by the nucleus, but not as a result of nuclear excitement from radioactive decay.
Energy in the gamma radiation range, often explicitly called gamma-radiation when it comes from astrophysical sources, is also produced by sub-atomic particle and particle-photon interactions. These include electron-positron annihilation
Electron-positron annihilation
Electron–positron annihilation occurs when an electron and a positron collide. The result of the collision is the annihilation of the electron and positron, and the creation of gamma ray photons or, at higher energies, other particles:...
, neutral pion decay, bremsstrahlung
Bremsstrahlung
Bremsstrahlung is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into a photon because energy is conserved. The term is...
, inverse Compton scattering
Compton scattering
In physics, Compton scattering is a type of scattering that X-rays and gamma rays undergo in matter. The inelastic scattering of photons in matter results in a decrease in energy of an X-ray or gamma ray photon, called the Compton effect...
and synchrotron radiation
Synchrotron radiation
The electromagnetic radiation emitted when charged particles are accelerated radially is called synchrotron radiation. It is produced in synchrotrons using bending magnets, undulators and/or wigglers...
. In a terrestrial gamma-ray flash
Terrestrial gamma-ray flash
Terrestrial gamma-ray flashes are bursts of gamma rays in the Earth's atmosphere. TGFs have been recorded to last 0.2 to 3.5 milliseconds, and have energies of up to 20 MeV. They are probably caused by electric fields produced above thunderstorms...
a brief pulse of gamma radiation occurring high in the atmosphere of Earth, gamma rays are thought to be produced by high intensity static electric fields accelerating electrons, which then produce gamma rays by bremsstrahlung interactions with atoms in the air they collide with.
High energy gamma rays in astronomy include a gamma ray background produced when cosmic ray
Cosmic ray
Cosmic rays are energetic charged subatomic particles, originating from outer space. They may produce secondary particles that penetrate the Earth's atmosphere and surface. The term ray is historical as cosmic rays were thought to be electromagnetic radiation...
s (either high speed electrons or protons) interact with ordinary matter, producing both pair-production gamma rays at 511 keV, or bremsstrahlung
Bremsstrahlung
Bremsstrahlung is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into a photon because energy is conserved. The term is...
at energies of tens of MeV or more, when cosmic ray electrons interact with nuclei of sufficiently high atomic number (see gamma ray image of the Moon at the beginning of this article, for illustration).

- Pulsars and magnetars. The gamma ray sky (see illustration at right) is dominated by the more common and longer-term production of gamma rays in beams that emanate from pulsarPulsarA pulsar is a highly magnetized, rotating neutron star that emits a beam of electromagnetic radiation. The radiation can only be observed when the beam of emission is pointing towards the Earth. This is called the lighthouse effect and gives rise to the pulsed nature that gives pulsars their name...
s within the Milky Way. Sources from the rest of the sky are mostly quasarQuasarA quasi-stellar radio source is a very energetic and distant active galactic nucleus. Quasars are extremely luminous and were first identified as being high redshift sources of electromagnetic energy, including radio waves and visible light, that were point-like, similar to stars, rather than...
s. Pulsars are thought to be neutron stars with magnetic fields that produce focused beams of radiation, and are far less energetic, more common, and much nearer (typically seen only in our own galaxy) than are quasarQuasarA quasi-stellar radio source is a very energetic and distant active galactic nucleus. Quasars are extremely luminous and were first identified as being high redshift sources of electromagnetic energy, including radio waves and visible light, that were point-like, similar to stars, rather than...
s (or the rarer sources of gamma ray bursts discussed below). In a pulsar, which produces gamma rays for much longer than a burst, the relatively long-lived magnetic field of the pulsar produces the focused beams of relativistic charged particles, which produce gamma rays in interaction with matter, when these charged particles strike gas or dust in the nearby medium, and are deflected or stopped. This is a similar mechanism to the production of high energy photons megavoltage radiation therapyRadiation therapyRadiation therapy , radiation oncology, or radiotherapy , sometimes abbreviated to XRT or DXT, is the medical use of ionizing radiation, generally as part of cancer treatment to control malignant cells.Radiation therapy is commonly applied to the cancerous tumor because of its ability to control...
machines (see bremsstrahlungBremsstrahlungBremsstrahlung is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into a photon because energy is conserved. The term is...
). The "inverse Compton effect," in which charged particles (usually electrons) scatter from low-energy photons to convert them to higher energy photons (the gamma rays) is another possible mechanism of gamma ray production from relativistic charged particle beams. Neutron stars with a very high magnetic field (magnetarMagnetarA magnetar is a type of neutron star with an extremely powerful magnetic field, the decay of which powers the emission of copious high-energy electromagnetic radiation, particularly X-rays and gamma rays...
s) are thought to produce astronomical soft gamma repeaterSoft gamma repeaterA soft gamma repeater is an astronomical object which emits large bursts of gamma-rays and X-rays at irregular intervals. It is conjectured that they are a type of magnetar or, alternatively, neutron stars with fossil disks around them....
s, which are another relatively long-lived neutron star-powered source of gamma radiation.
- Quasars and active galaxies. More powerful gamma rays from much farther quasars and other active galaxies probably have a roughly similar linear particle acceleratorParticle acceleratorA particle accelerator is a device that uses electromagnetic fields to propel charged particles to high speeds and to contain them in well-defined beams. An ordinary CRT television set is a simple form of accelerator. There are two basic types: electrostatic and oscillating field accelerators.In...
-like method of production, with high energy electrons produced by the quasar, followed again by inverse Compton scattering, synchrotron radiationSynchrotron radiationThe electromagnetic radiation emitted when charged particles are accelerated radially is called synchrotron radiation. It is produced in synchrotrons using bending magnets, undulators and/or wigglers...
, or bremsstrahlung, to produce gamma rays. However, quasar gamma rays are produced from a distance much further away, in distant galaxies. As the black holeBlack holeA black hole is a region of spacetime from which nothing, not even light, can escape. The theory of general relativity predicts that a sufficiently compact mass will deform spacetime to form a black hole. Around a black hole there is a mathematically defined surface called an event horizon that...
at the center of such galaxies intermittantly destroys stars and focuses charged particles derived from them into beams, these beams interact with gas, dust, and lower energy photons to produce X-ray and gamma ray radiation. These sources are known to fluctuate with durations of a few weeks, indicating their relatively small size (less than a few light-weeks across). The particle beams emerge from the rotatational poles of the supermassive black holeSupermassive black holeA supermassive black hole is the largest type of black hole in a galaxy, in the order of hundreds of thousands to billions of solar masses. Most, and possibly all galaxies, including the Milky Way, are believed to contain supermassive black holes at their centers.Supermassive black holes have...
at a galactic center, which is thought to form the power source of the quasar. Such sources of gamma and X-rays are the most commonly-visible high intensity sources outside our own galaxy, since they shine not as bursts (see illustration), but instead relatively continuously when viewed with gamma ray telescopes. The power of a typical quasar is about 1040 watts, of which only a small fraction is emitted as gamma radiation, and much of the rest is emitted as electromagnetic waves at all frequencies, including radio waves.
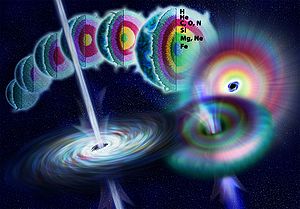
- Gamma-ray bursts. The most intense sources of gamma rays known, are also the most intense sources of any type of electromagnetic radiation presently known. They are rare compared with the sources discussed above. These intense sources are the "long duration burst" sources of gamma rays in astronomy ("long" in this context, meaning a few tens of seconds). By contrast, "short" gamma ray bursts, which are not associated with supernovae, are thought to produce gamma rays during the collision of pairs of neutron stars, or a neutron star and black holeBlack holeA black hole is a region of spacetime from which nothing, not even light, can escape. The theory of general relativity predicts that a sufficiently compact mass will deform spacetime to form a black hole. Around a black hole there is a mathematically defined surface called an event horizon that...
after they spiral toward each other by emission of gravitational waves; such bursts last two seconds or less, and are of far lower energy than the "long" bursts (they are often seen only in our own galaxy for this reason).
The so-called long duration gamma ray bursts produce events in which energies of ~ 1044 joules (as much energy as our Sun
Sun
The Sun is the star at the center of the Solar System. It is almost perfectly spherical and consists of hot plasma interwoven with magnetic fields...
will produce in its entire life-time) but over a period of only 20 to 40 seconds, accompanied by high-efficiency conversion to gamma rays (on the order of 50% total energy conversion). The leading hypotheses for the mechanism of production of these highest-known intensity beams of radiation, are inverse Compton scattering
Compton scattering
In physics, Compton scattering is a type of scattering that X-rays and gamma rays undergo in matter. The inelastic scattering of photons in matter results in a decrease in energy of an X-ray or gamma ray photon, called the Compton effect...
and synchrotron radiation
Synchrotron radiation
The electromagnetic radiation emitted when charged particles are accelerated radially is called synchrotron radiation. It is produced in synchrotrons using bending magnets, undulators and/or wigglers...
production of gamma rays from high-energy charged particles. These processes occur as relativistic charged particles leaving the region near the event horizon of the newly-formed black hole
Black hole
A black hole is a region of spacetime from which nothing, not even light, can escape. The theory of general relativity predicts that a sufficiently compact mass will deform spacetime to form a black hole. Around a black hole there is a mathematically defined surface called an event horizon that...
during the supernova explosion, and focused for a few tens of seconds into a relativistic beam by the magnetic field of the exploding hypernova
Hypernova
Hypernova , also known as a type 1c Supernova, refers to an incredibly large star that collapses at the end of its lifespan...
. The fusion explosion of the hypernova drives the energetics of the process. If the beam happens to be narrowly directed in the direction of the Earth, it shines with high gamma ray power even at distances of up to 10 billion light years—close to the edge of the visible universe.
Health effects
All ionizing radiationIonizing radiation
Ionizing radiation is radiation composed of particles that individually have sufficient energy to remove an electron from an atom or molecule. This ionization produces free radicals, which are atoms or molecules containing unpaired electrons...
causes similar damage at a cellular level, but because rays of alpha particles and beta particles are relatively non-penetrating, external exposure to them causes only localized damage, e.g. radiation burns to the skin. Gamma rays and neutrons are more penetrating, causing diffuse damage throughout the body (e.g. radiation sickness
Radiation Sickness
Radiation Sickness is a VHS by the thrash metal band Nuclear Assault. The video is a recording of a concert at the Hammersmith Odeon, London in 1988. It was released in 1991...
), increasing incidence of cancer rather than burns. External radiation exposure should also be distinguished from internal exposure, due to ingested or inhaled radioactive substances, which, depending on the substance's chemical nature, can produce both diffuse and localized internal damage. The most biological damaging forms of gamma radiation occur in the gamma ray window, between 3 and 10 MeV, with higher energy gamma rays being less harmful because the body is relatively transparent to them. See cobalt-60
Cobalt-60
Cobalt-60, , is a synthetic radioactive isotope of cobalt. Due to its half-life of 5.27 years, is not found in nature. It is produced artificially by neutron activation of . decays by beta decay to the stable isotope nickel-60...
.
Uses

Gamma-induced molecular changes can also be used to alter the properties of semi-precious stones, and is often used to change white topaz
Topaz
Topaz is a silicate mineral of aluminium and fluorine with the chemical formula Al2SiO42. Topaz crystallizes in the orthorhombic system and its crystals are mostly prismatic terminated by pyramidal and other faces.-Color and varieties:...
into blue topaz.
Non-contact industrial sensors used in the Refining, Mining, Chemical, Food, Soaps and Detergents, and Pulp and Paper industries, in applications measuring levels, density, and thicknesses commonly use sources of gamma. Typically these use Co-60 or Cs-137 isotopes as the radiation source.
In the US, gamma ray detectors are beginning to be used as part of the Container Security Initiative
Container Security Initiative
The Container Security Initiative was launched in 2002 by the U.S. Bureau of Customs and Border Protection , an agency of the Department of Homeland Security. Its purpose was to increase security for container cargo shipped to the United States...
(CSI). These US$
United States dollar
The United States dollar , also referred to as the American dollar, is the official currency of the United States of America. It is divided into 100 smaller units called cents or pennies....
5 million machines are advertised to scan 30 containers per hour. The objective of this technique is to screen merchant ship containers before they enter US ports.
Gamma radiation is often used to kill living organisms, in a process called irradiation
Irradiation
Irradiation is the process by which an object is exposed to radiation. The exposure can originate from various sources, including natural sources. Most frequently the term refers to ionizing radiation, and to a level of radiation that will serve a specific purpose, rather than radiation exposure to...
. Applications of this include sterilizing medical equipment (as an alternative to autoclave
Autoclave
An autoclave is an instrument used to sterilize equipment and supplies by subjecting them to high pressure saturated steam at 121 °C for around 15–20 minutes depending on the size of the load and the contents. It was invented by Charles Chamberland in 1879, although a precursor known as the...
s or chemical means), removing decay-causing bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
from many foods or preventing fruit and vegetables from sprouting to maintain freshness and flavor.
Despite their cancer-causing properties, gamma rays are also used to treat some types of cancer
Cancer
Cancer , known medically as a malignant neoplasm, is a large group of different diseases, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invade nearby parts of the body. The cancer may also spread to more distant parts of the...
, since the rays kill cancer cells also. In the procedure called gamma-knife surgery, multiple concentrated beams of gamma rays are directed on the growth in order to kill the cancerous cells. The beams are aimed from different angles to concentrate the radiation on the growth while minimizing damage to surrounding tissues.
Gamma rays are also used for diagnostic purposes in nuclear medicine
Nuclear medicine
In nuclear medicine procedures, elemental radionuclides are combined with other elements to form chemical compounds, or else combined with existing pharmaceutical compounds, to form radiopharmaceuticals. These radiopharmaceuticals, once administered to the patient, can localize to specific organs...
in imaging techniques. A number of different gamma-emitting radioisotopes are used. For example, in a PET scan a radiolabled sugar called fludeoxyglucose emits positrons that are converted to pairs of gamma rays that localize cancer (which often takes up more sugar than other surrounding tissues). The most common gamma emitter used in medical applications is the nuclear isomer
Nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus caused by the excitation of one or more of its nucleons . "Metastable" refers to the fact that these excited states have half-lives more than 100 to 1000 times the half-lives of the other possible excited nuclear states...
technetium-99m
Technetium-99m
Technetium-99m is a metastable nuclear isomer of technetium-99, symbolized as 99mTc. The "m" indicates that this is a metastable nuclear isomer, i.e., that its half-life of 6 hours is considerably longer than most nuclear isomers that undergo gamma decay...
which emits gamma rays in the same energy range as diagnostic X-rays. When this radionuclide tracer is administered to a patient, a gamma camera
Gamma camera
A gamma camera, also called a scintillation camera or Anger camera, is a device used to image gamma radiation emitting radioisotopes, a technique known as scintigraphy...
can be used to form an image of the radioisotope's distribution by detecting the gamma radiation emitted (see also SPECT). Depending on what molecule has been labeled with the tracer, such techniques can be employed to diagnose a wide range of conditions (for example, the spread of cancer to the bones in a bone scan
Bone scan
A bone scan or bone scintigraphy is a nuclear scanning test to find certain abnormalities in bone which are triggering the bone's attempts to heal. It is primarily used to help diagnose a number of conditions relating to bones, including: cancer of the bone or cancers that have spread to the bone,...
).
Body response
When gamma radiation breaks DNA molecules, a cell may be able to repair the damaged genetic material, within limits. However, a study of Rothkamm and Lobrich has shown that this repair process works well after high-dose exposure but is much slower in the case of a low-dose exposure.Risk assessment
The natural outdoor exposure in Great BritainGreat Britain
Great Britain or Britain is an island situated to the northwest of Continental Europe. It is the ninth largest island in the world, and the largest European island, as well as the largest of the British Isles...
ranges from 2 to 4 nSv/h (nanosieverts
Sievert
The sievert is the International System of Units SI derived unit of dose equivalent radiation. It attempts to quantitatively evaluate the biological effects of ionizing radiation as opposed to just the absorbed dose of radiation energy, which is measured in gray...
per hour
Hour
The hour is a unit of measurement of time. In modern usage, an hour comprises 60 minutes, or 3,600 seconds...
). Natural exposure to gamma rays is about 1 to 2 mSv per year, and the average total amount of radiation received in one year per inhabitant in the USA is 3.6 mSv. There is a small increase in the dose, due to naturally occurring gamma radiation, around small particles of high atomic number materials in the human body caused by the photoelectric effect.
By comparison, the radiation dose from chest radiography
Radiography
Radiography is the use of X-rays to view a non-uniformly composed material such as the human body. By using the physical properties of the ray an image can be developed which displays areas of different density and composition....
(about 0.06 mSv) is a fraction of the annual naturally occurring background radiation dose,. A chest CT delivers 5 to 8 mSv. A whole-body PET
Positron emission tomography
Positron emission tomography is nuclear medicine imaging technique that produces a three-dimensional image or picture of functional processes in the body. The system detects pairs of gamma rays emitted indirectly by a positron-emitting radionuclide , which is introduced into the body on a...
/CT scan can deliver 14 to 32 mSv depending on the protocol. The dose from fluoroscopy
Fluoroscopy
Fluoroscopy is an imaging technique commonly used by physicians to obtain real-time moving images of the internal structures of a patient through the use of a fluoroscope. In its simplest form, a fluoroscope consists of an X-ray source and fluorescent screen between which a patient is placed...
of the stomach is much higher, approximately 50 mSv (14 times the annual yearly background).
An acute full-body equivalent single exposure dose of 1 Sv (1000 mSv) causes slight blood changes, but 2.0–3.5 Sv (2.0–3.5 Gy) causes very severe syndrome of nausea, hair loss, and hemorrhaging, and will cause death in a sizable number of cases—-about 10% to 35% without medical treatment. A dose of 5 Sv (5 Gy) is considered approximately the LD50 (lethal dose for 50% of exposed population) for an acute exposure to radiation even with standard medical treatment. A dose higher than 5 Sv (5 Gy) brings an increasing chance of death above 50%. Above 7.5–10 Sv (7.5–10 Gy) to the entire body, even extraordinary treatment, such as bone-marrow transplants, will not prevent the death of the individual exposed (see Radiation poisoning
Radiation poisoning
Acute radiation syndrome also known as radiation poisoning, radiation sickness or radiation toxicity, is a constellation of health effects which occur within several months of exposure to high amounts of ionizing radiation...
).. (Doses much larger than this may, however, be delivered to selected parts of the body in the course of radiation therapy
Radiation therapy
Radiation therapy , radiation oncology, or radiotherapy , sometimes abbreviated to XRT or DXT, is the medical use of ionizing radiation, generally as part of cancer treatment to control malignant cells.Radiation therapy is commonly applied to the cancerous tumor because of its ability to control...
.)
For low dose exposure, for example among nuclear workers, who receive an average yearly radiation dose of 19 mSv, the risk of dying from cancer (excluding leukemia
Leukemia
Leukemia or leukaemia is a type of cancer of the blood or bone marrow characterized by an abnormal increase of immature white blood cells called "blasts". Leukemia is a broad term covering a spectrum of diseases...
) increases by 2 percent. For a dose of 100 mSv, that risk increase is at 10 percent. By comparison, risk of dying from cancer was increased by 32 percent for the survivors of the atomic bombing of Hiroshima and Nagasaki.
See also
- Alpha particleAlpha particleAlpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is classically produced in the process of alpha decay, but may be produced also in other ways and given the same name...
- Beta particleBeta particleBeta particles are high-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei such as potassium-40. The beta particles emitted are a form of ionizing radiation also known as beta rays. The production of beta particles is termed beta decay...
- AnnihilationAnnihilationAnnihilation is defined as "total destruction" or "complete obliteration" of an object; having its root in the Latin nihil . A literal translation is "to make into nothing"....
- Gamma cameraGamma cameraA gamma camera, also called a scintillation camera or Anger camera, is a device used to image gamma radiation emitting radioisotopes, a technique known as scintigraphy...
- Gamma-ray astronomyGamma-ray astronomyGamma-ray astronomy is the astronomical study of the cosmos with gamma rays. Gamma-rays are the most energetic form of "light" that travel across the universe, and gamma-rays thus have the smallest wavelength of any wave in the electromagnetic spectrum.Gamma-rays are created by celestial events...
- Gamma-ray burst
- Gamma spectroscopyGamma spectroscopyGamma-ray spectroscopy is the quantitative study of the energy spectra of gamma-ray sources, both nuclear laboratory, geochemical, and astrophysical. Gamma rays are the highest-energy form of electromagnetic radiation, being physically exactly like all other forms except for higher photon energy...
- Mössbauer effectMössbauer effectThe Mössbauer effect, or recoilless nuclear resonance fluorescence, is a physical phenomenon discovered by Rudolf Mössbauer in 1958. It involves the resonant and recoil-free emission and absorption of γ radiation by atomic nuclei bound in a solid...
- Nuclear fissionNuclear fissionIn nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
and fusionNuclear fusionNuclear fusion is the process by which two or more atomic nuclei join together, or "fuse", to form a single heavier nucleus. This is usually accompanied by the release or absorption of large quantities of energy... - Radioactive decayRadioactive decayRadioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
External links
- Basic reference on several types of radiation
- Radiation Q & A
- GCSE information
- Radiation information
- Gamma ray bursts
- The Lund/LBNL Nuclear Data Search – Contains information on gamma-ray energies from isotopes.
- Mapping soils with airborne detectors
-
 The LIVEChart of Nuclides – IAEA with filter on gamma-ray energy, in Java or HTML
The LIVEChart of Nuclides – IAEA with filter on gamma-ray energy, in Java or HTML - Health Physics Society Public Education Website

