
Electrochemistry
Encyclopedia

Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
that studies chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s which take place in a solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
at the interface of an electron conductor
Electrical conductor
In physics and electrical engineering, a conductor is a material which contains movable electric charges. In metallic conductors such as copper or aluminum, the movable charged particles are electrons...
(a metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
or a semiconductor
Semiconductor
A semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
) and an ionic conductor (the electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
), and which involve electron transfer between the electrode and the electrolyte or species in solution.
If a chemical reaction is driven by an external applied voltage
Voltage
Voltage, otherwise known as electrical potential difference or electric tension is the difference in electric potential between two points — or the difference in electric potential energy per unit charge between two points...
, as in electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
, or if a voltage is created by a chemical reaction as in a battery
Battery (electricity)
An electrical battery is one or more electrochemical cells that convert stored chemical energy into electrical energy. Since the invention of the first battery in 1800 by Alessandro Volta and especially since the technically improved Daniell cell in 1836, batteries have become a common power...
, it is an electrochemical reaction. In contrast, chemical reactions where electrons are transferred between molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s are called oxidation/reduction (redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
) reactions. In general, electrochemistry deals with situations where oxidation and reduction
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
reactions are separated in space or time, connected by an external electric circuit.
16th to 18th century developments

Magnetism
Magnetism is a property of materials that respond at an atomic or subatomic level to an applied magnetic field. Ferromagnetism is the strongest and most familiar type of magnetism. It is responsible for the behavior of permanent magnets, which produce their own persistent magnetic fields, as well...
and, to a lesser extent, electricity. For his work on magnets, Gilbert became known as the "Father of Magnetism." He discovered various methods for producing and strengthening magnets.
In 1663 the German
Germany
Germany , officially the Federal Republic of Germany , is a federal parliamentary republic in Europe. The country consists of 16 states while the capital and largest city is Berlin. Germany covers an area of 357,021 km2 and has a largely temperate seasonal climate...
physicist
Physicist
A physicist is a scientist who studies or practices physics. Physicists study a wide range of physical phenomena in many branches of physics spanning all length scales: from sub-atomic particles of which all ordinary matter is made to the behavior of the material Universe as a whole...
Otto von Guericke
Otto von Guericke
Otto von Guericke was a German scientist, inventor, and politician...
created the first electric generator, which produced static electricity by applying friction in the machine. The generator was made of a large sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
ball cast inside a glass globe, mounted on a shaft. The ball was rotated by means of a crank and a static electric
Static electricity
Static electricity refers to the build-up of electric charge on the surface of objects. The static charges remain on an object until they either bleed off to ground or are quickly neutralized by a discharge. Static electricity can be contrasted with current electricity, which can be delivered...
spark
Electric spark
An electric spark is a type of electrostatic discharge that occurs when an electric field creates an ionized electrically conductive channel in air producing a brief emission of light and sound. A spark is formed when the electric field strength exceeds the dielectric field strength of air...
was produced when a pad was rubbed against the ball as it rotated. The globe could be removed and used as source for experiments with electricity.
By the mid—18th century the French
France
The French Republic , The French Republic , The French Republic , (commonly known as France , is a unitary semi-presidential republic in Western Europe with several overseas territories and islands located on other continents and in the Indian, Pacific, and Atlantic oceans. Metropolitan France...
chemist
Chemist
A chemist is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties such as density and acidity. Chemists carefully describe the properties they study in terms of quantities, with detail on the level of molecules and their component atoms...
Charles François de Cisternay du Fay had discovered two types of static electricity, and that like charges repel each other whilst unlike charges attract. Du Fay announced that electricity consisted of two fluids: "vitreous" (from the Latin
Latin
Latin is an Italic language originally spoken in Latium and Ancient Rome. It, along with most European languages, is a descendant of the ancient Proto-Indo-European language. Although it is considered a dead language, a number of scholars and members of the Christian clergy speak it fluently, and...
for "glass"), or positive, electricity; and "resinous," or negative, electricity. This was the two-fluid theory of electricity, which was to be opposed by Benjamin Franklin's
Benjamin Franklin
Dr. Benjamin Franklin was one of the Founding Fathers of the United States. A noted polymath, Franklin was a leading author, printer, political theorist, politician, postmaster, scientist, musician, inventor, satirist, civic activist, statesman, and diplomat...
one-fluid theory later in the century.
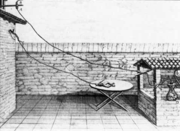
Charles-Augustin de Coulomb
Charles-Augustin de Coulomb was a French physicist. He is best known for developing Coulomb's law, the definition of the electrostatic force of attraction and repulsion. The [SI unit] of charge, the coulomb, was named after him....
developed the law of electrostatic attraction in 1785 as an outgrowth of his attempt to investigate the law of electrical repulsions as stated by Joseph Priestley
Joseph Priestley
Joseph Priestley, FRS was an 18th-century English theologian, Dissenting clergyman, natural philosopher, chemist, educator, and political theorist who published over 150 works...
in England.

Italy
Italy , officially the Italian Republic languages]] under the European Charter for Regional or Minority Languages. In each of these, Italy's official name is as follows:;;;;;;;;), is a unitary parliamentary republic in South-Central Europe. To the north it borders France, Switzerland, Austria and...
physician
Physician
A physician is a health care provider who practices the profession of medicine, which is concerned with promoting, maintaining or restoring human health through the study, diagnosis, and treatment of disease, injury and other physical and mental impairments...
and anatomist Luigi Galvani
Luigi Galvani
Luigi Aloisio Galvani was an Italian physician and physicist who lived and died in Bologna. In 1791, he discovered that the muscles of dead frogs legs twitched when struck by a spark...
marked the birth of electrochemistry by establishing a bridge between chemical reactions and electricity on his essay "De Viribus Electricitatis in Motu Musculari Commentarius" (Latin for Commentary on the Effect of Electricity on Muscular Motion) in 1791 where he proposed a "nerveo-electrical substance" on biological life forms.
In his essay Galvani concluded that animal tissue contained a here-to-fore neglected innate, vital force, which he termed "animal electricity," which activated nerve
Nerve
A peripheral nerve, or simply nerve, is an enclosed, cable-like bundle of peripheral axons . A nerve provides a common pathway for the electrochemical nerve impulses that are transmitted along each of the axons. Nerves are found only in the peripheral nervous system...
s and muscle
Muscle
Muscle is a contractile tissue of animals and is derived from the mesodermal layer of embryonic germ cells. Muscle cells contain contractile filaments that move past each other and change the size of the cell. They are classified as skeletal, cardiac, or smooth muscles. Their function is to...
s spanned by metal probes. He believed that this new force was a form of electricity in addition to the "natural" form produced by lightning
Lightning
Lightning is an atmospheric electrostatic discharge accompanied by thunder, which typically occurs during thunderstorms, and sometimes during volcanic eruptions or dust storms...
or by the electric eel
Electric eel
The electric eel , is an electric fish, and the only species of the genus Electrophorus. It is capable of generating powerful electric shocks, of up to six hundred volts, which it uses for both hunting and self-defense. It is an apex predator in its South American range...
and torpedo ray
Electric ray
The electric rays are a group of rays, flattened cartilaginous fish with enlarged pectoral fins, comprising the order Torpediniformes. They are known for being capable of producing an electric discharge, ranging from as little as 8 volts up to 220 volts depending on species, used to stun prey and...
as well as the "artificial" form produced by friction
Friction
Friction is the force resisting the relative motion of solid surfaces, fluid layers, and/or material elements sliding against each other. There are several types of friction:...
(i.e., static electricity).
Galvani's scientific colleagues generally accepted his views, but Alessandro Volta
Alessandro Volta
Count Alessandro Giuseppe Antonio Anastasio Gerolamo Umberto Volta was a Lombard physicist known especially for the invention of the battery in 1800.-Early life and works:...
rejected the idea of an "animal electric fluid," replying that the frog's legs responded to differences in metal temper, composition, and bulk. Galvani refuted this by obtaining muscular action with two pieces of the same material.
19th century

William Nicholson (chemist)
William Nicholson was a renowned English chemist and writer on "natural philosophy" and chemistry, as well as a translator, journalist, publisher, scientist, and inventor.-Early life:...
and Johann Wilhelm Ritter
Johann Wilhelm Ritter
Johann Wilhelm Ritter was a German chemist, physicist and philosopher. He was born in Samitz near Haynau in Silesia , and died in Munich.-Life and work:...
succeeded in decomposing water into hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
by electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
. Soon thereafter Ritter discovered the process of electroplating
Electroplating
Electroplating is a plating process in which metal ions in a solution are moved by an electric field to coat an electrode. The process uses electrical current to reduce cations of a desired material from a solution and coat a conductive object with a thin layer of the material, such as a metal...
. He also observed that the amount of metal deposited and the amount of oxygen produced during an electrolytic process depended on the distance between the electrode
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
s. By 1801 Ritter observed thermoelectric currents and anticipated the discovery of thermoelectricity by Thomas Johann Seebeck
Thomas Johann Seebeck
Thomas Johann Seebeck was a physicist who in 1821 discovered the thermoelectric effect.Seebeck was born in Reval to a wealthy Baltic German merchant family. He received a medical degree in 1802 from the University of Göttingen, but preferred to study physics...
.
By the 1810s William Hyde Wollaston
William Hyde Wollaston
William Hyde Wollaston FRS was an English chemist and physicist who is famous for discovering two chemical elements and for developing a way to process platinum ore.-Biography:...
made improvements to the galvanic cell
Galvanic cell
A Galvanic cell, or Voltaic cell, named after Luigi Galvani, or Alessandro Volta respectively, is an electrochemical cell that derives electrical energy from spontaneous redox reaction taking place within the cell...
.
Sir Humphry Davy
Humphry Davy
Sir Humphry Davy, 1st Baronet FRS MRIA was a British chemist and inventor. He is probably best remembered today for his discoveries of several alkali and alkaline earth metals, as well as contributions to the discoveries of the elemental nature of chlorine and iodine...
's work with electrolysis led to the conclusion that the production of electricity in simple electrolytic cell
Electrolytic cell
An electrolytic cell decomposes chemical compounds by means of electrical energy, in a process called electrolysis; the Greek word lysis means to break up. The result is that the chemical energy is increased...
s resulted from chemical action and that chemical combination occurred between substances of opposite charge. This work led directly to the isolation of sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
and potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
from their compounds and of the alkaline earth metal
Alkaline earth metal
The alkaline earth metals are a group in the periodic table. In the modern IUPAC nomenclature, the alkaline earth metals are called the group 2 elements. Previously, they were called the Group IIA elements . The alkaline earth metals contain beryllium , magnesium , calcium , strontium , barium and...
s from theirs in 1808.
Hans Christian Ørsted
Hans Christian Ørsted
Hans Christian Ørsted was a Danish physicist and chemist who discovered that electric currents create magnetic fields, an important aspect of electromagnetism...
's discovery of the magnetic effect of electrical currents in 1820 was immediately recognized as an epoch-making advance, although he left further work on electromagnetism
Electromagnetism
Electromagnetism is one of the four fundamental interactions in nature. The other three are the strong interaction, the weak interaction and gravitation...
to others. André-Marie Ampère
André-Marie Ampère
André-Marie Ampère was a French physicist and mathematician who is generally regarded as one of the main discoverers of electromagnetism. The SI unit of measurement of electric current, the ampere, is named after him....
quickly repeated Oestred's experiment, and formulated them mathematically.
In 1821, Estonian-German physicist
Physicist
A physicist is a scientist who studies or practices physics. Physicists study a wide range of physical phenomena in many branches of physics spanning all length scales: from sub-atomic particles of which all ordinary matter is made to the behavior of the material Universe as a whole...
Thomas Johann Seebeck
Thomas Johann Seebeck
Thomas Johann Seebeck was a physicist who in 1821 discovered the thermoelectric effect.Seebeck was born in Reval to a wealthy Baltic German merchant family. He received a medical degree in 1802 from the University of Göttingen, but preferred to study physics...
demonstrated the electrical potential in the juncture points of two dissimilar metals when there is a heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
difference between the joints.
In 1827, the German scientist Georg Ohm
Georg Ohm
Georg Simon Ohm was a German physicist. As a high school teacher, Ohm began his research with the recently-invented electrochemical cell, invented by Italian Count Alessandro Volta. Using equipment of his own creation, Ohm determined that there is a direct proportionality between the potential...
expressed his law
Ohm's law
Ohm's law states that the current through a conductor between two points is directly proportional to the potential difference across the two points...
in this famous book "Die galvanische Kette, mathematisch bearbeitet" (The Galvanic Circuit Investigated Mathematically) in which he gave his complete theory of electricity.
In 1832, Michael Faraday
Michael Faraday
Michael Faraday, FRS was an English chemist and physicist who contributed to the fields of electromagnetism and electrochemistry....
's experiments led him to state his two laws of electrochemistry. In 1836, John Daniell
John Frederic Daniell
John Frederic Daniell was an English chemist and physicist.Daniell was born in London, and in 1831 became the first professor of chemistry at the newly founded King's College London. His name is best known for his invention of the Daniell cell , an electric battery much better than voltaic cells...
invented a primary cell in which hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
was eliminated in the generation of the electricity. Daniell had solved the problem of polarization. Later results revealed that alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
ing the amalgam
Amalgam (chemistry)
An amalgam is a substance formed by the reaction of mercury with another metal. Almost all metals can form amalgams with mercury, notable exceptions being iron and platinum. Silver-mercury amalgams are important in dentistry, and gold-mercury amalgam is used in the extraction of gold from ore.The...
ated zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
with mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
would produce a better voltage.
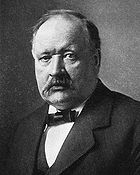
William Robert Grove
Sir William Robert Grove PC QC FRS was a judge and physical scientist. He anticipated the general theory of the conservation of energy, and was a pioneer of fuel cell technology.-Early life:...
produced the first fuel cell
Fuel cell
A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used...
in 1839. In 1846, Wilhelm Weber
Wilhelm Eduard Weber
Wilhelm Eduard Weber was a German physicist and, together with Carl Friedrich Gauss, inventor of the first electromagnetic telegraph.-Early years:...
developed the electrodynamometer. In 1868, Georges Leclanché
Georges Leclanché
Georges Leclanché was a French electrical engineer chiefly remembered for his invention of the Leclanché cell, one of the first modern electrical batteries and the forerunner of the modern dry cell battery.-Biography:...
patented a new cell which eventually became the forerunner to the world's first widely used battery, the zinc carbon cell
Zinc-carbon battery
A zinc–carbon dry cell or battery is packaged in a zinc can that serves as both a container and negative terminal. It was developed from the wet Leclanché cell . The positive terminal is a carbon rod surrounded by a mixture of manganese dioxide and carbon powder. The electrolyte used is a paste of...
.
Svante Arrhenius
Svante Arrhenius
Svante August Arrhenius was a Swedish scientist, originally a physicist, but often referred to as a chemist, and one of the founders of the science of physical chemistry...
published his thesis in 1884 on Recherches sur la conductibilité galvanique des électrolytes (Investigations on the galvanic conductivity of electrolytes). From his results the author concluded that electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
s, when dissolved in water, become to varying degrees split or dissociated into electrically opposite positive and negative ions.
In 1886, Paul Héroult
Paul Héroult
The French scientist Paul Héroult was the inventor of the aluminium electrolysis and of the electric steel furnace. He lived in Thury-Harcourt, Normandy.Christian Bickert said of him...
and Charles M. Hall
Charles Martin Hall
Charles Martin Hall was an American inventor, music enthusiast, and chemist. He is best known for his invention in 1886 of an inexpensive method for producing aluminium, which became the first metal to attain widespread use since the prehistoric discovery of iron.-Early years:Charles Martin Hall...
developed an efficient method (the Hall–Héroult process) to obtain aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
using electrolysis of molten alumina.
In 1894, Friedrich Ostwald
Wilhelm Ostwald
Friedrich Wilhelm Ostwald was a Baltic German chemist. He received the Nobel Prize in Chemistry in 1909 for his work on catalysis, chemical equilibria and reaction velocities...
concluded important studies of the conductivity
Conductivity (electrolytic)
The conductivity of an electrolyte solution is a measure of its ability to conduct electricity. The SI unit of conductivity is siemens per meter ....
and electrolytic dissociation of organic acid
Organic acid
An organic acid is an organic compound with acidic properties. The most common organic acids are the carboxylic acids, whose acidity is associated with their carboxyl group –COOH. Sulfonic acids, containing the group –SO2OH, are relatively stronger acids. The relative stability of the conjugate...
s.

Walther Nernst
Walther Hermann Nernst FRS was a German physical chemist and physicist who is known for his theories behind the calculation of chemical affinity as embodied in the third law of thermodynamics, for which he won the 1920 Nobel Prize in chemistry...
developed the theory of the electromotive force
Electromotive force
In physics, electromotive force, emf , or electromotance refers to voltage generated by a battery or by the magnetic force according to Faraday's Law, which states that a time varying magnetic field will induce an electric current.It is important to note that the electromotive "force" is not a...
of the voltaic cell in 1888. In 1889, he showed how the characteristics of the current produced could be used to calculate the free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
change in the chemical reaction producing the current. He constructed an equation, known as Nernst equation
Nernst equation
In electrochemistry, the Nernst equation is an equation that can be used to determine the equilibrium reduction potential of a half-cell in an electrochemical cell. It can also be used to determine the total voltage for a full electrochemical cell...
, which related the voltage of a cell to its properties.
In 1898, Fritz Haber
Fritz Haber
Fritz Haber was a German chemist, who received the Nobel Prize in Chemistry in 1918 for his development for synthesizing ammonia, important for fertilizers and explosives. Haber, along with Max Born, proposed the Born–Haber cycle as a method for evaluating the lattice energy of an ionic solid...
showed that definite reduction products can result from electrolytic processes if the potential at the cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
is kept constant. In 1898, he explained the reduction of nitrobenzene
Nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale as a precursor to aniline. Although occasionally used as a flavoring or perfume...
in stages at the cathode and this became the model for other similar reduction processes.
The 20th century and recent developments
In 1902, The Electrochemical SocietyThe Electrochemical Society
The Electrochemical Society is a learned society based in the United States that supports scientific inquiry in the field of electrochemistry and solid-state science and technology. It was founded in 1902 as the American Electrochemical Society...
(ECS) was founded.
In 1909, Robert Andrews Millikan began a series of experiments to determine the electric charge carried by a single electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
.
In 1923, Johannes Nicolaus Brønsted
Johannes Nicolaus Brønsted
Johannes Nicolaus Brønsted born in Varde was a Danish physical chemist.He received a degree in chemical engineering in 1899 and his Ph. D. in 1908 from the University of Copenhagen. He was immediately appointed professor of inorganic and physical chemistry at Copenhagen.In 1906 he published his...
and Martin Lowry
Martin Lowry
Thomas Martin Lowry CBE FRS was an English physical chemist. Independently from Johannes Nicolaus Brønsted he has developed the Brønsted–Lowry acid–base theory and was as a founder-member and president of the Faraday Society.-Biography:Lowry was born in Low Moor, Bradford, West Yorkshire,...
published essentially the same theory about how acids and bases behave, using an electrochemical basis.
Arne Tiselius
Arne Tiselius
Arne Wilhelm Kaurin Tiselius was a Swedish biochemist who won the Nobel Prize in Chemistry in 1948.- Biography:Tiselius was born in Stockholm...
developed the first sophisticated electrophoretic apparatus in 1937 and some years later he was awarded the 1948 Nobel Prize
Nobel Prize
The Nobel Prizes are annual international awards bestowed by Scandinavian committees in recognition of cultural and scientific advances. The will of the Swedish chemist Alfred Nobel, the inventor of dynamite, established the prizes in 1895...
for his work in protein electrophoresis
Electrophoresis
Electrophoresis, also called cataphoresis, is the motion of dispersed particles relative to a fluid under the influence of a spatially uniform electric field. This electrokinetic phenomenon was observed for the first time in 1807 by Reuss , who noticed that the application of a constant electric...
.
A year later, in 1949, the International Society of Electrochemistry
International Society of Electrochemistry
The International Society of Electrochemistry is a global scientific society founded in 1949. The Head Office of ISE is located now in Lausanne, Switzerland. ISE is a Member Organization of IUPAC...
(ISE) was founded.
By the 1960s–1970s quantum electrochemistry
Quantum electrochemistry
The scientific school of Quantum electrochemistry began to form in the 1960s under Revaz Dogonadze. Generally speaking, the field comprises the notions arising in electrodynamics, quantum mechanics, and electrochemistry; and so is studied by a very large array of different professional researchers...
was developed by Revaz Dogonadze
Revaz Dogonadze
Revaz Dogonadze was a notable Georgian scientist, Corresponding Member of the Georgian National Academy of Sciences , Dr.Sc. , Professor, one of the founders of quantum electrochemistry,-Life and works:...
and his pupils.
Redox reactions
RedoxRedox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
stands for reduction-oxidation, and are electrochemical processes involving electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
transfer to or from a molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
or ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
changing its oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
. This reaction can occur through the application of an external voltage
Voltage
Voltage, otherwise known as electrical potential difference or electric tension is the difference in electric potential between two points — or the difference in electric potential energy per unit charge between two points...
or through the release of chemical energy.
Oxidation and reduction
Oxidation and reduction describe the change of oxidation state that takes place in the atoms, ions or molecules involved in an electrochemical reactionChemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
. Formally, oxidation state is the hypothetical charge
Electric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
that an atom would have if all bonds to atoms of different elements were 100% ionic
Ionic bond
An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. Pure ionic bonding cannot exist: all ionic compounds have some...
. An atom or ion that gives up an electron to another atom or ion has its oxidation state increase, and the recipient of the negatively charged electron has its oxidation state decrease. Oxidation and reduction always occur in a paired fashion such that one species is oxidized when another is reduced. This paired electron transfer is called a redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
reaction.
For example, when atomic sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
reacts with atomic chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
, sodium donates one electron and attains an oxidation state of +1. Chlorine accepts the electron and its oxidation state is reduced to −1. The sign of the oxidation state (positive/negative) actually corresponds to the value of each ion's electronic charge. The attraction of the differently charged sodium and chlorine ions is the reason they then form an ionic bond
Ionic bond
An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. Pure ionic bonding cannot exist: all ionic compounds have some...
.
The loss of electrons from an atom or molecule is called oxidation, and the gain of electrons is reduction
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
. This can be easily remembered through the use of mnemonic
Mnemonic
A mnemonic , or mnemonic device, is any learning technique that aids memory. To improve long term memory, mnemonic systems are used to make memorization easier. Commonly encountered mnemonics are often verbal, such as a very short poem or a special word used to help a person remember something,...
devices. Two of the most popular are "OIL RIG" (Oxidation Is Loss, Reduction Is Gain) and "LEO" the lion says "GER" (Lose Electrons: Oxidization, Gain Electrons: Reduction). For cases where electrons are shared (covalent bonds) between atoms with large differences in electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
, the electron is assigned to the atom with the largest electronegativity in determining the oxidation state.
The atom or molecule which loses electrons is known as the reducing agent, or reductant, and the substance which accepts the electrons is called the oxidizing agent, or oxidant. The oxidizing agent is always being reduced in a reaction; the reducing agent is always being oxidized. Oxygen is a common oxidizing agent, but not the only one. Despite the name, an oxidation reaction does not necessarily need to involve oxygen. In fact, a fire
Fire
Fire is the rapid oxidation of a material in the chemical process of combustion, releasing heat, light, and various reaction products. Slower oxidative processes like rusting or digestion are not included by this definition....
can be fed by an oxidant other than oxygen; fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
fires are often unquenchable, as fluorine is an even stronger oxidant (it has a higher electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
) than oxygen.
For reactions involving oxygen, the gain of oxygen implies the oxidation of the atom or molecule to which the oxygen is added (and the oxygen is reduced). In organic compounds, such as butane
Butane
Butane is a gas with the formula C4H10 that is an alkane with four carbon atoms. The term may refer to any of two structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, butane refers only to the unbranched n-butane isomer; the other one being called "methylpropane" or...
or ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
, the loss of hydrogen implies oxidation of the molecule from which it is lost (and the hydrogen is reduced). This follows because the hydrogen donates its electron in covalent bonds with non-metals but it takes the electron along when it is lost. Conversely, loss of oxygen or gain of hydrogen implies reduction.
Balancing redox reactions
Electrochemical reactions in water are better understood by balancing redox reactions using the ion-electron method where H+Hydronium
In chemistry, a hydronium ion is the cation , a type of oxonium ion produced by protonation of water. This cation is often used to represent the nature of the proton in aqueous solution, where the proton is highly solvated...
, OH–
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
ion, H2O and electrons (to compensate the oxidation changes) are added to cell's half-reaction
Half-reaction
A half reaction is either the oxidation or reduction reaction component of a redox reaction. A half reaction is obtained by considering the change in oxidation states of individual substances involved in the redox reaction.-Example:...
s for oxidation and reduction.
Acidic medium
In acid medium H+Hydronium
In chemistry, a hydronium ion is the cation , a type of oxonium ion produced by protonation of water. This cation is often used to represent the nature of the proton in aqueous solution, where the proton is highly solvated...
ions and water are added to half-reaction
Half-reaction
A half reaction is either the oxidation or reduction reaction component of a redox reaction. A half reaction is obtained by considering the change in oxidation states of individual substances involved in the redox reaction.-Example:...
s to balance the overall reaction.
For example, when manganese
Manganese
Manganese is a chemical element, designated by the symbol Mn. It has the atomic number 25. It is found as a free element in nature , and in many minerals...
reacts with sodium bismuthate
Sodium bismuthate
Sodium bismuthate, also known as sodium bismuth oxide, is a slightly hygroscopic chemical compound with the chemical formula of NaBiO3.Sodium bismuthate is an oxidizer. It is not soluble in cold water, but decomposes when placed in hot water....
.
- Unbalanced reaction: Mn2+(aq) + NaBiO3(s) → Bi3+(aq) + MnO4–(aq)
- Oxidation: 4 H2O(l) + Mn2+(aq) → MnO4–(aq) + 8 H+(aq) + 5 e–
- Reduction: 2 e– + 6 H+(aq) + BiO3–(s) → Bi3+(aq) + 3 H2O(l)
Finally, the reaction is balanced by multiplying
Multiplication
Multiplication is the mathematical operation of scaling one number by another. It is one of the four basic operations in elementary arithmetic ....
the number of electrons from the reduction half reaction to oxidation half reaction and vice versa and adding both half reactions, thus solving the equation.
- 8 H2O(l) + 2 Mn2+(aq) → 2 MnO4–(aq) + 16 H+(aq) + 10 e–
- 10 e– + 30 H+(aq) + 5 BiO3–(s) → 5 Bi3+(aq) + 15 H2O(l)
Reaction balanced:
- 14 H+(aq) + 2 Mn2+(aq) + 5 NaBiO3(s) → 7 H2O(l) + 2 MnO4–(aq) + 5 Bi3+(aq) + 5 Na+(aq)
Basic medium
In basic medium OH–Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
ions and water are added to half reactions to balance the overall reaction. For example, on reaction between potassium permanganate
Potassium permanganate
Potassium permanganate is an inorganic chemical compound with the formula KMnO4. It is a salt consisting of K+ and MnO4− ions. Formerly known as permanganate of potash or Condy's crystals, it is a strong oxidizing agent. It dissolves in water to give intensely purple solutions, the...
and sodium sulfite
Sodium sulfite
Sodium sulfite is a soluble sodium salt of sulfurous acid. It is a product of sulfur dioxide scrubbing, a part of the flue gas desulfurization process...
.
- Unbalanced reaction: KMnO4 + Na2SO3 + H2O → MnO2 + Na2SO4 + KOH
- Reduction: 3 e– + 2 H2O + MnO4– → MnO2 + 4 OH–
- Oxidation: 2 OH– + SO32– → SO42– + H2O + 2 e–
The same procedure as followed on acid medium by multiplying electrons to opposite half reactions solve the equation thus balancing the overall reaction.
- 6 e– + 4 H2O + 2 MnO4– → 2 MnO2 + 8 OH–
- 6 OH– + 3 SO32– → 3 SO42– + 3 H2O + 6e–
Equation balanced:
- 2 KMnO4 + 3 Na2SO3 + H2O → 2 MnO2 + 3 Na2SO4 + 2 KOH
Neutral medium
The same procedure as used on acid medium is applied, for example on balancing using electron ion method to complete combustionCombustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
of propane
Propane
Propane is a three-carbon alkane with the molecular formula , normally a gas, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel for engines, oxy-gas torches, barbecues, portable stoves, and residential central...
.
- Unbalanced reaction: C3H8 + O2 → CO2 + H2O
- Reduction: 4 H+ + O2 + 4 e– → 2 H2O
- Oxidation: 6 H2O + C3H8 → 3 CO2 + 20 e– + 20 H+
As in acid and basic medium, electrons which were used to compensate oxidation changes are multiplied to opposite half reactions, thus solving the equation.
- 20 H+ + 5 O2 + 20 e– → 10 H2O
- 6 H2O + C3H8 → 3 CO2 + 20 e– + 20 H+
Equation balanced:
- C3H8 + 5 O2 → 3 CO2 + 4 H2O
Electrochemical cells
An electrochemical cell is a device that produces an electric current from energy released by a spontaneousSpontaneous process
A spontaneous process is the time-evolution of a system in which it releases free energy and moves to a lower, more thermodynamically stable energy state...
redox reaction. This kind of cell includes the Galvanic cell
Galvanic cell
A Galvanic cell, or Voltaic cell, named after Luigi Galvani, or Alessandro Volta respectively, is an electrochemical cell that derives electrical energy from spontaneous redox reaction taking place within the cell...
or Voltaic cell, named after Luigi Galvani
Luigi Galvani
Luigi Aloisio Galvani was an Italian physician and physicist who lived and died in Bologna. In 1791, he discovered that the muscles of dead frogs legs twitched when struck by a spark...
and Alessandro Volta, both scientists who conducted several experiments on chemical reactions and electric current during the late 18th century.
Electrochemical cells have two conductive electrodes (the anode and the cathode). The anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
is defined as the electrode where oxidation occurs and the cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
is the electrode where the reduction takes place. Electrodes can be made from any sufficiently conductive materials, such as metals, semiconductors, graphite, and even conductive polymer
Conductive polymer
Conductive polymers or, more precisely, intrinsically conducting polymers are organic polymers that conduct electricity. Such compounds may have metallic conductivity or can be semiconductors. The biggest advantage of conductive polymers is their processability, mainly by dispersion. Conductive...
s. In between these electrodes is the electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
, which contains ions that can freely move.
The Galvanic cell uses two different metal electrodes, each in an electrolyte where the positively charged ions are the oxidized form of the electrode metal. One electrode will undergo oxidation (the anode) and the other will undergo reduction (the cathode). The metal of the anode will oxidize, going from an oxidation state of 0 (in the solid form) to a positive oxidation state and become an ion. At the cathode, the metal ion in solution will accept one or more electrons from the cathode and the ion's oxidation state is reduced to 0. This forms a solid metal that electrodeposits
Electroplating
Electroplating is a plating process in which metal ions in a solution are moved by an electric field to coat an electrode. The process uses electrical current to reduce cations of a desired material from a solution and coat a conductive object with a thin layer of the material, such as a metal...
on the cathode. The two electrodes must be electrically connected to each other, allowing for a flow of electrons that leave the metal of the anode and flow through this connection to the ions at the surface of the cathode. This flow of electrons is an electrical current that can be used to do work, such as turn a motor or power a light.
A Galvanic cell whose electrode
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
s are zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
and copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
submerged in zinc sulfate
Zinc sulfate
Zinc sulfate is the inorganic compound with the formula ZnSO4 as well as any of three hydrates. It was historically known as "white vitriol". It is a colorless solid that is a common source of soluble zinc ions.-Production and reactivity:...
and copper sulfate, respectively, is known as a Daniell cell
Daniell cell
The Daniell cell was invented in 1836 by John Frederic Daniell, a British chemist and meteorologist, and consisted of a copper pot filled with a copper sulfate solution, in which was immersed an unglazed earthenware container filled with sulfuric acid and a zinc electrode...
.
Half reactions for a Daniell cell are these:
- Zinc electrode (anode): Zn(s) → Zn2+(aq) + 2 e–
- Copper electrode (cathode): Cu2+(aq) + 2 e– → Cu(s)

To provide a complete electric circuit, there must also be an ionic conduction path between the anode and cathode electrolytes in addition to the electron conduction path. The simplest ionic conduction path is to provide a liquid junction. To avoid mixing between the two electrolytes, the liquid junction can be provided through a porous plug that allows ion flow while reducing electrolyte mixing. To further minimize mixing of the electrolytes, a salt bridge
Salt bridge
A salt bridge, in chemistry, is a laboratory device used to connect the oxidation and reduction half-cells of a galvanic cell , a type of electrochemical cell...
can be used which consists of an electrolyte saturated gel in an inverted U-tube. As the negatively charged electrons flow in one direction around this circuit, the positively charged metal ions flow in the opposite direction in the electrolyte.
A voltmeter
Galvanometer
A galvanometer is a type of ammeter: an instrument for detecting and measuring electric current. It is an analog electromechanical transducer that produces a rotary deflection of some type of pointer in response to electric current flowing through its coil in a magnetic field. .Galvanometers were...
is capable of measuring the change of electrical potential
Electric potential
In classical electromagnetism, the electric potential at a point within a defined space is equal to the electric potential energy at that location divided by the charge there...
between the anode and the cathode.
Electrochemical cell voltage is also referred to as electromotive force
Electromotive force
In physics, electromotive force, emf , or electromotance refers to voltage generated by a battery or by the magnetic force according to Faraday's Law, which states that a time varying magnetic field will induce an electric current.It is important to note that the electromotive "force" is not a...
or emf.
A cell diagram can be used to trace the path of the electrons in the electrochemical cell. For example, here is a cell diagram of a Daniell cell:
- Zn(s) | Zn2+ (1M) || Cu2+ (1M) | Cu(s)
First, the reduced form of the metal to be oxidized at the anode (Zn) is written. This is separated from its oxidized form by a vertical line, which represents the limit between the phases (oxidation changes). The double vertical lines represent the saline bridge on the cell. Finally, the oxidized form of the metal to be reduced at the cathode, is written, separated from its reduced form by the vertical line. The electrolyte concentration is given as it is an important variable in determining the cell potential.
Standard electrode potential
To allow prediction of the cell potential, tabulations of standard electrode potentialStandard electrode potential
In electrochemistry, the standard electrode potential, abbreviated E° or E , is the measure of individual potential of a reversible electrode at standard state, which is with solutes at an effective concentration of 1 mol dm−3, and gases at a pressure of 1 atm...
are available. Such tabulations are referenced to the standard hydrogen electrode (SHE). The standard hydrogen electrode
Standard hydrogen electrode
The standard hydrogen electrode , is a redox electrode which forms the basis of the thermodynamic scale of oxidation-reduction potentials...
undergoes the reaction
- 2 H+(aq) + 2 e– → H2
which is shown as reduction but, in fact, the SHE can act as either the anode or the cathode, depending on the relative oxidation/reduction potential of the other electrode/electrolyte combination. The term standard in SHE requires a supply of hydrogen gas bubbled through the electrolyte at a pressure of 1 atm and an acidic electrolyte with H+ activity equal to 1 (usually assumed to be [H+] = 1 mol/liter).
The SHE electrode can be connected to any other electrode by a salt bridge to form a cell. If the second electrode is also at standard conditions, then the measured cell potential is called the standard electrode potential for the electrode. The standard electrode potential for the SHE is zero, by definition. The polarity of the standard electrode potential provides information about the relative reduction potential of the electrode compared to the SHE. If the electrode has a positive potential with respect to the SHE, then that means it is a strongly reducing electrode which forces the SHE to be the anode (an example is Cu in aqueous CuSO4 with a standard electrode potential of 0.337 V). Conversely, if the measured potential is negative, the electrode is more oxidizing than the SHE (such as Zn in ZnSO4 where the standard electrode potential is −0.76 V).
Standard electrode potentials are usually tabulated as reduction potentials. However, the reactions are reversible and the role of a particular electrode in a cell depends on the relative oxidation/reduction potential of both electrodes. The oxidation potential for a particular electrode is just the negative of the reduction potential. A standard cell potential can be determined by looking up the standard electrode potentials for both electrodes (sometimes called half cell potentials). The one that is smaller will be the anode and will undergo oxidation. The cell potential is then calculated as the sum of the reduction potential for the cathode and the oxidation potential for the anode.
- E°cell = E°red(cathode) – E°red(anode) = E°red(cathode) + E°oxi(anode)
For example, the standard electrode potential for a copper electrode is:
- Cell diagram
- Pt(s) | H2(1 atm) | H+(1 M) || Cu2+ (1 M) | Cu(s)
- E°cell = E°red(cathode) – E°red(anode)
At standard temperature, pressure and concentration conditions, the cell's emf
Electromotive force
In physics, electromotive force, emf , or electromotance refers to voltage generated by a battery or by the magnetic force according to Faraday's Law, which states that a time varying magnetic field will induce an electric current.It is important to note that the electromotive "force" is not a...
(measured by a multimeter
Multimeter
A multimeter or a multitester, also known as a VOM , is an electronic measuring instrument that combines several measurement functions in one unit. A typical multimeter may include features such as the ability to measure voltage, current and resistance...
) is 0.34 V. By definition, the electrode potential for the SHE is zero. Thus, the Cu is the cathode and the SHE is the anode giving
- Ecell = E°(Cu2+/Cu) – E°(H+/H2)
Or,
- E°(Cu2+/Cu) = 0.34 V
Changes in the stoichiometric coefficients of a balanced cell equation will not change E°red value because the standard electrode potential is an intensive property
Intensive and extensive properties
In the physical sciences, an intensive property , is a physical property of a system that does not depend on the system size or the amount of material in the system: it is scale invariant.By contrast, an extensive property In the physical sciences, an intensive property (also called a bulk...
.
Spontaneity of redox reaction
During operation of electrochemical cellElectrochemical cell
An electrochemical cell is a device capable of either deriving electrical energy from chemical reactions, or facilitating chemical reactions through the introduction of electrical energy. A common example of an electrochemical cell is a standard 1.5-volt "battery"...
s, chemical energy
Chemical energy
Chemical energy is the potential of a chemical substance to undergo a transformation through a chemical reaction or, to transform other chemical substances...
is transformed into electrical energy and is expressed mathematically as the product of the cell's emf and the electric charge
Electric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
transferred through the external circuit.
- Electrical energy = EcellCtrans
where Ecell is the cell potential measured in volts (V) and Ctrans is the cell current integrated over time and measured in coulombs (C); Ctrans can also be determined by multiplying the total number of electrons transferred (measured in moles) times Faraday's constant (F).
The emf of the cell at zero current is the maximum possible emf. It is used to calculate the maximum possible electrical energy that could be obtained from a chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
. This energy is referred to as electrical work and is expressed by the following equation:
- Wmax = Welectrical = –nF·Ecell,
where work is defined as positive into the system.
Since the free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
is the maximum amount of work that can be extracted from a system, one can write:
- ΔG = –nF·Ecell
A positive cell potential gives a negative change in Gibbs free energy. This is consistent with the cell production of an electric current
Electric current
Electric current is a flow of electric charge through a medium.This charge is typically carried by moving electrons in a conductor such as wire...
from the cathode to the anode through the external circuit. If the current is driven in the opposite direction by imposing an external potential, then work is done on the cell to drive electrolysis.
A spontaneous
Spontaneous process
A spontaneous process is the time-evolution of a system in which it releases free energy and moves to a lower, more thermodynamically stable energy state...
electrochemical reaction (change in Gibbs free energy less than zero) can be used to generate an electric current in electrochemical cells. This is the basis of all batteries and fuel cell
Fuel cell
A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used...
s. For example, gaseous oxygen (O2) and
hydrogen (H2) can be combined in a fuel cell to form water and energy, typically a combination of heat and electrical energy.
Conversely, non-spontaneous electrochemical reactions can be driven forward by the application of a current at sufficient voltage
Voltage
Voltage, otherwise known as electrical potential difference or electric tension is the difference in electric potential between two points — or the difference in electric potential energy per unit charge between two points...
. The electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
of water into gaseous oxygen and hydrogen is a typical example.
The relation between the equilibrium constant, K, and the Gibbs free energy for an electrochemical cell is expressed as follows:
- ΔG° = –RT ln(K) = –nF·E°cell
Rearranging to express the relation between standard potential and equilibrium constant yields
 .
.The previous equation can use Briggsian logarithm as shown below:

Nernst equation
The standard potential of an electrochemical cell requires standard conditions for all of the reactants. When reactant concentrations differ from standard conditions, the cell potential will deviate from the standard potential. In the 20th century German chemistChemist
A chemist is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties such as density and acidity. Chemists carefully describe the properties they study in terms of quantities, with detail on the level of molecules and their component atoms...
Walther Nernst
Walther Nernst
Walther Hermann Nernst FRS was a German physical chemist and physicist who is known for his theories behind the calculation of chemical affinity as embodied in the third law of thermodynamics, for which he won the 1920 Nobel Prize in chemistry...
proposed a mathematical model to determine the effect of reactant concentration on electrochemical cell potential.
In the late 19th century, Josiah Willard Gibbs
Josiah Willard Gibbs
Josiah Willard Gibbs was an American theoretical physicist, chemist, and mathematician. He devised much of the theoretical foundation for chemical thermodynamics as well as physical chemistry. As a mathematician, he invented vector analysis . Yale University awarded Gibbs the first American Ph.D...
had formulated a theory to predict whether a chemical reaction is spontaneous based on the free energy
- ΔG = ΔG° + RT·ln(Q)
Here ΔG is change in Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
, T is absolute temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
, R is the gas constant
Gas constant
The gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
and Q is reaction quotient
Reaction quotient
In chemistry, a reaction quotient: Qr is a function of the activities or concentrations of the chemical species involved in a chemical reaction. In the special case that the reaction is at equilibrium the reaction quotient is equal to the equilibrium constant....
.
Gibbs' key contribution was to formalize the understanding of the effect of reactant concentration on spontaneity.
Based on Gibbs' work, Nernst extended the theory to include the contribution from electric potential on charged species. As shown in the previous section, the change in Gibbs free energy for an electrochemical cell can be related to the cell potential. Thus, Gibbs' theory becomes
- nFΔE = nFΔE° – RT ln(Q)
Here n is the number of electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s/mole
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
product, F is the Faraday constant (coulombs/mole
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
), and ΔE is cell potential.
Finally, Nernst divided through by the amount of charge transferred to arrive at a new equation which now bears his name:
- ΔE = ΔE° – (RT/nF)ln(Q)
Assuming standard conditions (T = 25 °C) and R = 8.3145 J/(K·mol), the equation above can be expressed on base—10 logarithm
Common logarithm
The common logarithm is the logarithm with base 10. It is also known as the decadic logarithm, named after its base. It is indicated by log10, or sometimes Log with a capital L...
as shown below:

Concentration cells
A concentration cell is an electrochemical cell where the two electrodes are the same material, the electrolytes on the two half-cells involve the same ions, but the electrolyte concentration differs between the two half-cells.For example an electrochemical cell, where two copper electrodes are submerged in two copper(II) sulfate solutions, whose concentrations are 0.05 M and 2.0 M, connected through a salt bridge. This type of cell will generate a potential that can be predicted by the Nernst equation. Both electrodes undergo the same chemistry (although the reaction proceeds in reverse at the cathode)
- Cu2+(aq) + 2 e– → Cu(s)
Le Chatelier's principle
Le Châtelier's principle
In chemistry, Le Chatelier's principle, also called the Chatelier's principle, can be used to predict the effect of a change in conditions on a chemical equilibrium. The principle is named after Henry Louis Le Chatelier and sometimes Karl Ferdinand Braun who discovered it independently...
indicates that the reaction is more favorable to reduction as the concentration of Cu2+ ions increases. Reduction will take place in the cell's compartment where concentration is higher and oxidation will occur on the more dilute side.
The following cell diagram describes the cell mentioned above:
- Cu(s) | Cu2+ (0.05 M) || Cu2+ (2.0 M) | Cu(s)
Where the half cell reactions for oxidation and reduction are:
- Oxidation: Cu(s) → Cu2+ (0.05 M) + 2 e–
- Reduction: Cu2+ (2.0 M) + 2 e– → Cu(s)
- Overall reaction: Cu2+ (2.0 M) → Cu2+ (0.05 M)
The cell's emf is calculated through Nernst equation as follows:

The value of E° in this kind of cell is zero, as electrodes and ions are the same in both half-cells.
After replacing values from the case mentioned, it is possible to calculate cell's potential:

or by:

However, this value is only approximate, as reaction quotient is defined in terms of ion activities which can be approximated with the concentrations as calculated here.
The Nernst equation plays an important role in understanding electrical effects in cells and organelles. Such effects include nerve synapses and cardiac beat
Cardiac cycle
The cardiac cycle is a term referring to all or any of the events related to the flow or blood pressure that occurs from the beginning of one heartbeat to the beginning of the next. The frequency of the cardiac cycle is described by the heart rate. Each beat of the heart involves five major stages...
as well as the resting potential of a somatic cell.
Battery
A battery is a number of cells combined and is used to supply electrical energy that is stored chemically. The term battery is often, and incorrectly, used to describe a single cell. In a battery, cells are usually wired in series to increase the supply voltage but sometimes wired in parallel to allow greater current to be supplied. Batteries are optimized to produce a constant electric current for as long as possible. Although the cells discussed previously are useful for theoretical purposes and some laboratory experiments, the large internal resistance of the salt bridge make them inappropriate battery technologies. Various alternative battery technologies have been commercialized as discussed next.Dry cell

Fluid
In physics, a fluid is a substance that continually deforms under an applied shear stress. Fluids are a subset of the phases of matter and include liquids, gases, plasmas and, to some extent, plastic solids....
electrolyte. Instead, they use a moist electrolyte paste. Leclanché's cell
Zinc-carbon battery
A zinc–carbon dry cell or battery is packaged in a zinc can that serves as both a container and negative terminal. It was developed from the wet Leclanché cell . The positive terminal is a carbon rod surrounded by a mixture of manganese dioxide and carbon powder. The electrolyte used is a paste of...
is a good example of this, where the anode is a zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
container surrounded by a thin layer of manganese dioxide and a moist electrolyte paste of ammonium chloride
Ammonium chloride
Ammonium chloride NH4Cl is an inorganic compound with the formula NH4Cl. It is a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. Sal ammoniac is a name of natural, mineralogical form of ammonium chloride...
and zinc chloride
Zinc chloride
Zinc chloride is the name of chemical compound with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. ZnCl2 itself is hygroscopic and even deliquescent. Samples should therefore be protected from...
mixed with starch
Starch
Starch or amylum is a carbohydrate consisting of a large number of glucose units joined together by glycosidic bonds. This polysaccharide is produced by all green plants as an energy store...
. The cell's cathode is represented by a carbon bar inserted on the cell's electrolyte, usually placed in the middle.
Leclanché's
Georges Leclanché
Georges Leclanché was a French electrical engineer chiefly remembered for his invention of the Leclanché cell, one of the first modern electrical batteries and the forerunner of the modern dry cell battery.-Biography:...
simplified half reactions are shown below:
- Anode: Zn(s) → Zn2+(aq) + 2 e–
- Cathode: 2 NH4+(aq) + 2 MnO2(s) + 2 e– → Mn2O3(s) + 2 NH3(aq) + H2O(l)
- Overall reaction: Zn(s) + 2 NH4+(aq) + 2 MnO2(s) → Zn2+(aq) + Mn2O3(s) + 2 NH3(aq) + H2O(l)
The voltage obtained from the zinc-carbon battery
Zinc-carbon battery
A zinc–carbon dry cell or battery is packaged in a zinc can that serves as both a container and negative terminal. It was developed from the wet Leclanché cell . The positive terminal is a carbon rod surrounded by a mixture of manganese dioxide and carbon powder. The electrolyte used is a paste of...
is around 1.5 V
Volt
The volt is the SI derived unit for electric potential, electric potential difference, and electromotive force. The volt is named in honor of the Italian physicist Alessandro Volta , who invented the voltaic pile, possibly the first chemical battery.- Definition :A single volt is defined as the...
.
Mercury battery
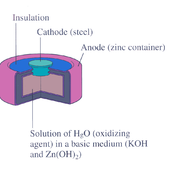
Medicine
Medicine is the science and art of healing. It encompasses a variety of health care practices evolved to maintain and restore health by the prevention and treatment of illness....
and electronics
Electronics
Electronics is the branch of science, engineering and technology that deals with electrical circuits involving active electrical components such as vacuum tubes, transistors, diodes and integrated circuits, and associated passive interconnection technologies...
. The battery consists of a steel
Steel
Steel is an alloy that consists mostly of iron and has a carbon content between 0.2% and 2.1% by weight, depending on the grade. Carbon is the most common alloying material for iron, but various other alloying elements are used, such as manganese, chromium, vanadium, and tungsten...
—made container in the shape of a cylinder acting as the cathode, where an amalgamated anode of mercury and zinc is surrounded by a stronger alkaline electrolyte and a paste of zinc oxide
Zinc oxide
Zinc oxide is an inorganic compound with the formula ZnO. It is a white powder that is insoluble in water. The powder is widely used as an additive into numerous materials and products including plastics, ceramics, glass, cement, rubber , lubricants, paints, ointments, adhesives, sealants,...
and mercury(II) oxide
Mercury(II) oxide
Mercury oxide, also called mercuric oxide or simply mercury oxide, has a formula of HgO. It has a red or orange color. Mercury oxide is a solid at room temperature and pressure...
.
Mercury battery half reactions are shown below:
- Anode: Zn(Hg) + 2 OH–(aq) → ZnO(s) + H2O(l) + 2 e–
- Cathode: HgO(s) + H2O(l) + 2 e– → Hg(l) + 2 OH–(aq)
- Overall reaction: Zn(Hg) + HgO(s) → ZnO(s) + Hg(l)
There are no changes in the electrolyte's composition when the cell works. Such batteries provide 1.35 V of direct current
Direct current
Direct current is the unidirectional flow of electric charge. Direct current is produced by such sources as batteries, thermocouples, solar cells, and commutator-type electric machines of the dynamo type. Direct current may flow in a conductor such as a wire, but can also flow through...
.
Lead–acid battery

Automobile
An automobile, autocar, motor car or car is a wheeled motor vehicle used for transporting passengers, which also carries its own engine or motor...
s, consists of a series of six identical cells assembled in series. Each cell has a lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
anode and a cathode made from lead dioxide packed in a metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
plaque. Cathode and anode are submerged in a solution of sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
acting as the electrolyte.
Lead–acid battery half cell reactions are shown below:
- Anode: Pb(s) + SO42–(aq) → PbSO4(s) + 2 e–
- Cathode: PbO2(s) + 4 H+(aq) + SO42–(aq) + 2 e– → PbSO4(s) + 2 H2O(l)
- Overall reaction: Pb(s) + PbO2(s) + 4 H+(aq) + 2 SO42–(aq) → 2 PbSO4(s) + 2 H2O(l)
At standard conditions, each cell may produce a potential of 2 V
Volt
The volt is the SI derived unit for electric potential, electric potential difference, and electromotive force. The volt is named in honor of the Italian physicist Alessandro Volta , who invented the voltaic pile, possibly the first chemical battery.- Definition :A single volt is defined as the...
, hence overall voltage produced is 12 V. Differing from mercury and zinc-carbon batteries, lead-acid batteries are rechargeable
Rechargeable battery
A rechargeable battery or storage battery is a group of one or more electrochemical cells. They are known as secondary cells because their electrochemical reactions are electrically reversible. Rechargeable batteries come in many different shapes and sizes, ranging anything from a button cell to...
. If an external voltage is supplied to the battery it will produce an electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
of the products in the overall reaction (discharge), thus recovering initial components which made the battery work.
Lithium rechargeable battery
Instead of an aqueous electrolyte or a moist electrolyte paste, a solid state battery operates using a solid electrolyte. LithiumLithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
polymer batteries are an example of this; a graphite bar acts as the anode, a bar of lithium cobaltate acts as the cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
, and a polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
, swollen with a lithium salt, allows the passage of ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s and serves as the electrolyte. In this cell, the carbon in the anode can reversibly form a lithium-carbon alloy. Upon discharging, lithium ions spontaneously leave the lithium cobaltate cathode and travel through the polymer and into the carbon anode forming the alloy. This flow of positive lithium ions is the electrical current that the battery provides. By charging the cell, the lithium dealloys and travels back into the cathode. The advantage of this kind of battery is that Lithium possess the highest negative value of standard reduction potential. It is also a light metal
Light metal
Light metals are metals of low atomic weight. The cut off between light metals and heavy metals varies. Lithium, beryllium, sodium, magnesium and aluminium are almost always included. Additional metals up to nickel are often included as well. Metals heavier than nickel are usually called heavy...
and therefore less mass is required to generate 1 mole of
electrons. Lithium ion battery technologies are widely used in portable electronic devices because they have high energy storage density and are rechargeable. These technologies show promise for future automotive applications, with new materials such as iron phosphates and lithium vanadates.
Flow battery
Most batteries have all of the electrolyte and electrodes within a single housing. A flow battery is unusual in that the majority of the electrolyte, including dissolved reactive species, is stored in separate tanks. The electrolytes are pumped through a reactor, which houses the electrodes, when the battery is charged or discharged. Because the electrodes are preserved, and the electrolyte combusted, a "flow battery" is better described as a reversible fuel cell.These types of batteries are typically used for large-scale energy storage (kWh – multi MWh). Of the several different types that have been developed, some are of current commercial interest, including the iron/chromium flow battery, vanadium redox battery
Vanadium redox battery
The vanadium redox battery is a type of rechargeable flow battery that employs vanadium ions in different oxidation states to store chemical potential energy...
and zinc-bromine flow battery
Zinc-bromine flow battery
The zinc–bromine flow battery is a type of hybrid flow battery. A solution of zinc bromide is stored in two tanks. When the battery is charged or discharged the solutions are pumped through a reactor stack and back into the tanks. One tank is used to store the electrolyte for the positive...
.
Fuel cells
Fossil fuelFossil fuel
Fossil fuels are fuels formed by natural processes such as anaerobic decomposition of buried dead organisms. The age of the organisms and their resulting fossil fuels is typically millions of years, and sometimes exceeds 650 million years...
s are used in power plants to supply electrical needs, however their conversion into electricity is an inefficient process. The most efficient electrical power plant may only convert about 40%
Percentage
In mathematics, a percentage is a way of expressing a number as a fraction of 100 . It is often denoted using the percent sign, “%”, or the abbreviation “pct”. For example, 45% is equal to 45/100, or 0.45.Percentages are used to express how large/small one quantity is, relative to another quantity...
of the original chemical energy
Chemical energy
Chemical energy is the potential of a chemical substance to undergo a transformation through a chemical reaction or, to transform other chemical substances...
into electricity when burned
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
or processed.
To enhance electrical production, scientists have developed fuel cells where combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
is replaced by electrochemical methods, similar to a battery but requiring continuous replenishment of the reactants consumed.
The most popular is the oxygen-hydrogen fuel cell, where two inert electrodes (porous electrodes of nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
and nickel oxide) are placed in an electrolytic solution such as hot caustic potash, in both compartments (anode and cathode) gaseous hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
are bubbled into solution.
Oxygen-hydrogen fuel cell reactions are shown bellow:
- Anode: 2 H2(g) → 4 H+ + 4 e–
- Cathode: O2(g) + 4 e– + 4 H+ → 2 H2O(l)
- Overall reaction: 2 H2(g) + O2(g) → 2 H2O(l)
The overall reaction is identical to hydrogen combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
. Oxidation and reduction take place in the anode and cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
separately. This is similar to the electrode used in the cell for measuring standard reduction potential which has a double function acting as electrical conductor
Electrical conductor
In physics and electrical engineering, a conductor is a material which contains movable electric charges. In metallic conductors such as copper or aluminum, the movable charged particles are electrons...
s providing a surface required to decomposition of the molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s into atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s before electron transferring, thus named electrocatalyst
Electrocatalyst
An electrocatalyst is a catalyst that participates in electrochemical reactions. Catalyst materials modify and increase the rate of chemical reactions without being consumed in the process. Electrocatalysts are a specific form of catalysts that function at electrode surfaces or may be the...
s. Platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
, nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
, and rhodium
Rhodium
Rhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
are good electrocatalysts.
Corrosion
Corrosion is the term applied to steelSteel
Steel is an alloy that consists mostly of iron and has a carbon content between 0.2% and 2.1% by weight, depending on the grade. Carbon is the most common alloying material for iron, but various other alloying elements are used, such as manganese, chromium, vanadium, and tungsten...
rust
Rust
Rust is a general term for a series of iron oxides. In colloquial usage, the term is applied to red oxides, formed by the reaction of iron and oxygen in the presence of water or air moisture...
caused by an electrochemical process. Most people are likely familiar with the corrosion of iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
, in the form of reddish rust. Other examples include the black tarnish on silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
, and red or green corrosion that may appear on copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
and its alloys, such as brass
Brass
Brass is an alloy of copper and zinc; the proportions of zinc and copper can be varied to create a range of brasses with varying properties.In comparison, bronze is principally an alloy of copper and tin...
. The cost of replacing metals lost to corrosion is in the multi-billions of dollars
United States dollar
The United States dollar , also referred to as the American dollar, is the official currency of the United States of America. It is divided into 100 smaller units called cents or pennies....
per year.
Iron corrosion
For iron rust to occur the metal has to be in contact with oxygenOxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
, although chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s for this process are relatively complex and not all of them are completely understood, it is believed the causes are the following:
Electron transferring (reduction-oxidation)
- One area on the surface of the metal acts as the anode, which is where the oxidation (corrosion) occurs. At the anode, the metal gives up electrons.
- Fe(s) → Fe2+(aq) + 2 e–
- ElectronElectronThe electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s are transferred from ironIronIron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
reducing oxygen in the atmosphereAtmosphereAn atmosphere is a layer of gases that may surround a material body of sufficient mass, and that is held in place by the gravity of the body. An atmosphere may be retained for a longer duration, if the gravity is high and the atmosphere's temperature is low...
into water on the cathode, which is placed in another region of the metal.- O2(g) + 4 H+(aq) + 4 e– → 2 H2O(l)
- Global reaction for the process:
- 2 Fe(s) + O2(g) + 4 H+(aq) → 2 Fe2+(aq) + 2 H2O(l)
- Standard emf for iron rusting:
- E° = E°cathode – E°anode
- E° = 1.23V – (−0.44 V) = 1.67 V
Iron corrosion takes place on acid medium; H+
Hydronium
In chemistry, a hydronium ion is the cation , a type of oxonium ion produced by protonation of water. This cation is often used to represent the nature of the proton in aqueous solution, where the proton is highly solvated...
ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s come from reaction between carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
in the atmosphere and water, forming carbonic acid
Carbonic acid
Carbonic acid is the inorganic compound with the formula H2CO3 . It is also a name sometimes given to solutions of carbon dioxide in water, because such solutions contain small amounts of H2CO3. Carbonic acid forms two kinds of salts, the carbonates and the bicarbonates...
. Fe2+ ions oxides, following this equation:
- 4 Fe2+(aq) + O2(g) + (4+2x)H2O(l) → 2 Fe2O3·xH2O + 8 H+(aq)
Iron(III) oxide
Iron(III) oxide
Iron oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron oxide , which is rare, and iron oxide , which also occurs naturally as the mineral magnetite. As the mineral known as hematite, Fe2O3 is the main...
hydrated is known as rust. The concentration of water associated with iron oxide varies, thus chemical representation is presented as Fe2O3·xH2O.
The electric circuit works as passage of electrons and ions occurs, thus if an electrolyte is present it will facilitate oxidation, this explains why rusting is quicker on salt water
Brine
Brine is water, saturated or nearly saturated with salt .Brine is used to preserve vegetables, fruit, fish, and meat, in a process known as brining . Brine is also commonly used to age Halloumi and Feta cheeses, or for pickling foodstuffs, as a means of preserving them...
.
Corrosion of common metals
Coinage metals, such as copper and silver, slowly corrode through use.A patina
Patina
Patina is a tarnish that forms on the surface of bronze and similar metals ; a sheen on wooden furniture produced by age, wear, and polishing; or any such acquired change of a surface through age and exposure...
of green-blue copper carbonate forms on the surface of copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
with exposure to the water and carbon dioxide in the air. Silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
coins or cutlery
Cutlery
Cutlery refers to any hand implement used in preparing, serving, and especially eating food in the Western world. It is more usually known as silverware or flatware in the United States, where cutlery can have the more specific meaning of knives and other cutting instruments. This is probably the...
that are exposed to high sulfur foods such as egg
Egg (food)
Eggs are laid by females of many different species, including birds, reptiles, amphibians, and fish, and have probably been eaten by mankind for millennia. Bird and reptile eggs consist of a protective eggshell, albumen , and vitellus , contained within various thin membranes...
s or the low levels of sulfur species in the air develop a layer of black Silver sulfide
Silver sulfide
Silver sulfide, Ag2S, is the sulfide of silver. This dense black solid constitutes the tarnish that forms over time on silverware and other silver objects. Silver sulfide is insoluble in all solvents, but is degraded by strong acids. Silver sulfide features a covalent bond, as it is made up of...
.
Gold
Gold
Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a...
and platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
are extremely difficult to oxidize under normal circumstances, and require exposure to a powerful chemical oxidizing agent such as aqua regia
Aqua regia
Aqua regia or aqua regis is a highly corrosive mixture of acids, fuming yellow or red solution, also called nitro-hydrochloric acid. The mixture is formed by freshly mixing concentrated nitric acid and hydrochloric acid, usually in a volume ratio of 1:3, respectively...
.
Some common metals oxidize extremely rapidly in air. Titanium
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color....
and aluminium oxidize instantaneously in contact with the oxygen in the air. These metals form an extremely thin layer of oxidized metal on the surface. This thin layer of oxide protects the underlying layers of the metal from the air preventing the entire metal from oxidizing. These metals are used in applications where corrosion resistance is important. Iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
, in contrast, has an oxide that forms in air and water, called rust
Rust
Rust is a general term for a series of iron oxides. In colloquial usage, the term is applied to red oxides, formed by the reaction of iron and oxygen in the presence of water or air moisture...
, that does not stop the further oxidation of the iron. Thus iron left exposed to air and water will continue to rust until all of the iron is oxided.
Prevention of corrosion
Attempts to save a metal from becoming anodic are of two general types. Anodic regions dissolve and destroy the structural integrity of the metal.While it is almost impossible to prevent anode/cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
formation, if a non-conducting material covers the metal, contact with the electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
is not possible and corrosion will not occur.
Coating
Metals can be coated with paintPaint
Paint is any liquid, liquefiable, or mastic composition which after application to a substrate in a thin layer is converted to an opaque solid film. One may also consider the digital mimicry thereof...
or other less conductive metals (passivation
Passivation
Passivation is the process of making a material "passive", and thus less reactive with surrounding air, water, or other gases or liquids. The goal is to inhibit corrosion, whether for structural or cosmetic reasons. Passivation of metals is usually achieved by the deposition of a layer of oxide...
). This prevents the metal surface from being exposed to electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
s. Scratches exposing the metal substrate will result in corrosion. The region under the coating adjacent to the scratch acts as the anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
of the reaction.
Sacrificial anodes
A method commonly used to protect a structural metal is to attach a metal which is more anodic than the metal to be protected. This forces the structural metal to be cathodic, thus spared corrosion. It is called "sacrificial" because the anode dissolves and has to be replaced periodically.Zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
bars are attached to various locations on steel ship
Ship
Since the end of the age of sail a ship has been any large buoyant marine vessel. Ships are generally distinguished from boats based on size and cargo or passenger capacity. Ships are used on lakes, seas, and rivers for a variety of activities, such as the transport of people or goods, fishing,...
hulls
Hull (watercraft)
A hull is the watertight body of a ship or boat. Above the hull is the superstructure and/or deckhouse, where present. The line where the hull meets the water surface is called the waterline.The structure of the hull varies depending on the vessel type...
to render the ship hull cathodic
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
. The zinc bars are replaced periodically. Other metals, such as magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
, would work very well but zinc is the least expensive useful metal.
To protect pipelines, an ingot of buried or exposed magnesium (or zinc) is buried
Bury
Bury is a town in Greater Manchester, England. It lies on the River Irwell, east of Bolton, west-southwest of Rochdale, and north-northwest of the city of Manchester...
beside the pipeline
Pipe (material)
A pipe is a tubular section or hollow cylinder, usually but not necessarily of circular cross-section, used mainly to convey substances which can flow — liquids and gases , slurries, powders, masses of small solids...
and is connected electrically
Wire
A wire is a single, usually cylindrical, flexible strand or rod of metal. Wires are used to bear mechanical loads and to carry electricity and telecommunications signals. Wire is commonly formed by drawing the metal through a hole in a die or draw plate. Standard sizes are determined by various...
to the pipe above ground. The pipeline is forced to be a cathode and is protected from being oxidized and rusting. The magnesium anode is sacrificed. At intervals new ingot
Ingot
An ingot is a material, usually metal, that is cast into a shape suitable for further processing. Non-metallic and semiconductor materials prepared in bulk form may also be referred to as ingots, particularly when cast by mold based methods.-Uses:...
s are buried to replace those lost.
Electrolysis
The spontaneous redox reactions of a conventional battery produce electricity through the different chemical potentials of the cathode and anode in the electrolyte. However, electrolysis requires an external source of electrical energy to induce a chemical reaction, and this process takes place in a compartment called an electrolytic cellElectrolytic cell
An electrolytic cell decomposes chemical compounds by means of electrical energy, in a process called electrolysis; the Greek word lysis means to break up. The result is that the chemical energy is increased...
.
Electrolysis of molten sodium chloride
When molten, the salt sodium chlorideSodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
can be electrolyzed to yield metallic sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
and gaseous chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
. Industrially this process takes place in a special cell named Down's cell. The cell is connected to an electrical power supply, allowing electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s to migrate from the power supply to the electrolytic cell.
Reactions that take place at Down's cell are the following:
- Anode (oxidation): 2 Cl– → Cl2(g) + 2 e–
- Cathode (reduction): 2 Na+(l) + 2 e– → 2 Na(l)
- Overall reaction: 2 Na+ + 2 Cl–(l) → 2 Na(l) + Cl2(g)
This process can yield large amounts of metallic sodium and gaseous chlorine, and is widely used on mineral dressing and metallurgy
Metallurgy
Metallurgy is a domain of materials science that studies the physical and chemical behavior of metallic elements, their intermetallic compounds, and their mixtures, which are called alloys. It is also the technology of metals: the way in which science is applied to their practical use...
industries
Industry
Industry refers to the production of an economic good or service within an economy.-Industrial sectors:There are four key industrial economic sectors: the primary sector, largely raw material extraction industries such as mining and farming; the secondary sector, involving refining, construction,...
.
The emf
Electromotive force
In physics, electromotive force, emf , or electromotance refers to voltage generated by a battery or by the magnetic force according to Faraday's Law, which states that a time varying magnetic field will induce an electric current.It is important to note that the electromotive "force" is not a...
for this process is approximately −4 V
Volt
The volt is the SI derived unit for electric potential, electric potential difference, and electromotive force. The volt is named in honor of the Italian physicist Alessandro Volta , who invented the voltaic pile, possibly the first chemical battery.- Definition :A single volt is defined as the...
indicating a (very) non-spontaneous process. In order for this reaction to occur the power supply should provide at least a potential of 4 V. However, larger voltages must be used for this reaction to occur at a high rate.
Electrolysis of water
Water can be converted to its component elemental gasses, H2 and O2 through the application of an external voltage. WaterWater
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
doesn't decompose into hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
spontaneously
Spontaneous process
A spontaneous process is the time-evolution of a system in which it releases free energy and moves to a lower, more thermodynamically stable energy state...
as the Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
for the process at standard conditions is about 474.4 kJ. The decomposition of water into hydrogen and oxygen can be performed in an electrolytic cell. In it, a pair of inert electrode
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
s usually made of platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
immersed in water act as anode and cathode in the electrolytic process. The electrolysis starts with the application of an external voltage between the electrodes. This process will not occur except at extremely high voltages without an electrolyte such as sodium chloride
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
or sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
(most used 0.1 M).
Bubbles from the gases will be seen near both electrodes. The following half reactions describe the process mentioned above:
- Anode (oxidation): 2 H2O(l) → O2(g) + 4 H+(aq) + 4 e–
- Cathode (reduction): 2 H2O(g) + 2 e– → H2(g) + 2 OH–(aq)
- Overall reaction: 2 H2O(l) → 2 H2(g) + O2(g)
Although strong acids may be used in the apparatus, the reaction will not net consume the acid. While this reaction will work at any conductive electrode at a sufficiently large potential, platinum catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
both hydrogen and oxygen formation, allowing for relatively mild voltages (~2 V depending on the pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
).
Electrolysis of aqueous solutions
Electrolysis in an aqueous is a similar process as mentioned in electrolysis of water. However, it is considered to be a complex process because the contents in solution have to be analyzed in half reactionsChemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
, whether reduced or oxidized.
Electrolysis of a solution of sodium chloride
The presence of water in a solution of sodium chlorideSodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
must be examined in respect to its reduction and oxidation in both electrodes. Usually, water is electrolysed as mentioned in electrolysis of water yielding gaseous oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
in the anode and gaseous hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
in the cathode. On the other hand, sodium chloride in water dissociates
Dissociation (chemistry)
Dissociation in chemistry and biochemistry is a general process in which ionic compounds separate or split into smaller particles, ions, or radicals, usually in a reversible manner...
in Na+ and Cl– ions, cation, which is the positive ion, will be attracted to the cathode (+), thus reducing the sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
ion. The anion will then be attracted to the anode (–) oxidizing chloride
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
ion.
The following half reactions describes the process mentioned:
- 1. Cathode: Na+(aq) + e– → Na(s) E°red = –2.71 V
- 2. Anode: 2 Cl–(aq) → Cl2(g) + 2 e– E°red = +1.36 V
- 3. Cathode: 2 H2O(l) + 2 e– → H2(g) + 2 OH–(aq) E°red = –0.83 V
- 4. Anode: 2 H2O(l) → O2(g) + 4 H+(aq) + 4 e– E°red = +1.23 V
Reaction 1 is discarded as it has the most negative value on standard reduction potential thus making it less thermodynamically favorable in the process.
When comparing the reduction potentials in reactions 2 and 4, the reduction of chloride ion is favored. Thus, if the Cl– ion is favored for reduction
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
, then the water reaction is favored for oxidation producing gaseous oxygen, however experiments show gaseous chlorine is produced and not oxygen.
Although the initial analysis is correct, there is another effect that can happen, known as the overvoltage effect
Overvoltage
When the voltage in a circuit or part of it is raised above its upper design limit, this is known as overvoltage. The conditions may be hazardous...
. Additional voltage is sometimes required, beyond the voltage predicted by the E°cell. This may be due to kinetic
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
rather than thermodynamic
Thermochemistry
Thermochemistry is the study of the energy and heat associated with chemical reactions and/or physical transformations. A reaction may release or absorb energy, and a phase change may do the same, such as in melting and boiling. Thermochemistry focuses on these energy changes, particularly on the...
considerations. In fact, it has been proven that the activation energy
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
for the chloride ion is very low, hence favorable in kinetic terms
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
. In other words, although the voltage applied is thermodynamically sufficient to drive electrolysis, the rate is so slow that to make the process proceed in a reasonable time frame, the voltage
Voltage
Voltage, otherwise known as electrical potential difference or electric tension is the difference in electric potential between two points — or the difference in electric potential energy per unit charge between two points...
of the external source has to be increased (hence, overvoltage).
Finally, reaction 3 is favorable because it describes the proliferation of OH–
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
ions thus letting a probable reduction of H+
Hydronium
In chemistry, a hydronium ion is the cation , a type of oxonium ion produced by protonation of water. This cation is often used to represent the nature of the proton in aqueous solution, where the proton is highly solvated...
ions less favorable an option.
The overall reaction for the process according to the analysis would be the following:
- Anode (oxidation): 2 Cl–(aq) → Cl2(g) + 2 e–
- Cathode (reduction): 2 H2O(l) + 2 e– → H2(g) + 2 OH–(aq)
- Overall reaction: 2 H2O + 2 Cl–(aq) → H2(g) + Cl2(g) + 2 OH–(aq)
As the overall reaction indicates, the concentration
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
of chloride ions is reduced in comparison to OH– ions (whose concentration increases). The reaction also shows the production of gaseous hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
and aqueous sodium hydroxide.
Quantitative electrolysis and Faraday's Laws
Quantitative aspects of electrolysis were originally developed by Michael FaradayMichael Faraday
Michael Faraday, FRS was an English chemist and physicist who contributed to the fields of electromagnetism and electrochemistry....
in 1834. Faraday is also credited to have coined the terms electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
, electrolysis, among many others while he studied quantitative analysis of electrochemical reactions. Also he was an advocate of the law of conservation of energy.
First law
Faraday concluded after several experiments on electrical current in non-spontaneous processSpontaneous process
A spontaneous process is the time-evolution of a system in which it releases free energy and moves to a lower, more thermodynamically stable energy state...
, the mass
Mass
Mass can be defined as a quantitive measure of the resistance an object has to change in its velocity.In physics, mass commonly refers to any of the following three properties of matter, which have been shown experimentally to be equivalent:...
of the products yielded on the electrodes was proportional to the value of current supplied to the cell, the length of time the current existed, and the molar mass of the substance analyzed. In other words, the amount of a substance deposited on each electrode of an electrolytic cell is directly proportional to the quantity of electricity
Electric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
passed through the cell.
Below is a simplified equation of Faraday's first law:

Where
- m is the mass of the substance produced at the electrode (in gramGramThe gram is a metric system unit of mass....
s), - Q is the total electric charge that passed through the solution (in coulombs),
- n is the valence number of the substance as an ion in solution (electrons per ion),
- M is the molar mass of the substance (in grams per moleMole (unit)The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
).
Second law
Faraday devised the laws of chemical electrodeposition of metals from solutions in 1857. He formulated the second law of electrolysis stating "the amounts of bodies which are equivalent to each other in their ordinary chemical action have equal quantities of electricity naturally associated with them." In other terms, the quantities of different elements deposited by a given amount of electricity are in the ratioRatio
In mathematics, a ratio is a relationship between two numbers of the same kind , usually expressed as "a to b" or a:b, sometimes expressed arithmetically as a dimensionless quotient of the two which explicitly indicates how many times the first number contains the second In mathematics, a ratio is...
of their chemical equivalent weight
Equivalent weight
Equivalent weight is a term which has been used in several contexts in chemistry. In its most general usage, it is the mass of one equivalent, that is the mass of a given substance which will:...
s.
An important aspect of the second law of electrolysis is electroplating
Electroplating
Electroplating is a plating process in which metal ions in a solution are moved by an electric field to coat an electrode. The process uses electrical current to reduce cations of a desired material from a solution and coat a conductive object with a thin layer of the material, such as a metal...
which together with the first law of electrolysis, has a significant number of applications in the industry, as when used to protect metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
s to avoid corrosion
Corrosion
Corrosion is the disintegration of an engineered material into its constituent atoms due to chemical reactions with its surroundings. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen...
.
Applications
There are various extremely important electrochemical processes in both nature and industry, like the coating of objects with metals or metal oxides through electrodeposition and the detection of alcohol in drunken drivers through the redox reaction of ethanol. The generation of chemical energy through photosynthesisPhotosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
is inherently an electrochemical process, as is production of metals like aluminum and titanium from their ores. Certain diabetes blood sugar meters measure the amount of glucose in the blood through its redox potential.
The nervous impulses in neurons are based on electric current generated by the movement of sodium and potassium ions into and out of cells, and certain animals like eels can generate a powerful voltage from certain cells that can disable much larger animals.
See also
- Reactivity seriesReactivity seriesIn introductory chemistry, the reactivity series or activity series is an empirical series of metals, in order of "reactivity" from highest to lowest...
- BioelectromagnetismBioelectromagnetismBioelectromagnetism refers to the electrical, magnetic or electromagnetic fields produced by living cells, tissues or organisms. Examples include the cell membrane potential and the electric currents that flow in nerves and muscles, as a result of action potentials...
- BioelectrochemistryBioelectrochemistryBioelectrochemistry is a branch of electrochemistry concerned with topics like cell electron-proton transport, cell membrane potentials and electrode reactions of redox enzymes.-History:...
- Contact tension – a historical forerunner to the theory of electrochemistry.
- Electrochemical impedance spectroscopy
- Electroanalytical methodElectroanalytical methodElectroanalytical methods are a class of techniques in analytical chemistry which study an analyte by measuring the potential and/or current in an electrochemical cell containing the analyte. These methods can be broken down into several categories depending on which aspects of the cell are...
- Electrochemical potential
- ElectrochemiluminescenceElectrochemiluminescenceElectrochemiluminescence or electrogenerated chemiluminescence is a kind of luminescence produced during electrochemical reactions in solutions. In electrogenerated chemiluminescence, electrochemically generated intermediates undergo a highly exergonic reaction to produce an electronically excited...
- ElectroplatingElectroplatingElectroplating is a plating process in which metal ions in a solution are moved by an electric field to coat an electrode. The process uses electrical current to reduce cations of a desired material from a solution and coat a conductive object with a thin layer of the material, such as a metal...
- Electrochemical engineeringElectrochemical engineeringElectrochemical engineering is the branch of engineering dealing with the technological applications of electrochemical phenomena...
- Electrochemical energy conversionElectrochemical energy conversionElectrochemical energy conversion is a field of energy technology concerned with electrochemical methods of energy conversion and storage like batteries, fuel cells, supercapacitors and photoelectrochemical energy conversion devices...
- Frost diagramFrost diagramA Frost diagram is used in electrochemistry to illustrate the relative stability of a number of different oxidation states of a particular substance...
- Important publications in electrochemistry
- MagnetoelectrochemistryMagnetoelectrochemistryMagnetoelectrochemistry is a branch of electrochemistry dealing with magnetic effects in electrochemistry.-History:These effects have been supposed to exist since the time of Michael Faraday....
- NanoelectrochemistryNanoelectrochemistryNanoelectrochemistry is a branch of electrochemistry that investigates the electrical and electrochemical properties of materials at the nanometer size regime. Nanoelectrochemistry plays significant role in the fabrication of various sensors, and devices for detecting molecules at very low...
- PhotoelectrochemistryPhotoelectrochemistryPhotoelectrochemistry is a subfield of study within physical chemistry concerned with the interaction of light with electrochemical systems. It is active domain of investigation. One of the pioneers of this field of electrochemistry was the german electrochemist Heinz Gerischer...
- Pourbaix diagramPourbaix diagramIn chemistry, a Pourbaix diagram, also known as a potential/pH diagram, maps out possible stable phases of an aqueous electrochemical system. Predominant ion boundaries are represented by lines. As such a Pourbaix diagram can be read much like a standard phase diagram with a different set of axes...
- Redox titrationRedox titrationRedox titration is a type of titration based on a redox reaction between the analyte and titrant.Redox titration may involve the use of a redox indicator and/or a potentiometer.-Example:...
- Standard electrode potential (data page)Standard electrode potential (data page)The values of standard electrode potentials are given in the table below in volts relative to the standard hydrogen electrode and are assembled from referencesThe values are for the following conditions:* the temperature of 298.15 K ;...
- VoltammetryVoltammetryVoltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes. In voltammetry, information about an analyte is obtained by measuring the current as the potential is varied.- Three electrode system :...
- ITIESITIESITIES is an acronym used in electrochemistry for the "Interface between Two Immiscible Electrolyte Solutions". Usually, one electrolyte is an aqueous electrolyte composed of hydrophilic ions such as NaCl dissolved in water and the other electrolyte is a lipophilic salt such as tetrabutylammonium...
External links
- All about electrochemistry (online Reference Text for General Chemistry)
- The Electrochemical Society
- Electrochemical Science and Technology Information Resource (ESTIR)
- International Society of Electrochemistry (ISE)

