
Copper
Encyclopedia
Copper is a chemical element
with the symbol Cu (from ) and atomic number
29. It is a ductile
metal with very high thermal
and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish. It is used as a conductor of heat and electricity, a building material, and a constituent of various metal alloy
s.
The metal and its alloys have been used for thousands of years. In the Roman era, copper was principally mined on Cyprus
, hence the origin of the name of the metal as сyprium (metal of Cyprus), later shortened to сuprum. Its compounds are commonly encountered as copper(II) salts, which often impart blue or green colors to minerals such as turquoise
and have been widely used historically as pigments. Architectural structures built with copper corrode to give green verdigris
(or patina
). Decorative art
prominently features copper, both by itself and as part of pigments.
Copper(II) ions are water-soluble, where they function at low concentration as bacteriostatic substances
, fungicide
s, and wood preservatives. In sufficient amounts, they are poisonous to higher organisms; at lower concentrations it is an essential trace nutrient
to all higher plant and animal life. The main areas where copper is found in animals are tissues, liver, muscle and bone.
of the periodic table, and they share certain attributes: they have one s-orbital electron on top of a filled d-electron shell
and are characterized by high ductility and electrical conductivity. The filled d-shells in these elements do not contribute much to the interatomic interactions, which are dominated by the s-electrons through metallic bond
s. Contrary to metals with incomplete d-shells, metallic bonds in copper are lacking a covalent
character and are relatively weak. This explains the low hardness and high ductility
of single crystals of copper. At the macroscopic scale, introduction of extended defects to the crystal lattice, such as grain boundaries, hinders flow of the material under applied stress thereby increasing its hardness. For this reason, copper is usually supplied in a fine-grained polycrystalline
form, which has greater strength than monocrystalline forms.
The low hardness of copper partly explains its high electrical (59.6×106 S
/m) and thus also high thermal conductivity, which are the second highest among pure metals at room temperature. This is because the resistivity to electron transport in metals at room temperature mostly originates from scattering of electrons on thermal vibrations of the lattice, which are relatively weak for a soft metal. The maximum permissible current density of copper in open air is approximately 3.1×106 A/m2 of cross-sectional area, above which it begins to heat excessively. As with other metals, if copper is placed against another metal, galvanic corrosion will occur.
Together with osmium
(bluish), and gold
(yellow), copper is one of only three elemental metals with a natural color other than gray or silver. Pure copper is orange-red and acquires a reddish tarnish
when exposed to air. The characteristic color of copper results from the electronic transitions between the filled 3d and half-empty 4s atomic shells – the energy difference between these shells is such that it corresponds to orange light. The same mechanism accounts for the yellow color of gold.
s +1 and +2, which are often called cuprous and cupric, respectively. It does not react with water, but it slowly reacts with atmospheric oxygen forming a layer of brown-black copper oxide. In contrast to the oxidation of iron by wet air, this oxide layer stops the further, bulk corrosion. A green layer of verdigris (copper carbonate) can often be seen on old copper constructions, such as the Statue of Liberty
, the largest copper statue in the world build using repoussé and chasing
. Hydrogen sulfide
s and sulfide
s react with copper to form various copper sulfide
s on the surface. In the latter case, the copper corrodes, as is seen when copper is exposed to air containing sulfur compounds. Oxygen-containing ammonia solutions give water-soluble complexes with copper, as do oxygen and hydrochloric acid to form copper chlorides and acidified hydrogen peroxide
to form copper(II) salts. Copper(II) chloride and copper comproportionate to form copper(I) chloride.
s of copper. 63Cu and 65Cu are stable, with 63Cu comprising approximately 69% of naturally occurring copper; they both have a spin
of 3/2. The other isotopes are radioactive, with the most stable being 67Cu with a half-life
of 61.83 hours. Seven metastable isotopes
have been characterized, with 68mCu the longest-lived with a half-life of 3.8 minutes. Isotopes with a mass number
above 64 decay by β-
, whereas those with a mass number below 64 decay by β+
. 64Cu
, which has a half-life of 12.7 hours, decays both ways.
62Cu and 64Cu have significant applications. 64Cu is a radiocontrast
for X-ray imaging, and complexed with a chelate
can be used for treating
cancer. 62Cu is used in 62Cu-PTSM that is a radioactive tracer
for positron emission tomography.
or as part of minerals. Native copper is a polycrystal, with the largest described single crystal measuring 4.4×3.2×3.2 cm. The largest mass of elemental copper weighed 420 tonnes and was found in 1857 on the Keweenaw Peninsula
in Michigan
, US. There are many examples of copper-containing minerals: chalcopyrite
and chalcocite
are copper sulfides, azurite
and malachite
are copper carbonates and cuprite
is a copper oxide. Copper is present in the Earth's crust at a concentration of about 50 parts per million (ppm), and is also synthesized in massive stars.

.png)
 Most copper is mined or extracted as copper sulfides from large open pit mines in porphyry copper deposits that contain 0.4 to 1.0% copper. Examples include Chuquicamata
Most copper is mined or extracted as copper sulfides from large open pit mines in porphyry copper deposits that contain 0.4 to 1.0% copper. Examples include Chuquicamata
in Chile
, Bingham Canyon Mine
in Utah, United States
and El Chino Mine
in New Mexico, United States. According to the British Geological Survey
, in 2005, Chile was the top mine producer of copper with at least one-third world share followed by the United States, Indonesia and Peru. The amount of copper in use is increasing and the quantity available is barely sufficient to allow all countries to reach developed world levels of usage.
has been extracted since 1900. As with many natural resources, the total amount of copper on Earth is vast (around 1014 tons just in the top kilometer of Earth's crust, or about 5 million years worth at the current rate of extraction). However, only a tiny fraction of these reserves is economically viable, given present-day prices and technologies. Various estimates of existing copper reserves available for mining vary from 25 years to 60 years, depending on core assumptions such as the growth rate. Recycling is a major source of copper in the modern world. Because of these and other factors, the future of copper production and supply is the subject of much debate, including the concept of Peak copper
, analogue to Peak Oil
.
The price of copper has historically been unstable, and it quintupled from the 60-year low of US$0.60/lb (US$1.32/kg) in June 1999 to US$3.75 per pound (US$8.27/kg) in May 2006. It dropped to US$2.40/lb (US$5.29/kg) in February 2007, then rebounded to US$3.50/lb (US$7.71/kg) in April 2007. In February 2009, weakening global demand and a steep fall in commodity prices since the previous year's highs left copper prices at US$1.51/lb.
ores to the level of 10–15% copper by froth flotation
or bioleaching
. Heating this material with silica in flash smelting
removes much of the iron as slag. The process exploits the greater ease of converting iron sulfides into its oxides, which in turn react with the silica to form the silicate slag, which floats on top of the heated mass. The resulting copper matte consisting of Cu2S is then roasted
to convert all sulfides into oxides:
The cuprous oxide is converted to blister copper upon heating:
This step exploits the relatively easy reduction of copper oxides to copper metal. Natural gas is blown across the blister to remove most of the remaining oxygen and electrorefining is performed on the resulting material to produce pure copper:
's Metal Stocks in Society report
, the global per capita stock of Copper in use in society is 35–55 kg. Much of this is in more-developed countries (140–300 kg per capita) rather than less-developed countries (30–40 kg per capita).
The process of recycling copper follows roughly the same steps as is used to extract copper, but requires fewer steps. High purity scrap copper is melted in a furnace and then reduced
and cast into billets and ingot
s; lower purity scrap is refined by electroplating in a bath of sulfuric acid.
s. Both cuprous
and cupric oxides
are known. Among the numerous copper sulfide
s, important examples include copper(I) sulfide
and copper(II) sulfide.
The cuprous halides with chlorine
, bromine
, and iodine
are known, as are the cupric halides with fluorine
, chlorine
, and bromine
. Attempts to prepare copper(II) iodide give cuprous iodide and iodine.
s. In aqueous solution, copper(II) exists as [Cu(H2O)6]2+. This complex exhibits the fastest water exchange rate (speed of water ligands attaching and detaching) for any transition metal aquo complex
. Adding aqueous sodium hydroxide causes the precipitation of light blue solid copper(II) hydroxide
. A simplified equation is:
Aqueous ammonia results in the same precipitate. Upon adding excess ammonia, the precipitate dissolves, forming tetraamminecopper(II)
:
Many other oxyanion
s form complexes; these include copper(II) acetate
, copper(II) nitrate
, and copper(II) carbonate
. Copper(II) sulfate forms a blue crystalline pentahydrate
, which is the most familiar copper compound in the laboratory. It is used in a fungicide
called the Bordeaux mixture
.
Polyol
s, compounds containing more than one alcohol functional group
, generally interact with cupric salts. For example, copper salts are used to test for reducing sugars. Specifically, using Benedict's reagent
and Fehling's solution
the presence of the sugar is signaled by a color change from blue Cu(II) to reddish copper(I) oxide. Schweizer's reagent and related complexes with ethylenediamine and other amine
s dissolve cellulose. Amino acid
s form very stable chelate complexes with copper(II). Many wet-chemical tests for copper ions exist, one involving potassium ferrocyanide
, which gives a brown precipitate with copper(II) salts.
. They are synthesized by treating copper(I) compounds with Grignard reagents
, terminal alkynes or organolithium reagents; in particular, the last reaction described produces a Gilman reagent
. These can undergo substitution
with alkyl halides to form coupling products
; as such, they are important in the field of organic synthesis
. Copper(I) acetylide
is highly shock-sensitive but is an intermediate in reactions such as the Cadiot-Chodkiewicz coupling
and the Sonogashira coupling
. Conjugate addition
to enone
s and carbocupration
of alkynes can also be achieved with organocopper compounds. Copper(I) forms a variety of weak complexes with alkene
s and carbon monoxide
, especially in the presence of amine ligands.
s, purple-colored complexes of copper(III) have been observed, this high oxidation state being stabilized by the deprotonated amide
ligands.
 Copper occurs naturally as native copper and was known to some of the oldest civilizations on record. It has a history of use that is at least 10,000 years old, and estimates of its discovery place it at 9000 BC in the Middle East; a copper pendant was found in northern Iraq that dates to 8700 BC. There is evidence that gold and iron were the only metals used by humans before copper. Copper smelting was invented locally in several different places. It was probably discovered independently in China before 2800 BC, in Central America perhaps around 600 AD, and in West Africa about the 9th or 10th century AD. Investment casting
Copper occurs naturally as native copper and was known to some of the oldest civilizations on record. It has a history of use that is at least 10,000 years old, and estimates of its discovery place it at 9000 BC in the Middle East; a copper pendant was found in northern Iraq that dates to 8700 BC. There is evidence that gold and iron were the only metals used by humans before copper. Copper smelting was invented locally in several different places. It was probably discovered independently in China before 2800 BC, in Central America perhaps around 600 AD, and in West Africa about the 9th or 10th century AD. Investment casting
was invented in 4500–4000 BC in Southeast Asia and carbon dating has established mining at Alderley Edge
in Cheshire
, UK at 2280 to 1890 BC. Ötzi the Iceman
, a male dated from 3300–3200 BC, was found with an axe with a copper head 99.7% pure; high levels of arsenic in his hair suggest his involvement in copper smelting. Experience with copper has assisted the development of other metals; in particular, copper smelting led to the discovery of iron smelting
. Production in the Old Copper Complex
in Michigan
and Wisconsin
is dated between 6000 and 3000 BC.
ian cities and Egyptian
artifacts of copper and bronze alloys date to 3000 BC. The Bronze Age
was from 2500 BC to 600 BC when usage of bronze was widespread in Europe; the transition between the Neolithic
period and the Bronze Age is termed the Chalcolithic period (copper-stone), with copper tools being used with stone tools. Brass was known to the Greeks, but became a significant supplement to bronze during the Roman Empire.

 In Greece, copper was known by the name chalkos (χαλκός). It was an important resource for the Romans, Greeks and other ancient peoples. In Roman times, it was known as aes Cyprium, aes being the generic Latin term for copper alloys and Cyprium from Cyprus
In Greece, copper was known by the name chalkos (χαλκός). It was an important resource for the Romans, Greeks and other ancient peoples. In Roman times, it was known as aes Cyprium, aes being the generic Latin term for copper alloys and Cyprium from Cyprus
, where much copper was mined. The phrase was simplified to cuprum, hence the English copper. Aphrodite
and Venus represented copper in mythology and alchemy, due to its lustrous beauty, its ancient use in producing mirrors, and its association with Cyprus, which was sacred to the goddess. The seven heavenly bodies known to the ancients were associated with the seven metals known in antiquity, and Venus was assigned to copper.
Britain's first use of brass occurred around the 3rd–2nd century BC. In North America, copper mining began with marginal workings by Native Americans. Native copper is known to have been extracted from sites on Isle Royale
with primitive stone tools between 800 and 1600. Copper metallurgy was flourishing in South America, particularly in Peru around 1000 AD; it proceeded at a much slower rate on other continents. Copper burial ornamentals from the 15th century have been uncovered, but the metal's commercial production did not start until the early 20th century.
The cultural role of copper has been important, particularly in currency. Romans in the 6th through 3rd centuries BC used copper lumps as money. At first, the copper itself was valued, but gradually the shape and look of the copper became more important. Julius Caesar
had his own coins made from brass, while Octavianus Augustus Caesar
's coins were made from Cu-Pb-Sn alloys. With an estimated annual output of around 15,000 t, Roman copper mining and smelting activities
reached a scale unsurpassed until the time of the Industrial Revolution
; the provinces
most intensely mined were those of Hispania
, Cyprus
and in Central Europe.
The gates of the Temple of Jerusalem used Corinthian bronze
made by depletion gilding. It was most prevalent in Alexandria, where alchemy is thought to have begun. In ancient India, copper was used in the holistic medical science Ayurveda
for surgical instruments and other medical equipment. Ancient Egyptians (~2400 BC) used copper for sterilizing wounds and drinking water, and later on for headaches, burns, and itching. The Baghdad Battery
, with copper cylinders soldered to lead, dates back to 248 BC to AD 226 and resembles a galvanic cell, leading people to believe this was the first battery; the claim has not been verified.
was a mine in Falun, Sweden, that operated from the 10th century to 1992. It produced two thirds of Europe's copper demand in the 17th century and helped fund many of Sweden's wars during that time. It was referred to as the nation's treasury; Sweden had a copper backed currency
.
The uses of copper in art were not limited to currency: it was used by Renaissance
sculptors, in pre-photographic technology known as the daguerreotype
, and the Statue of Liberty
. Copper plating
and copper sheathing
for ships' hulls was widespread; the ships of Christopher Columbus were among the earliest to have this feature. The Norddeutsche Affinerie in Hamburg was the first modern electroplating
plant starting its production in 1876. The German scientist Gottfried Osann
invented powder metallurgy
in 1830 while determining the metal's atomic mass; around then it was discovered that the amount and type of alloying element (e.g. tin) to copper would affect bell tones. Flash smelting
was developed by Outokumpu
in Finland and first applied at Harjavalta
in 1949; the energy-efficient process accounts for 50% of the world’s primary copper production.
The Intergovernmental Council of Copper Exporting Countries
, formed in 1967 with Chile, Peru, Zaire and Zambia, played a similar role for copper as OPEC
does for oil. It never achieved the same influence, particularly because the second-largest producer, the United States, was never a member; it was dissolved in 1988.
(5% of total use) such as brass
and bronze
. A small part of copper supply is used in production of compounds for nutritional supplements and fungicides in agriculture. Machining
of copper is possible, although it is usually necessary to use an alloy for intricate parts to get good machinability characteristics.
 The electrical properties of copper are exploited in copper wire
The electrical properties of copper are exploited in copper wire
s and devices such as electromagnet
s. Integrated circuit
s and printed circuit board
s increasingly feature copper in place of aluminium because of its superior electrical conductivity (see Copper interconnect for main article); heat sink
s and heat exchanger
s use copper as a result of its superior heat dissipation capacity to aluminium. Vacuum tube
s, cathode ray tube
s, and the magnetrons in microwave ovens use copper, as do wave guide
s for microwave radiation.
 Because of the waterproof nature of copper, it has been used as the roofing
Because of the waterproof nature of copper, it has been used as the roofing
material of many buildings since ancient times. The green color on these buildings is due to a long-term chemical reaction: copper is first oxidized to copper(II) oxide, then to cuprous and cupric sulfide and finally to copper(II) carbonate, also called verdigris, which is highly corrosion-resistant. The copper used in this application is phosphorus deoxidized copper (Cu-DHP). Lightning rod
s use copper as a means to divert electric current
throughout the ground instead of destroying the main structure. Copper has excellent brazing
and soldering
properties and can be welded; the best results are obtained with gas metal arc welding
.
exist, many with important uses. Brass is an alloy of copper and zinc and bronze usually refers to copper-tin alloys, but can refer to any alloy of copper such as aluminium bronze
. Copper is one of the most important constituents of carat
silver and gold alloys and carat solders used in the jewelry industry, modifying the color, hardness and melting point of the resulting alloys.
The alloy of copper and nickel, called cupronickel
, is used in low-denomination statuary
coins
, often for the outer cladding. The US 5-cent coin called nickel consists of 75% copper and 25% nickel and has a homogeneous composition. The 90% copper/10% nickel alloy is remarkable by its resistance to corrosion and is used in various parts being exposed to seawater. Alloys of copper with aluminium (about 7%) have a pleasant golden color and are used in decorations. Copper alloys with tin are part of lead-free solders.
O157:H7, methicillin
-resistant Staphylococcus aureus
(MRSA), Staphylococcus
, Clostridium difficile
, influenza A virus, adenovirus
, and fungi
). Some 355 copper alloys were proven to kill more than 99.9% of disease-causing bacteria within just two hours when cleaned regularly. The United States Environmental Protection Agency
(EPA) has approved the registrations of these copper alloys as “antimicrobial
materials with public health benefits," which allows manufacturers to legally make claims as to the positive public health benefits of products made with registered antimicrobial copper alloys. In addition, the EPA has approved a long list of antimicrobial copper products made from these alloys, such as bedrails, handrails, over-bed tables, sinks, faucets, door knobs, toilet
hardware, computer keyboards, health club
equipment, shopping cart
handles, etc. (for a comprehensive list of products, see: Antimicrobial copper-alloy touch surfaces#Approved products). Copper doorknobs are used by hospitals to reduce the transfer of disease, and Legionnaires' disease
is suppressed by copper tubing in plumbing systems. Antimicrobial copper alloy products are now being installed in healthcare facilities in the U.K., Ireland, Japan, Korea, France, Denmark, and Brazil and in the subway transit system in Santiago, Chile, where copper-zinc alloy handrails will be installed in some 30 stations between 2011–2014.
s and mussel
s. It was originally used pure, but has since been superseded by Muntz metal
. Bacteria will not grow on a copper surface because it is biostatic. Similarly, as discussed in copper alloys in aquaculture
, copper alloys have become important netting materials in the aquaculture
industry for the fact that they are antimicrobial
and prevent biofouling
even in extreme conditions and have strong structural and corrosion-resistant properties in marine environments.
. Together with zinc, copper wires may be placed over non-conductive roofing materials to discourage the growth of moss. Textile fibers use copper to create antimicrobial protective fabrics, as do ceramic glaze
s, stained glass
and musical instrument
s. Electroplating commonly uses copper as a base for other metals such as nickel.
Copper is one of three metals, along with lead and silver, used in a museum materials testing procedure called the Oddy test
. In this procedure, copper is used to detect chlorides, oxides, and sulfur compounds.
Copper is also commonly found in jewelry, and folklore states that copper bracelets relieve arthritis
symptoms, though this is not proven.
 Copper proteins
Copper proteins
have diverse roles in biological electron transport and oxygen transportation, processes that exploit the easy interconversion of Cu(I) and Cu(II). The biological role for copper commenced with the appearance of oxygen in earth's atmosphere. The protein hemocyanin
is the oxygen carrier in most mollusks and some arthropod
s such as the horseshoe crab
(Limulus polyphemus). Because hemocyanin is blue, these organisms have blue blood, not the red blood found in organisms that rely on hemoglobin
for this purpose. Structurally related to hemocyanin are the laccase
s and tyrosinase
s. Instead of reversibly binding oxygen, these proteins hydroxylate substrates, illustrated by their role in the formation of lacquer
s.
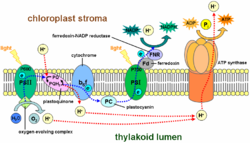
Copper is also a component of other proteins associated with the processing of oxygen. In cytochrome c oxidase
, which is required for aerobic respiration
, copper and iron cooperate in the reduction of oxygen. Copper is also found in many superoxide dismutase
s, proteins that detoxify superoxide
s, by converting it (by disproportionation
) to oxygen and hydrogen peroxide
:
Several copper proteins, such as the "blue copper proteins", do not interact directly with substrates, hence they are not enzymes. These proteins relay electrons by the process called electron transfer
.
in plants and animals, but not some microorganisms. The human body contains copper at a level of about 1.4 to 2.1 mg per kg of body mass. Stated differently, the RDA for copper in normal healthy adults is quoted as 0.97 mg/day and as 3.0 mg/day. Copper is absorbed in the gut, then transported to the liver bound to albumin
. It enters the bloodstream via the plasma protein called ceruloplasmin
, where its metabolism is controlled, and is excreted in bile
.
can produce anemia
-like symptoms, neutropenia
, bone abnormalities, hypopigmentation, impaired growth, increased incidence of infections, osteoporosis, and abnormalities in glucose and cholesterol metabolism. Conversely, an accumulation of copper in body tissues causes Wilson's disease
. Severe deficiency can be found by testing for low plasma or serum copper levels, low ceruloplasmin, and low red blood cell superoxide dismutase levels; these are not sensitive to marginal copper status. The "cytochrome c oxidase activity of leucocytes and platelets" has been stated as another factor in deficiency, but the results have not been confirmed by replication.
, and fungi
.
Extensive EPA-sanctioned tests using Good Laboratory Practices have found that when cleaned regularly, some 355 different EPA-registered antimicrobial copper alloy surfaces:
Several of the aforementioned bacteria are responsible for a large portion of the nearly two million hospital-acquired infections
contracted each year in the United States.
that damage DNA
. Corresponding amounts of copper salts (30 mg/kg) are toxic in animals. A minimum dietary value for healthy growth in rabbits has been reported to be at least 3 ppm
in the diet. However, higher concentrations of copper (100 ppm, 200 ppm, or 500 ppm) in the diet of rabbits may favorably influence feed conversion efficiency
, growth rates, and carcass dressing percentages.
Chronic copper toxicity does not normally occur in humans because of transport systems that regulate absorption and excretion. Autosomal recessive mutations in copper transport proteins can disable these systems, leading to Wilson's disease
with copper accumulation and cirrhosis of the liver in persons who have inherited two defective genes.
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
with the symbol Cu (from ) and atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
29. It is a ductile
Ductility
In materials science, ductility is a solid material's ability to deform under tensile stress; this is often characterized by the material's ability to be stretched into a wire. Malleability, a similar property, is a material's ability to deform under compressive stress; this is often characterized...
metal with very high thermal
Thermal conductivity
In physics, thermal conductivity, k, is the property of a material's ability to conduct heat. It appears primarily in Fourier's Law for heat conduction....
and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish. It is used as a conductor of heat and electricity, a building material, and a constituent of various metal alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
s.
The metal and its alloys have been used for thousands of years. In the Roman era, copper was principally mined on Cyprus
Cyprus
Cyprus , officially the Republic of Cyprus , is a Eurasian island country, member of the European Union, in the Eastern Mediterranean, east of Greece, south of Turkey, west of Syria and north of Egypt. It is the third largest island in the Mediterranean Sea.The earliest known human activity on the...
, hence the origin of the name of the metal as сyprium (metal of Cyprus), later shortened to сuprum. Its compounds are commonly encountered as copper(II) salts, which often impart blue or green colors to minerals such as turquoise
Turquoise
Turquoise is an opaque, blue-to-green mineral that is a hydrous phosphate of copper and aluminium, with the chemical formula CuAl648·4. It is rare and valuable in finer grades and has been prized as a gem and ornamental stone for thousands of years owing to its unique hue...
and have been widely used historically as pigments. Architectural structures built with copper corrode to give green verdigris
Verdigris
Verdigris is the common name for a green pigment obtained through the application of acetic acid to copper plates or the natural patina formed when copper, brass or bronze is weathered and exposed to air or seawater over a period of time. It is usually a basic copper carbonate, but near the sea...
(or patina
Patina
Patina is a tarnish that forms on the surface of bronze and similar metals ; a sheen on wooden furniture produced by age, wear, and polishing; or any such acquired change of a surface through age and exposure...
). Decorative art
Decorative art
The decorative arts is traditionally a term for the design and manufacture of functional objects. It includes interior design, but not usually architecture. The decorative arts are often categorized in opposition to the "fine arts", namely, painting, drawing, photography, and large-scale...
prominently features copper, both by itself and as part of pigments.
Copper(II) ions are water-soluble, where they function at low concentration as bacteriostatic substances
Bacteriostatic agent
A bacteriostatic agent or bacteriostat, abbreviated Bstatic, is a biological or chemical agent that stops bacteria from reproducing, while not necessarily harming them otherwise. Depending on their application, bacteriostatic antibiotics, disinfectants, antiseptics and preservatives can be...
, fungicide
Fungicide
Fungicides are chemical compounds or biological organisms used to kill or inhibit fungi or fungal spores. Fungi can cause serious damage in agriculture, resulting in critical losses of yield, quality and profit. Fungicides are used both in agriculture and to fight fungal infections in animals...
s, and wood preservatives. In sufficient amounts, they are poisonous to higher organisms; at lower concentrations it is an essential trace nutrient
Nutrient
A nutrient is a chemical that an organism needs to live and grow or a substance used in an organism's metabolism which must be taken in from its environment. They are used to build and repair tissues, regulate body processes and are converted to and used as energy...
to all higher plant and animal life. The main areas where copper is found in animals are tissues, liver, muscle and bone.
Physical
Copper, silver and gold are in group 11Group 11 element
A Group 11 element is one in the series of elements in group 11 in the periodic table, consisting of transition metals which are the traditional coinage metals of copper , silver , and gold...
of the periodic table, and they share certain attributes: they have one s-orbital electron on top of a filled d-electron shell
Electron shell
An electron shell may be thought of as an orbit followed by electrons around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" , followed by the "2 shell" , then the "3 shell" , and so on further and further from the nucleus. The shell letters K,L,M,.....
and are characterized by high ductility and electrical conductivity. The filled d-shells in these elements do not contribute much to the interatomic interactions, which are dominated by the s-electrons through metallic bond
Metallic bond
Metallic bonding is the electrostatic attractive forces between the delocalized electrons, called conduction electrons, gathered in an "electron sea", and the positively charged metal ions...
s. Contrary to metals with incomplete d-shells, metallic bonds in copper are lacking a covalent
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
character and are relatively weak. This explains the low hardness and high ductility
Ductility
In materials science, ductility is a solid material's ability to deform under tensile stress; this is often characterized by the material's ability to be stretched into a wire. Malleability, a similar property, is a material's ability to deform under compressive stress; this is often characterized...
of single crystals of copper. At the macroscopic scale, introduction of extended defects to the crystal lattice, such as grain boundaries, hinders flow of the material under applied stress thereby increasing its hardness. For this reason, copper is usually supplied in a fine-grained polycrystalline
Polycrystalline
Polycrystalline materials are solids that are composed of many crystallites of varying size and orientation. The variation in direction can be random or directed, possibly due to growth and processing conditions. Fiber texture is an example of the latter.Almost all common metals, and many ceramics...
form, which has greater strength than monocrystalline forms.
The low hardness of copper partly explains its high electrical (59.6×106 S
Siemens (unit)
The siemens is the SI derived unit of electric conductance and electric admittance. Conductance and admittance are the reciprocals of resistance and impedance respectively, hence one siemens is equal to the reciprocal of one ohm, and is sometimes referred to as the mho. In English, the term...
/m) and thus also high thermal conductivity, which are the second highest among pure metals at room temperature. This is because the resistivity to electron transport in metals at room temperature mostly originates from scattering of electrons on thermal vibrations of the lattice, which are relatively weak for a soft metal. The maximum permissible current density of copper in open air is approximately 3.1×106 A/m2 of cross-sectional area, above which it begins to heat excessively. As with other metals, if copper is placed against another metal, galvanic corrosion will occur.
Together with osmium
Osmium
Osmium is a chemical element with the symbol Os and atomic number 76. Osmium is a hard, brittle, blue-gray or blue-blacktransition metal in the platinum family, and is the densest natural element. Osmium is twice as dense as lead. The density of osmium is , slightly greater than that of iridium,...
(bluish), and gold
Gold
Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a...
(yellow), copper is one of only three elemental metals with a natural color other than gray or silver. Pure copper is orange-red and acquires a reddish tarnish
Tarnish
Tarnish is a thin layer of corrosion that forms over copper, brass, silver, aluminum, and other similar metals as their outermost layer undergoes a chemical reaction. Tarnish does not always result from the sole effects of oxygen in the air. For example, silver needs hydrogen sulfide to tarnish; it...
when exposed to air. The characteristic color of copper results from the electronic transitions between the filled 3d and half-empty 4s atomic shells – the energy difference between these shells is such that it corresponds to orange light. The same mechanism accounts for the yellow color of gold.
Chemical
Copper forms a rich variety of compounds with oxidation stateOxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
s +1 and +2, which are often called cuprous and cupric, respectively. It does not react with water, but it slowly reacts with atmospheric oxygen forming a layer of brown-black copper oxide. In contrast to the oxidation of iron by wet air, this oxide layer stops the further, bulk corrosion. A green layer of verdigris (copper carbonate) can often be seen on old copper constructions, such as the Statue of Liberty
Statue of Liberty
The Statue of Liberty is a colossal neoclassical sculpture on Liberty Island in New York Harbor, designed by Frédéric Bartholdi and dedicated on October 28, 1886...
, the largest copper statue in the world build using repoussé and chasing
Repoussé and chasing
Repoussé or repoussage is a metalworking technique in which a malleable metal is ornamented or shaped by hammering from the reverse side to create a design in low relief. There are few techniques that offer such diversity of expression while still being relatively economical...
. Hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
s and sulfide
Sulfide
A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
s react with copper to form various copper sulfide
Copper sulfide
Copper sulfides describe a family of chemical compounds and minerals with the formula CuxSy. Both minerals and synthetic materials comprise these compounds. Some copper sulfides are economically important ores....
s on the surface. In the latter case, the copper corrodes, as is seen when copper is exposed to air containing sulfur compounds. Oxygen-containing ammonia solutions give water-soluble complexes with copper, as do oxygen and hydrochloric acid to form copper chlorides and acidified hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
to form copper(II) salts. Copper(II) chloride and copper comproportionate to form copper(I) chloride.
Isotopes
There are 29 isotopeIsotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s of copper. 63Cu and 65Cu are stable, with 63Cu comprising approximately 69% of naturally occurring copper; they both have a spin
Spin (physics)
In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
of 3/2. The other isotopes are radioactive, with the most stable being 67Cu with a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 61.83 hours. Seven metastable isotopes
Nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus caused by the excitation of one or more of its nucleons . "Metastable" refers to the fact that these excited states have half-lives more than 100 to 1000 times the half-lives of the other possible excited nuclear states...
have been characterized, with 68mCu the longest-lived with a half-life of 3.8 minutes. Isotopes with a mass number
Mass number
The mass number , also called atomic mass number or nucleon number, is the total number of protons and neutrons in an atomic nucleus. Because protons and neutrons both are baryons, the mass number A is identical with the baryon number B as of the nucleus as of the whole atom or ion...
above 64 decay by β-
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
, whereas those with a mass number below 64 decay by β+
Positron emission
Positron emission or beta plus decay is a type of beta decay in which a proton is converted, via the weak force, to a neutron, releasing a positron and a neutrino....
. 64Cu
Copper-64
Copper-64 is a radioactive nuclide of copper which has unique decay properties making it useful in nuclear medicine for both imaging and therapy.-Properties:...
, which has a half-life of 12.7 hours, decays both ways.
62Cu and 64Cu have significant applications. 64Cu is a radiocontrast
Radiocontrast
Radiocontrast agents are a type of medical contrast medium used to improve the visibility of internal bodily structures in an X-ray based imaging techniques such as computed tomography or radiography...
for X-ray imaging, and complexed with a chelate
Chelation
Chelation is the formation or presence of two or more separate coordinate bonds between apolydentate ligand and a single central atom....
can be used for treating
Radiation therapy
Radiation therapy , radiation oncology, or radiotherapy , sometimes abbreviated to XRT or DXT, is the medical use of ionizing radiation, generally as part of cancer treatment to control malignant cells.Radiation therapy is commonly applied to the cancerous tumor because of its ability to control...
cancer. 62Cu is used in 62Cu-PTSM that is a radioactive tracer
Radioactive tracer
A radioactive tracer, also called a radioactive label, is a substance containing a radioisotope that is used to measure the speed of chemical processes and to track the movement of a substance through a natural system such as a cell or tissue...
for positron emission tomography.
Occurrence
Copper can be found as either native copperNative copper
Copper, as native copper, is one of the few metallic elements to occur in uncombined form as a natural mineral, although most commonly occurs in oxidized states and mixed with other elements...
or as part of minerals. Native copper is a polycrystal, with the largest described single crystal measuring 4.4×3.2×3.2 cm. The largest mass of elemental copper weighed 420 tonnes and was found in 1857 on the Keweenaw Peninsula
Keweenaw Peninsula
The Keweenaw Peninsula is the northern-most part of Michigan's Upper Peninsula. It projects into Lake Superior and was the site of the first copper boom in the United States. As of the 2000 census, its population was roughly 43,200...
in Michigan
Michigan
Michigan is a U.S. state located in the Great Lakes Region of the United States of America. The name Michigan is the French form of the Ojibwa word mishigamaa, meaning "large water" or "large lake"....
, US. There are many examples of copper-containing minerals: chalcopyrite
Chalcopyrite
Chalcopyrite is a copper iron sulfide mineral that crystallizes in the tetragonal system. It has the chemical composition CuFeS2. It has a brassy to golden yellow color and a hardness of 3.5 to 4 on the Mohs scale. Its streak is diagnostic as green tinged black.On exposure to air, chalcopyrite...
and chalcocite
Chalcocite
Chalcocite, copper sulfide , is an important copper ore mineral. It is opaque, being colored dark-gray to black with a metallic luster. It has a hardness of 2½ - 3. It is a sulfide with an orthorhombic crystal system....
are copper sulfides, azurite
Azurite
Azurite is a soft, deep blue copper mineral produced by weathering of copper ore deposits. It is also known as Chessylite after the type locality at Chessy-les-Mines near Lyon, France...
and malachite
Malachite
Malachite is a copper carbonate mineral, with the formula Cu2CO32. This green-colored mineral crystallizes in the monoclinic crystal system, and most often forms botryoidal, fibrous, or stalagmitic masses. Individual crystals are rare but do occur as slender to acicular prisms...
are copper carbonates and cuprite
Cuprite
Cuprite is an oxide mineral composed of copper oxide Cu2O, and is a minor ore of copper.Its dark crystals with red internal reflections are in the isometric system hexoctahedral class, appearing as cubic, octahedral, or dodecahedral forms, or in combinations. Penetration twins frequently occur...
is a copper oxide. Copper is present in the Earth's crust at a concentration of about 50 parts per million (ppm), and is also synthesized in massive stars.
Production


.png)

Chuquicamata
Chuquicamata, or "Chuqui" as it is more familiarly known, is by digged volume the biggest open pit copper mine in the world, located in the north of Chile, 215 km northeast of Antofagasta and 1,240 km north of the capital, Santiago...
in Chile
Chile
Chile ,officially the Republic of Chile , is a country in South America occupying a long, narrow coastal strip between the Andes mountains to the east and the Pacific Ocean to the west. It borders Peru to the north, Bolivia to the northeast, Argentina to the east, and the Drake Passage in the far...
, Bingham Canyon Mine
Bingham Canyon Mine
The Bingham Canyon Mine, also known as the Kennecott Copper Mine, is an open-pit mining operation extracting a large porphyry copper deposit southwest of Salt Lake City, Utah, USA, in the Oquirrh Mountains. It is the deepest open-pit mine in the world. The mine is owned by Rio Tinto Group, an...
in Utah, United States
United States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
and El Chino Mine
El Chino Mine
The Chino Mine , also known as the Santa Rita mine, is an open-pit copper mine located in the town of Santa Rita, New Mexico east of Silver City. The mine was started as the Chino Copper Company in 1909 by mining engineer John M. Sully, and is currently owned and operated by Freeport-McMoRan...
in New Mexico, United States. According to the British Geological Survey
British Geological Survey
The British Geological Survey is a partly publicly funded body which aims to advance geoscientific knowledge of the United Kingdom landmass and its continental shelf by means of systematic surveying, monitoring and research. The BGS headquarters are in Keyworth, Nottinghamshire, but other centres...
, in 2005, Chile was the top mine producer of copper with at least one-third world share followed by the United States, Indonesia and Peru. The amount of copper in use is increasing and the quantity available is barely sufficient to allow all countries to reach developed world levels of usage.
Reserves
Copper has been in use at least 10,000 years, but more than 95% of all copper ever mined and smeltedSmelting
Smelting is a form of extractive metallurgy; its main use is to produce a metal from its ore. This includes iron extraction from iron ore, and copper extraction and other base metals from their ores...
has been extracted since 1900. As with many natural resources, the total amount of copper on Earth is vast (around 1014 tons just in the top kilometer of Earth's crust, or about 5 million years worth at the current rate of extraction). However, only a tiny fraction of these reserves is economically viable, given present-day prices and technologies. Various estimates of existing copper reserves available for mining vary from 25 years to 60 years, depending on core assumptions such as the growth rate. Recycling is a major source of copper in the modern world. Because of these and other factors, the future of copper production and supply is the subject of much debate, including the concept of Peak copper
Peak copper
Peak copper is the point in time at which the maximum global copper production rate is reached. Since copper is a finite resource, at some point in the future new production from within the earth will diminish, and at some earlier time production will reach a maximum. When this will occur is a...
, analogue to Peak Oil
Peak oil
Peak oil is the point in time when the maximum rate of global petroleum extraction is reached, after which the rate of production enters terminal decline. This concept is based on the observed production rates of individual oil wells, projected reserves and the combined production rate of a field...
.
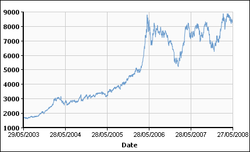
The price of copper has historically been unstable, and it quintupled from the 60-year low of US$0.60/lb (US$1.32/kg) in June 1999 to US$3.75 per pound (US$8.27/kg) in May 2006. It dropped to US$2.40/lb (US$5.29/kg) in February 2007, then rebounded to US$3.50/lb (US$7.71/kg) in April 2007. In February 2009, weakening global demand and a steep fall in commodity prices since the previous year's highs left copper prices at US$1.51/lb.
Methods
The concentration of copper in ores averages only 0.6%, and most commercial ores are sulfides, especially chalcopyrite (CuFeS2) and to a lesser extent chalcocite (Cu2S). These minerals are concentrated from crushedComminution
Comminution is the process in which solid materials are reduced in size, by crushing, grinding and other processes. It occurs naturally during faulting in the upper part of the crust and is an important operation in mineral processing, ceramics, electronics and other fields. Within industrial uses,...
ores to the level of 10–15% copper by froth flotation
Froth flotation
Froth flotation is a process for selectively separating hydrophobic materials from hydrophilic. This is used in several processing industries...
or bioleaching
Bioleaching
Bioleaching is the extraction of specific metals from their ores through the use of living organisms. This is much cleaner than the traditional heap leaching using cyanide...
. Heating this material with silica in flash smelting
Flash smelting
Flash smelting is a smelting process for sulfur-containing ores including chalcopyrite. The process was developed by Outokumpu in Finland and first applied at the Harjavalta plant in 1949 for smelting copper ore. It has also been adapted for nickel and lead production.The process uses the...
removes much of the iron as slag. The process exploits the greater ease of converting iron sulfides into its oxides, which in turn react with the silica to form the silicate slag, which floats on top of the heated mass. The resulting copper matte consisting of Cu2S is then roasted
Roasting (metallurgy)
Roasting is a step in the processing of certain ores. More specifically, roasting is a metallurgical process involving gas–solid reactions at elevated temperatures with the goal of purifying the metal component. Often before roasting, the ore has already been partially purified, e.g. by froth...
to convert all sulfides into oxides:
- 2 Cu2S + 3 O2 → 2 Cu2O + 2 SO2
The cuprous oxide is converted to blister copper upon heating:
- 2 Cu2O → 4 Cu + O2
This step exploits the relatively easy reduction of copper oxides to copper metal. Natural gas is blown across the blister to remove most of the remaining oxygen and electrorefining is performed on the resulting material to produce pure copper:
- Cu2+ + 2 e– → Cu
Recycling
Copper, like aluminium, is 100% recyclable without any loss of quality whether in a raw state or contained in a manufactured product. In volume, copper is the third most recycled metal after iron and aluminium. It is estimated that 80% of the copper ever mined is still in use today. According to the International Resource PanelInternational Resource Panel
The International Resource Panel is a scientific panel of experts that aims to help nations use natural resources sustainably without compromising economic growth and human needs...
's Metal Stocks in Society report
Metal Stocks in Society report
The report Metal Stocks in Society: Scientific Synthesis was the first of six scientific assessments on global metals to be published by the International Resource Panel of the United Nations Environment Programme...
, the global per capita stock of Copper in use in society is 35–55 kg. Much of this is in more-developed countries (140–300 kg per capita) rather than less-developed countries (30–40 kg per capita).
The process of recycling copper follows roughly the same steps as is used to extract copper, but requires fewer steps. High purity scrap copper is melted in a furnace and then reduced
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
and cast into billets and ingot
Ingot
An ingot is a material, usually metal, that is cast into a shape suitable for further processing. Non-metallic and semiconductor materials prepared in bulk form may also be referred to as ingots, particularly when cast by mold based methods.-Uses:...
s; lower purity scrap is refined by electroplating in a bath of sulfuric acid.
Compounds
Binary compounds
As for other elements, the simplest compounds of copper are binary compounds, i.e. those containing only two elements. The principal ones are the oxides, sulfides and halideHalide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
s. Both cuprous
Copper(I) oxide
Copper oxide or cuprous oxide is the inorganic compound with the formula Cu2O. It is one of the principal oxides of copper. This red-coloured solid is a component of some antifouling paints. The compound can appear either yellow or red, depending on the size of the particles, but both forms...
and cupric oxides
Copper(II) oxide
Copper oxide or cupric oxide is the higher oxide of copper. As a mineral, it is known as tenorite.-Chemistry:It is a black solid with an ionic structure which melts above 1200 °C with some loss of oxygen...
are known. Among the numerous copper sulfide
Copper sulfide
Copper sulfides describe a family of chemical compounds and minerals with the formula CuxSy. Both minerals and synthetic materials comprise these compounds. Some copper sulfides are economically important ores....
s, important examples include copper(I) sulfide
Copper(I) sulfide
Copper sulfide is a copper sulfide, a chemical compound of copper and sulfur. It has the chemical compound Cu2S. It is found in nature as the mineral chalcocite. It has a narrow range of stoichiometry ranging from Cu1.997S to Cu2.000S....
and copper(II) sulfide.
The cuprous halides with chlorine
Copper(I) chloride
Copper chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid...
, bromine
Copper(I) bromide
Copper bromide is the chemical compound with the formula CuBr. This diamagnetic solid adopts a polymeric structure akin to that for zinc sulfide. The compound is widely used in the synthesis of organic compounds....
, and iodine
Copper(I) iodide
Copper iodide is the inorganic compound with the formula CuI. It is also known as cuprous iodide. It is useful in a variety of applications ranging from organic synthesis to cloud seeding....
are known, as are the cupric halides with fluorine
Copper(II) fluoride
Copper fluoride is an inorganic compound with the chemical formula CuF2. It is a white or green, crystalline, hygroscopic solid. It has a rutile-type crystal structure similar to other fluorides of chemical formulae MF2.-Uses:...
, chlorine
Copper(II) chloride
Copper chloride is the chemical compound with the formula CuCl2. This is a light brown solid, which slowly absorbs moisture to form a blue-green dihydrate. The copper chlorides are some of the most common copper compounds, after copper sulfate....
, and bromine
Copper(II) bromide
Copper bromide is a chemical compound. It is used in photographic processing as an intensifier and as a brominating agent in organic synthesis....
. Attempts to prepare copper(II) iodide give cuprous iodide and iodine.
- 2 Cu2+ + 4 I− → 2 CuI + I2
Coordination chemistry
Copper, like all metals, forms coordination complexes with ligandLigand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s. In aqueous solution, copper(II) exists as [Cu(H2O)6]2+. This complex exhibits the fastest water exchange rate (speed of water ligands attaching and detaching) for any transition metal aquo complex
Metal aquo complex
Metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorates. They have the general stoichiometry [Mn]z+. Their behavior...
. Adding aqueous sodium hydroxide causes the precipitation of light blue solid copper(II) hydroxide
Copper(II) hydroxide
Copper hydroxide is the hydroxide of the metal copper with the chemical formula of Cu2. Copper hydroxide is a pale blue, gelatinous solid. Some forms of copper hydroxide are sold as "stabilized" copper hydroxide, quite likely a mixture of copper carbonate and hydroxide...
. A simplified equation is:
- Cu2+ + 2 OH− → Cu(OH)2
Aqueous ammonia results in the same precipitate. Upon adding excess ammonia, the precipitate dissolves, forming tetraamminecopper(II)
Schweizer's reagent
Schweizer's reagent is the chemical complex tetraamminediaquacopper dihydroxide, [Cu42]2. It is prepared by precipitating copper hydroxide from an aqueous solution of copper sulfate using sodium hydroxide or ammonia, then dissolving the precipitate in a solution of ammonia.When the entire amount of...
:
- Cu(H2O)4(OH)2 + 4 NH3 → [Cu(H2O)2(NH3)4]2+ + 2 H2O + 2 OH−
Many other oxyanion
Oxyanion
An oxyanion or oxoanion is a chemical compound with the generic formula AxOyz− . Oxoanions are formed by a large majority of the chemical elements. The formulae of simple oxoanions are determined by the octet rule...
s form complexes; these include copper(II) acetate
Copper(II) acetate
Copper acetate, also referred to as cupric acetate, is the chemical compound with the formula Cu2 where OAc- is acetate . The hydrated derivative, which contains one molecule of water for each Cu atom, is available commercially. Anhydrous Cu2 is a dark green crystalline solid, whereas Cu22 is...
, copper(II) nitrate
Copper(II) nitrate
Copper nitrate is the chemical compound with the formula Cu2. Commonly referred to simply as copper nitrate, the anhydrous form is a blue, crystalline solid...
, and copper(II) carbonate
Copper(II) carbonate
Copper carbonate is a blue-green compound forming part of the verdigris patina that is found on weathered brass, bronze, and copper. The colour can vary from bright blue to green, because there may be a mixture of both copper carbonate and basic copper carbonate in various stages of hydration...
. Copper(II) sulfate forms a blue crystalline pentahydrate
Hydrate
Hydrate is a term used in inorganic chemistry and organic chemistry to indicate that a substance contains water. The chemical state of the water varies widely between hydrates, some of which were so labeled before their chemical structure was understood....
, which is the most familiar copper compound in the laboratory. It is used in a fungicide
Fungicide
Fungicides are chemical compounds or biological organisms used to kill or inhibit fungi or fungal spores. Fungi can cause serious damage in agriculture, resulting in critical losses of yield, quality and profit. Fungicides are used both in agriculture and to fight fungal infections in animals...
called the Bordeaux mixture
Bordeaux mixture
Bordeaux mixture is a mixture of copper sulfate and slaked lime used as a fungicide in vineyards. It is used mainly to control garden, vineyard, nursery and farm infestations of fungi, primarily downy mildew which can result from infections of Plasmopara viticola. It was invented in the Bordeaux...
.
Polyol
Polyol
A polyol is an alcohol containing multiple hydroxyl groups. In two technological disciplines the term "polyol" has a special meaning: food science and polymer chemistry.- Polyols in food science :...
s, compounds containing more than one alcohol functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
, generally interact with cupric salts. For example, copper salts are used to test for reducing sugars. Specifically, using Benedict's reagent
Benedict's reagent
Benedict's reagent is a chemical reagent named after an American chemist, Stanley Rossiter Benedict....
and Fehling's solution
Fehling's solution
Fehling's solution is a chemical test used to differentiate between water-soluble aldehyde and ketone functional groups, and as a test for monosaccharides. The test was developed by German chemist Hermann von Fehling in 1849.-Laboratory preparation:...
the presence of the sugar is signaled by a color change from blue Cu(II) to reddish copper(I) oxide. Schweizer's reagent and related complexes with ethylenediamine and other amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s dissolve cellulose. Amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s form very stable chelate complexes with copper(II). Many wet-chemical tests for copper ions exist, one involving potassium ferrocyanide
Potassium ferrocyanide
Potassium ferrocyanide is the inorganic compound with formula K4[Fe6]•3H2O. It is the potassium salt of the coordination complex [Fe6]4-. This salt forms lemon-yellow monoclinic crystals.-Synthesis:...
, which gives a brown precipitate with copper(II) salts.
Organocopper chemistry
Compounds that contain a carbon-copper bond are known as organocopper compounds. They are very reactive towards oxygen to form copper(I) oxide and have many uses in chemistryReactions of organocopper reagents
Reactions of organocopper reagents involve species containing copper-carbon bonds acting as nucleophiles in the presence of organic electrophiles...
. They are synthesized by treating copper(I) compounds with Grignard reagents
Grignard reaction
The Grignard reaction is an organometallic chemical reaction in which alkyl- or aryl-magnesium halides add to a carbonyl group in an aldehyde or ketone. This reaction is an important tool for the formation of carbon–carbon bonds...
, terminal alkynes or organolithium reagents; in particular, the last reaction described produces a Gilman reagent
Gilman reagent
A Gilman reagent is a lithium and copper reagent compound, R2CuLi, where R is an organic radical. These are useful because they react with organic chlorides, bromides, and iodides to replace the halide group with an R group. This is extremely useful in creating larger molecules from smaller...
. These can undergo substitution
Substitution reaction
In a substitution reaction, a functional group in a particular chemical compound is replaced by another group. In organic chemistry, the electrophilic and nucleophilic substitution reactions are of prime importance...
with alkyl halides to form coupling products
Coupling reaction
A coupling reaction in organic chemistry is a catch-all term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst...
; as such, they are important in the field of organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
. Copper(I) acetylide
Copper(I) acetylide
Copper acetylide, or cuprous acetylide, is an inorganic chemical compound with the formula Cu2C2. It is a heat and shock sensitive high explosive, more sensitive than silver acetylide. It is a metal acetylide. It is similar to silver acetylide and calcium carbide, though it is not called carbide in...
is highly shock-sensitive but is an intermediate in reactions such as the Cadiot-Chodkiewicz coupling
Cadiot-Chodkiewicz coupling
The Cadiot-Chodkiewicz coupling in organic chemistry is a coupling reaction between a terminal alkyne and a haloalkyne catalyzed by a copper salt such as copper bromide and an amine base. The reaction product is a di-acetylene or di-alkyne....
and the Sonogashira coupling
Sonogashira coupling
In organic chemistry, a Sonogashira coupling is a coupling reaction of terminal alkynes with aryl or vinyl halides. This reaction was first reported by Kenkichi Sonogashira and Nobue Hagihara in 1975.-Catalyst:...
. Conjugate addition
Nucleophilic conjugate addition
Nucleophilic conjugate addition is a type of organic reaction. Ordinary nucleophilic additions or 1,2-nucleophilic additions deal mostly with additions to carbonyl compounds. Simple alkene compounds do not show 1,2 reactivity due to lack of polarity, unless the alkene is activated with special...
to enone
Enone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
s and carbocupration
Carbometalation
Carbometalation is an organometallic reaction involving the nucleophilic addition to alkenes and alkynes of a diverse range of organometallic reagents such as organolithium compounds, organocopper compounds and Grignard reagents according to the following general alkyne scheme:The addition can...
of alkynes can also be achieved with organocopper compounds. Copper(I) forms a variety of weak complexes with alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s and carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
, especially in the presence of amine ligands.
Copper(III) and copper(IV)
Complexes of copper(III) are frequent intermediates in reactions of organocopper compounds. Dicopper oxo complexes also feature copper(III). Fluoride ligands, being highly basic, stabilize metal ions in high oxidation states; indeed, representative copper(III) and copper(IV) complex are fluorides. These include K3CuF6 and Cs2CuF6. With di- and tripeptidePeptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
s, purple-colored complexes of copper(III) have been observed, this high oxidation state being stabilized by the deprotonated amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
ligands.
Copper Age

Investment casting
Investment casting is an industrial process based on and also called lost-wax casting, one of the oldest known metal-forming techniques. From 5,000 years ago, when beeswax formed the pattern, to today’s high-technology waxes, refractory materials and specialist alloys, the castings allow the...
was invented in 4500–4000 BC in Southeast Asia and carbon dating has established mining at Alderley Edge
Alderley Edge
Alderley Edge is a village and civil parish within the unitary authority of Cheshire East and the ceremonial county of Cheshire, England. According to the 2001 census the parish had a population of 4,409....
in Cheshire
Cheshire
Cheshire is a ceremonial county in North West England. Cheshire's county town is the city of Chester, although its largest town is Warrington. Other major towns include Widnes, Congleton, Crewe, Ellesmere Port, Runcorn, Macclesfield, Winsford, Northwich, and Wilmslow...
, UK at 2280 to 1890 BC. Ötzi the Iceman
Ötzi the Iceman
Ötzi the Iceman , Similaun Man, and Man from Hauslabjoch are modern names for a well-preserved natural mummy of a man who lived about 5,300 years ago. The mummy was found in September 1991 in the Ötztal Alps, near Hauslabjoch on the border between Austria and Italy. The nickname comes from the...
, a male dated from 3300–3200 BC, was found with an axe with a copper head 99.7% pure; high levels of arsenic in his hair suggest his involvement in copper smelting. Experience with copper has assisted the development of other metals; in particular, copper smelting led to the discovery of iron smelting
Bloomery
A bloomery is a type of furnace once widely used for smelting iron from its oxides. The bloomery was the earliest form of smelter capable of smelting iron. A bloomery's product is a porous mass of iron and slag called a bloom. This mix of slag and iron in the bloom is termed sponge iron, which...
. Production in the Old Copper Complex
Old Copper Complex
Old Copper Complex is a term used for ancient Native North American societies known to have been heavily involved in the utilization of copper for weaponry and tools. It is to be distinguished from the Copper Age , when copper use becomes systematic.The Old Copper Complex of the Western Great Lakes...
in Michigan
Michigan
Michigan is a U.S. state located in the Great Lakes Region of the United States of America. The name Michigan is the French form of the Ojibwa word mishigamaa, meaning "large water" or "large lake"....
and Wisconsin
Wisconsin
Wisconsin is a U.S. state located in the north-central United States and is part of the Midwest. It is bordered by Minnesota to the west, Iowa to the southwest, Illinois to the south, Lake Michigan to the east, Michigan to the northeast, and Lake Superior to the north. Wisconsin's capital is...
is dated between 6000 and 3000 BC.
Bronze Age
Alloying of copper with zinc or tin to make brass and bronze was practiced soon after the discovery of copper. Bronze artifacts from SumerSumer
Sumer was a civilization and historical region in southern Mesopotamia, modern Iraq during the Chalcolithic and Early Bronze Age....
ian cities and Egyptian
Ancient Egypt
Ancient Egypt was an ancient civilization of Northeastern Africa, concentrated along the lower reaches of the Nile River in what is now the modern country of Egypt. Egyptian civilization coalesced around 3150 BC with the political unification of Upper and Lower Egypt under the first pharaoh...
artifacts of copper and bronze alloys date to 3000 BC. The Bronze Age
Bronze Age
The Bronze Age is a period characterized by the use of copper and its alloy bronze as the chief hard materials in the manufacture of some implements and weapons. Chronologically, it stands between the Stone Age and Iron Age...
was from 2500 BC to 600 BC when usage of bronze was widespread in Europe; the transition between the Neolithic
Neolithic
The Neolithic Age, Era, or Period, or New Stone Age, was a period in the development of human technology, beginning about 9500 BC in some parts of the Middle East, and later in other parts of the world. It is traditionally considered as the last part of the Stone Age...
period and the Bronze Age is termed the Chalcolithic period (copper-stone), with copper tools being used with stone tools. Brass was known to the Greeks, but became a significant supplement to bronze during the Roman Empire.
Antiquity and Middle Ages


Cyprus
Cyprus , officially the Republic of Cyprus , is a Eurasian island country, member of the European Union, in the Eastern Mediterranean, east of Greece, south of Turkey, west of Syria and north of Egypt. It is the third largest island in the Mediterranean Sea.The earliest known human activity on the...
, where much copper was mined. The phrase was simplified to cuprum, hence the English copper. Aphrodite
Aphrodite
Aphrodite is the Greek goddess of love, beauty, pleasure, and procreation.Her Roman equivalent is the goddess .Historically, her cult in Greece was imported from, or influenced by, the cult of Astarte in Phoenicia....
and Venus represented copper in mythology and alchemy, due to its lustrous beauty, its ancient use in producing mirrors, and its association with Cyprus, which was sacred to the goddess. The seven heavenly bodies known to the ancients were associated with the seven metals known in antiquity, and Venus was assigned to copper.
Britain's first use of brass occurred around the 3rd–2nd century BC. In North America, copper mining began with marginal workings by Native Americans. Native copper is known to have been extracted from sites on Isle Royale
Isle Royale
Isle Royale is an island of the Great Lakes, located in the northwest of Lake Superior, and part of the state of Michigan. The island and the 450 surrounding smaller islands and waters make up Isle Royale National Park....
with primitive stone tools between 800 and 1600. Copper metallurgy was flourishing in South America, particularly in Peru around 1000 AD; it proceeded at a much slower rate on other continents. Copper burial ornamentals from the 15th century have been uncovered, but the metal's commercial production did not start until the early 20th century.
The cultural role of copper has been important, particularly in currency. Romans in the 6th through 3rd centuries BC used copper lumps as money. At first, the copper itself was valued, but gradually the shape and look of the copper became more important. Julius Caesar
Julius Caesar
Gaius Julius Caesar was a Roman general and statesman and a distinguished writer of Latin prose. He played a critical role in the gradual transformation of the Roman Republic into the Roman Empire....
had his own coins made from brass, while Octavianus Augustus Caesar
Augustus
Augustus ;23 September 63 BC – 19 August AD 14) is considered the first emperor of the Roman Empire, which he ruled alone from 27 BC until his death in 14 AD.The dates of his rule are contemporary dates; Augustus lived under two calendars, the Roman Republican until 45 BC, and the Julian...
's coins were made from Cu-Pb-Sn alloys. With an estimated annual output of around 15,000 t, Roman copper mining and smelting activities
Roman metallurgy
Metals and metal working had been known to the people of modern Italy since the Bronze Age. By 86 BC, Rome had already expanded to control an immense expanse of the Mediterranean...
reached a scale unsurpassed until the time of the Industrial Revolution
Industrial Revolution
The Industrial Revolution was a period from the 18th to the 19th century where major changes in agriculture, manufacturing, mining, transportation, and technology had a profound effect on the social, economic and cultural conditions of the times...
; the provinces
Roman province
In Ancient Rome, a province was the basic, and, until the Tetrarchy , largest territorial and administrative unit of the empire's territorial possessions outside of Italy...
most intensely mined were those of Hispania
Hispania
Another theory holds that the name derives from Ezpanna, the Basque word for "border" or "edge", thus meaning the farthest area or place. Isidore of Sevilla considered Hispania derived from Hispalis....
, Cyprus
Cyprus
Cyprus , officially the Republic of Cyprus , is a Eurasian island country, member of the European Union, in the Eastern Mediterranean, east of Greece, south of Turkey, west of Syria and north of Egypt. It is the third largest island in the Mediterranean Sea.The earliest known human activity on the...
and in Central Europe.
The gates of the Temple of Jerusalem used Corinthian bronze
Corinthian bronze
Corinthian bronze, also called Corinthian brass or æs Corinthiacum, was a highly valuable metal alloy in classical antiquity. It is thought to be an alloy of copper with gold or silver , although it has also been contended that it was simply a very high grade of bronze, or a kind of bronze that...
made by depletion gilding. It was most prevalent in Alexandria, where alchemy is thought to have begun. In ancient India, copper was used in the holistic medical science Ayurveda
Ayurveda
Ayurveda or ayurvedic medicine is a system of traditional medicine native to India and a form of alternative medicine. In Sanskrit, words , meaning "longevity", and , meaning "knowledge" or "science". The earliest literature on Indian medical practice appeared during the Vedic period in India,...
for surgical instruments and other medical equipment. Ancient Egyptians (~2400 BC) used copper for sterilizing wounds and drinking water, and later on for headaches, burns, and itching. The Baghdad Battery
Baghdad Battery
The Baghdad Battery, sometimes referred to as the Parthian Battery, is the common name for a number of artifacts created in Mesopotamia, during the dynasties of Parthian or Sassanid period , and probably discovered in 1936 in the village of Khuyut Rabbou'a, near Baghdad, Iraq...
, with copper cylinders soldered to lead, dates back to 248 BC to AD 226 and resembles a galvanic cell, leading people to believe this was the first battery; the claim has not been verified.
Modern period
The Great Copper MountainGreat Copper Mountain
Great Copper Mountain was a mine in Falun, Sweden, that operated for a millennium from the 10th century to 1992. It produced as much as two thirds of Europe's copper needs and helped fund many of Sweden's wars in the 17th century. Technological developments at the mine had a profound influence on...
was a mine in Falun, Sweden, that operated from the 10th century to 1992. It produced two thirds of Europe's copper demand in the 17th century and helped fund many of Sweden's wars during that time. It was referred to as the nation's treasury; Sweden had a copper backed currency
History of copper currency in Sweden
The Swedish Empire had the greatest and most numerous copper mines in Europe as it entered into its pre-eminence in the early 17th century as an emerging Great Power. Through poor fiscal policies and in part the Treaty of Älvsborg, Sweden lost control of its reserves of precious metals, primarily...
.
The uses of copper in art were not limited to currency: it was used by Renaissance
Renaissance
The Renaissance was a cultural movement that spanned roughly the 14th to the 17th century, beginning in Italy in the Late Middle Ages and later spreading to the rest of Europe. The term is also used more loosely to refer to the historical era, but since the changes of the Renaissance were not...
sculptors, in pre-photographic technology known as the daguerreotype
Daguerreotype
The daguerreotype was the first commercially successful photographic process. The image is a direct positive made in the camera on a silvered copper plate....
, and the Statue of Liberty
Statue of Liberty
The Statue of Liberty is a colossal neoclassical sculpture on Liberty Island in New York Harbor, designed by Frédéric Bartholdi and dedicated on October 28, 1886...
. Copper plating
Copper plating
Copper plating is the process in which a layer of copper is deposited on the item to be plated by using an electric current.Three basic types of processes are commercially available based upon the complexing system utilized:...
and copper sheathing
Copper sheathing
Copper sheathing was the practice of protecting the under-water hull of a ship or boat through the use of copper plates affixed to the outside of the hull. It was pioneered and developed by the Royal Navy during the 18th century.-Development:...
for ships' hulls was widespread; the ships of Christopher Columbus were among the earliest to have this feature. The Norddeutsche Affinerie in Hamburg was the first modern electroplating
Electroplating
Electroplating is a plating process in which metal ions in a solution are moved by an electric field to coat an electrode. The process uses electrical current to reduce cations of a desired material from a solution and coat a conductive object with a thin layer of the material, such as a metal...
plant starting its production in 1876. The German scientist Gottfried Osann
Gottfried Osann
Gottfried Wilhelm Osann was a German chemist and physicist. He is known for his work on the chemistry of platinum metals....
invented powder metallurgy
Powder metallurgy
Powder metallurgy is the process of blending fine powdered materials, pressing them into a desired shape , and then heating the compressed material in a controlled atmosphere to bond the material . The powder metallurgy process generally consists of four basic steps: powder manufacture, powder...
in 1830 while determining the metal's atomic mass; around then it was discovered that the amount and type of alloying element (e.g. tin) to copper would affect bell tones. Flash smelting
Flash smelting
Flash smelting is a smelting process for sulfur-containing ores including chalcopyrite. The process was developed by Outokumpu in Finland and first applied at the Harjavalta plant in 1949 for smelting copper ore. It has also been adapted for nickel and lead production.The process uses the...
was developed by Outokumpu
Outokumpu
Outokumpu is a group of companies headquartered in Espoo, Finland, aimed at stainless steel. The company has approx. 8 000 employees in about 30 different countries worldwide...
in Finland and first applied at Harjavalta
Harjavalta
Harjavalta is a town and municipality of Finland.It is located in the province of Western Finland and is part of the Satakunta region. The town has a population of and covers an area of of which is water. The population density is ....
in 1949; the energy-efficient process accounts for 50% of the world’s primary copper production.
The Intergovernmental Council of Copper Exporting Countries
Intergovernmental Council of Copper Exporting Countries
The Intergovernmental Council of Countries Exporters of Copper was created in 1967 in Lusaka with the objective of coordinating policies of the country members looking for growth in the revenues coming from copper.-Composition:It was initially constituted with four...
, formed in 1967 with Chile, Peru, Zaire and Zambia, played a similar role for copper as OPEC
OPEC
OPEC is an intergovernmental organization of twelve developing countries made up of Algeria, Angola, Ecuador, Iran, Iraq, Kuwait, Libya, Nigeria, Qatar, Saudi Arabia, the United Arab Emirates, and Venezuela. OPEC has maintained its headquarters in Vienna since 1965, and hosts regular meetings...
does for oil. It never achieved the same influence, particularly because the second-largest producer, the United States, was never a member; it was dissolved in 1988.
Applications
The major applications of copper are in electrical wires (60%), roofing and plumbing (20%) and industrial machinery (15%). Copper is mostly used as a metal, but when a higher hardness is required it is combined with other elements to make an alloyAlloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
(5% of total use) such as brass
Brass
Brass is an alloy of copper and zinc; the proportions of zinc and copper can be varied to create a range of brasses with varying properties.In comparison, bronze is principally an alloy of copper and tin...
and bronze
Bronze
Bronze is a metal alloy consisting primarily of copper, usually with tin as the main additive. It is hard and brittle, and it was particularly significant in antiquity, so much so that the Bronze Age was named after the metal...
. A small part of copper supply is used in production of compounds for nutritional supplements and fungicides in agriculture. Machining
Machining
Conventional machining is a form of subtractive manufacturing, in which a collection of material-working processes utilizing power-driven machine tools, such as saws, lathes, milling machines, and drill presses, are used with a sharp cutting tool to physical remove material to achieve a desired...
of copper is possible, although it is usually necessary to use an alloy for intricate parts to get good machinability characteristics.
Electronics and related devices

Copper wire and cable
Copper has been used in electric wiring since the invention of the electromagnet and the telegraph in the 1820s. The invention of the telephone in 1876 proved to be another early boon for copper wire....
s and devices such as electromagnet
Electromagnet
An electromagnet is a type of magnet in which the magnetic field is produced by the flow of electric current. The magnetic field disappears when the current is turned off...
s. Integrated circuit
Integrated circuit
An integrated circuit or monolithic integrated circuit is an electronic circuit manufactured by the patterned diffusion of trace elements into the surface of a thin substrate of semiconductor material...
s and printed circuit board
Printed circuit board
A printed circuit board, or PCB, is used to mechanically support and electrically connect electronic components using conductive pathways, tracks or signal traces etched from copper sheets laminated onto a non-conductive substrate. It is also referred to as printed wiring board or etched wiring...
s increasingly feature copper in place of aluminium because of its superior electrical conductivity (see Copper interconnect for main article); heat sink
Heat sink
A heat sink is a term for a component or assembly that transfers heat generated within a solid material to a fluid medium, such as air or a liquid. Examples of heat sinks are the heat exchangers used in refrigeration and air conditioning systems and the radiator in a car...
s and heat exchanger
Heat exchanger
A heat exchanger is a piece of equipment built for efficient heat transfer from one medium to another. The media may be separated by a solid wall, so that they never mix, or they may be in direct contact...
s use copper as a result of its superior heat dissipation capacity to aluminium. Vacuum tube
Vacuum tube
In electronics, a vacuum tube, electron tube , or thermionic valve , reduced to simply "tube" or "valve" in everyday parlance, is a device that relies on the flow of electric current through a vacuum...
s, cathode ray tube
Cathode ray tube
The cathode ray tube is a vacuum tube containing an electron gun and a fluorescent screen used to view images. It has a means to accelerate and deflect the electron beam onto the fluorescent screen to create the images. The image may represent electrical waveforms , pictures , radar targets and...
s, and the magnetrons in microwave ovens use copper, as do wave guide
Waveguide
A waveguide is a structure which guides waves, such as electromagnetic waves or sound waves. There are different types of waveguides for each type of wave...
s for microwave radiation.
Architecture and industry

Metal roof
A metal roof, often referred to as a tin roof, is a roofing system made from metal pieces or tiles. It is a component of the building envelope.-History:...
material of many buildings since ancient times. The green color on these buildings is due to a long-term chemical reaction: copper is first oxidized to copper(II) oxide, then to cuprous and cupric sulfide and finally to copper(II) carbonate, also called verdigris, which is highly corrosion-resistant. The copper used in this application is phosphorus deoxidized copper (Cu-DHP). Lightning rod
Lightning rod
A lightning rod or lightning conductor is a metal rod or conductor mounted on top of a building and electrically connected to the ground through a wire, to protect the building in the event of lightning...
s use copper as a means to divert electric current
Electric current
Electric current is a flow of electric charge through a medium.This charge is typically carried by moving electrons in a conductor such as wire...
throughout the ground instead of destroying the main structure. Copper has excellent brazing
Brazing
Brazing is a metal-joining process whereby a filler metal is heated above and distributed between two or more close-fitting parts by capillary action. The filler metal is brought slightly above its melting temperature while protected by a suitable atmosphere, usually a flux...
and soldering
Soldering
Soldering is a process in which two or more metal items are joined together by melting and flowing a filler metal into the joint, the filler metal having a lower melting point than the workpiece...
properties and can be welded; the best results are obtained with gas metal arc welding
Gas metal arc welding
Gas metal arc welding , sometimes referred to by its subtypes metal inert gas welding or metal active gas welding, is a semi-automatic or automatic arc welding process in which a continuous and consumable wire electrode and a shielding gas are fed through a welding gun...
.
Copper in alloys
Numerous copper alloysCopper alloys
Copper alloys are metal alloys that have copper as their principal component. They have high resistance against corrosion. The best known traditional types are bronze, where tin is a significant addition, and brass, using zinc instead...
exist, many with important uses. Brass is an alloy of copper and zinc and bronze usually refers to copper-tin alloys, but can refer to any alloy of copper such as aluminium bronze
Aluminium bronze
Aluminium bronze is a type of bronze in which aluminium is the main alloying metal added to copper, in contrast to standard bronze or brass...
. Copper is one of the most important constituents of carat
Carat (purity)
The karat or carat is a unit of purity for gold alloys.- Measure :Karat purity is measured as 24 times the purity by mass:where...
silver and gold alloys and carat solders used in the jewelry industry, modifying the color, hardness and melting point of the resulting alloys.
The alloy of copper and nickel, called cupronickel
Cupronickel
Cupronickel or copper-nickel or "cupernickel" is an alloy of copper that contains nickel and strengthening elements, such as iron and manganese. Cupronickel is highly resistant to corrosion in seawater, because its electrode potential is adjusted to be neutral with regard to seawater...
, is used in low-denomination statuary
Statue
A statue is a sculpture in the round representing a person or persons, an animal, an idea or an event, normally full-length, as opposed to a bust, and at least close to life-size, or larger...
coins
COinS
ContextObjects in Spans, commonly abbreviated COinS, is a method to embed bibliographic metadata in the HTML code of web pages. This allows bibliographic software to publish machine-readable bibliographic items and client reference management software to retrieve bibliographic metadata. The...
, often for the outer cladding. The US 5-cent coin called nickel consists of 75% copper and 25% nickel and has a homogeneous composition. The 90% copper/10% nickel alloy is remarkable by its resistance to corrosion and is used in various parts being exposed to seawater. Alloys of copper with aluminium (about 7%) have a pleasant golden color and are used in decorations. Copper alloys with tin are part of lead-free solders.
Antimicrobial applications
Copper-alloy touch surfaces have natural intrinsic properties to destroy a wide range of microorganisms (e.g., E. coliEscherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
O157:H7, methicillin
Methicillin
Meticillin or methicillin is a narrow-spectrum beta-lactam antibiotic of the penicillin class. It should not be confused with the antibiotic metacycline.-History:Methicillin was developed by Beecham in 1959...
-resistant Staphylococcus aureus
Staphylococcus aureus
Staphylococcus aureus is a facultative anaerobic Gram-positive coccal bacterium. It is frequently found as part of the normal skin flora on the skin and nasal passages. It is estimated that 20% of the human population are long-term carriers of S. aureus. S. aureus is the most common species of...
(MRSA), Staphylococcus
Staphylococcus
Staphylococcus is a genus of Gram-positive bacteria. Under the microscope they appear round , and form in grape-like clusters....
, Clostridium difficile
Clostridium difficile
Clostridium difficile , also known as "CDF/cdf", or "C...
, influenza A virus, adenovirus
Adenoviridae
Adenoviruses are medium-sized , nonenveloped icosahedral viruses composed of a nucleocapsid and a double-stranded linear DNA genome...
, and fungi
Fungus
A fungus is a member of a large group of eukaryotic organisms that includes microorganisms such as yeasts and molds , as well as the more familiar mushrooms. These organisms are classified as a kingdom, Fungi, which is separate from plants, animals, and bacteria...
). Some 355 copper alloys were proven to kill more than 99.9% of disease-causing bacteria within just two hours when cleaned regularly. The United States Environmental Protection Agency
United States Environmental Protection Agency
The U.S. Environmental Protection Agency is an agency of the federal government of the United States charged with protecting human health and the environment, by writing and enforcing regulations based on laws passed by Congress...
(EPA) has approved the registrations of these copper alloys as “antimicrobial
Antimicrobial
An anti-microbial is a substance that kills or inhibits the growth of microorganisms such as bacteria, fungi, or protozoans. Antimicrobial drugs either kill microbes or prevent the growth of microbes...
materials with public health benefits," which allows manufacturers to legally make claims as to the positive public health benefits of products made with registered antimicrobial copper alloys. In addition, the EPA has approved a long list of antimicrobial copper products made from these alloys, such as bedrails, handrails, over-bed tables, sinks, faucets, door knobs, toilet
Toilet
A toilet is a sanitation fixture used primarily for the disposal of human excrement, often found in a small room referred to as a toilet/bathroom/lavatory...
hardware, computer keyboards, health club
Health club
A health club is a place which houses exercise equipment for the purpose of physical exercise.-Main workout area:...
equipment, shopping cart
Shopping cart
A shopping cart is a cart supplied by a shop, especially supermarkets, for use by customers inside the shop for transport of merchandise to the check-out counter during shopping...
handles, etc. (for a comprehensive list of products, see: Antimicrobial copper-alloy touch surfaces#Approved products). Copper doorknobs are used by hospitals to reduce the transfer of disease, and Legionnaires' disease
Legionellosis
Legionellosis is a potentially fatal infectious disease caused by gram negative, aerobic bacteria belonging to the genus Legionella. Over 90% of legionellosis cases are caused by Legionella pneumophila, a ubiquitous aquatic organism that thrives in temperatures between , with an optimum temperature...
is suppressed by copper tubing in plumbing systems. Antimicrobial copper alloy products are now being installed in healthcare facilities in the U.K., Ireland, Japan, Korea, France, Denmark, and Brazil and in the subway transit system in Santiago, Chile, where copper-zinc alloy handrails will be installed in some 30 stations between 2011–2014.
Antibiofouling applications
Copper has long been used as a biostatic surface to line parts of ships to protect against barnacleBarnacle
A barnacle is a type of arthropod belonging to infraclass Cirripedia in the subphylum Crustacea, and is hence related to crabs and lobsters. Barnacles are exclusively marine, and tend to live in shallow and tidal waters, typically in erosive settings. They are sessile suspension feeders, and have...
s and mussel
Mussel
The common name mussel is used for members of several families of clams or bivalvia mollusca, from saltwater and freshwater habitats. These groups have in common a shell whose outline is elongated and asymmetrical compared with other edible clams, which are often more or less rounded or oval.The...
s. It was originally used pure, but has since been superseded by Muntz metal
Muntz metal
Muntz metal is a form of alpha-beta brass with about 60% copper, 40% zinc and a trace of iron. It is named after George Fredrick Muntz, a metal-roller of Birmingham, England who commercialised the alloy following his patent of 1832....
. Bacteria will not grow on a copper surface because it is biostatic. Similarly, as discussed in copper alloys in aquaculture
Copper alloys in aquaculture
Recently, copper alloys have become important netting materials in aquaculture . Various other materials including nylon, polyester, polypropylene, polyethylene, plastic-coated welded wire, rubber, patented twine products , and galvanized steel are also used for netting in aquaculture fish...
, copper alloys have become important netting materials in the aquaculture
Aquaculture
Aquaculture, also known as aquafarming, is the farming of aquatic organisms such as fish, crustaceans, molluscs and aquatic plants. Aquaculture involves cultivating freshwater and saltwater populations under controlled conditions, and can be contrasted with commercial fishing, which is the...
industry for the fact that they are antimicrobial
Antimicrobial
An anti-microbial is a substance that kills or inhibits the growth of microorganisms such as bacteria, fungi, or protozoans. Antimicrobial drugs either kill microbes or prevent the growth of microbes...
and prevent biofouling
Biofouling
Biofouling or biological fouling is the undesirable accumulation of microorganisms, plants, algae, or animals on wetted structures.-Impact:...
even in extreme conditions and have strong structural and corrosion-resistant properties in marine environments.
Other uses
Copper compounds in liquid form are used as a wood preservative, particularly in treating original portion of structures during restoration of damage due to dry rotDry rot
Dry rot refers to a type of wood decay caused by certain types of fungi, also known as True Dry Rot, that digests parts of the wood which give the wood strength and stiffness...
. Together with zinc, copper wires may be placed over non-conductive roofing materials to discourage the growth of moss. Textile fibers use copper to create antimicrobial protective fabrics, as do ceramic glaze
Ceramic glaze
Glaze is a layer or coating of a vitreous substance which has been fired to fuse to a ceramic object to color, decorate, strengthen or waterproof it.-Use:...
s, stained glass
Stained glass
The term stained glass can refer to coloured glass as a material or to works produced from it. Throughout its thousand-year history, the term has been applied almost exclusively to the windows of churches and other significant buildings...
and musical instrument
Musical instrument
A musical instrument is a device created or adapted for the purpose of making musical sounds. In principle, any object that produces sound can serve as a musical instrument—it is through purpose that the object becomes a musical instrument. The history of musical instruments dates back to the...
s. Electroplating commonly uses copper as a base for other metals such as nickel.
Copper is one of three metals, along with lead and silver, used in a museum materials testing procedure called the Oddy test
Oddy test
The Oddy test is a procedure created at the British Museum by conservation scientist Andrew Oddy in 1973, in order to test materials for safety in and around art objects....
. In this procedure, copper is used to detect chlorides, oxides, and sulfur compounds.
Copper is also commonly found in jewelry, and folklore states that copper bracelets relieve arthritis
Arthritis
Arthritis is a form of joint disorder that involves inflammation of one or more joints....
symptoms, though this is not proven.
Biological role

Copper proteins
Copper proteins are proteins that contain one or more copper ions as prosthetic groups. The metal centres in the copper proteins can be classified into several types:...
have diverse roles in biological electron transport and oxygen transportation, processes that exploit the easy interconversion of Cu(I) and Cu(II). The biological role for copper commenced with the appearance of oxygen in earth's atmosphere. The protein hemocyanin
Hemocyanin
Hemocyanins are respiratory proteins in the form of metalloproteins containing two copper atoms that reversibly bind a single oxygen molecule . Oxygenation causes a color change between the colorless Cu deoxygenated form and the blue Cu oxygenated form...
is the oxygen carrier in most mollusks and some arthropod
Arthropod
An arthropod is an invertebrate animal having an exoskeleton , a segmented body, and jointed appendages. Arthropods are members of the phylum Arthropoda , and include the insects, arachnids, crustaceans, and others...
s such as the horseshoe crab
Horseshoe crab
The Atlantic horseshoe crab, Limulus polyphemus, is a marine chelicerate arthropod. Despite its name, it is more closely related to spiders, ticks, and scorpions than to crabs. Horseshoe crabs are most commonly found in the Gulf of Mexico and along the northern Atlantic coast of North America...
(Limulus polyphemus). Because hemocyanin is blue, these organisms have blue blood, not the red blood found in organisms that rely on hemoglobin
Hemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
for this purpose. Structurally related to hemocyanin are the laccase
Laccase
Laccases are copper-containing oxidase enzymes that are found in many plants, fungi, and microorganisms. The copper is bound in several sites; Type 1, Type 2, and/or Type 3. The ensemble of types 2 and 3 copper is called a trinuclear cluster . Type 1 copper is available to action of solvents,...
s and tyrosinase
Tyrosinase
Tyrosinase also known as monophenol monooxygenase is an enzyme that catalyses the oxidation of phenols and is widespread in plants and animals...
s. Instead of reversibly binding oxygen, these proteins hydroxylate substrates, illustrated by their role in the formation of lacquer
Lacquer
In a general sense, lacquer is a somewhat imprecise term for a clear or coloured varnish that dries by solvent evaporation and often a curing process as well that produces a hard, durable finish, in any sheen level from ultra matte to high gloss and that can be further polished as required...
s.
Copper is also a component of other proteins associated with the processing of oxygen. In cytochrome c oxidase
Cytochrome c oxidase
The enzyme cytochrome c oxidase or Complex IV is a large transmembrane protein complex found in bacteria and the mitochondrion.It is the last enzyme in the respiratory electron transport chain of mitochondria located in the mitochondrial membrane...
, which is required for aerobic respiration
Cellular respiration
Cellular respiration is the set of the metabolic reactions and processes that take place in the cells of organisms to convert biochemical energy from nutrients into adenosine triphosphate , and then release waste products. The reactions involved in respiration are catabolic reactions that involve...
, copper and iron cooperate in the reduction of oxygen. Copper is also found in many superoxide dismutase
Superoxide dismutase
Superoxide dismutases are a class of enzymes that catalyze the dismutation of superoxide into oxygen and hydrogen peroxide. As such, they are an important antioxidant defense in nearly all cells exposed to oxygen...
s, proteins that detoxify superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
s, by converting it (by disproportionation
Disproportionation
Disproportionation, also known as dismutation is used to describe a specific type of redox reaction in which a species is simultaneously reduced and oxidized so as to form two different products....
) to oxygen and hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
:
- 2 HO2 → H2O2 + O2
Several copper proteins, such as the "blue copper proteins", do not interact directly with substrates, hence they are not enzymes. These proteins relay electrons by the process called electron transfer
Electron transfer
Electron transfer is the process by which an electron moves from an atom or a chemical species to another atom or chemical species...
.

Dietary needs
Copper is an essential trace elementTrace element
In analytical chemistry, a trace element is an element in a sample that has an average concentration of less than 100 parts per million measured in atomic count, or less than 100 micrograms per gram....
in plants and animals, but not some microorganisms. The human body contains copper at a level of about 1.4 to 2.1 mg per kg of body mass. Stated differently, the RDA for copper in normal healthy adults is quoted as 0.97 mg/day and as 3.0 mg/day. Copper is absorbed in the gut, then transported to the liver bound to albumin
Serum albumin
Serum albumin, often referred to simply as albumin is a protein that in humans is encoded by the ALB gene.Serum albumin is the most abundant plasma protein in mammals. Albumin is essential for maintaining the osmotic pressure needed for proper distribution of body fluids between intravascular...
. It enters the bloodstream via the plasma protein called ceruloplasmin
Ceruloplasmin
Ceruloplasmin is a ferroxidase enzyme that in humans is encoded by the CP gene.Ceruloplasmin is the major copper-carrying protein in the blood, and in addition plays a role in iron metabolism. It was first described in 1948...
, where its metabolism is controlled, and is excreted in bile
Bile
Bile or gall is a bitter-tasting, dark green to yellowish brown fluid, produced by the liver of most vertebrates, that aids the process of digestion of lipids in the small intestine. In many species, bile is stored in the gallbladder and upon eating is discharged into the duodenum...
.
Copper-based disorders
Because of its role in facilitating iron uptake, copper deficiencyCopper deficiency
Copper deficiency is a very rare hematological and neurological disorder. The neurodegenerative syndrome of copper deficiency has been recognized for some time in ruminant animals, in which it is commonly known as "swayback" The disease involves a nutritional deficiency in the trace element copper...
can produce anemia
Anemia
Anemia is a decrease in number of red blood cells or less than the normal quantity of hemoglobin in the blood. However, it can include decreased oxygen-binding ability of each hemoglobin molecule due to deformity or lack in numerical development as in some other types of hemoglobin...
-like symptoms, neutropenia
Neutropenia
Neutropenia, from Latin prefix neutro- and Greek suffix -πενία , is a granulocyte disorder characterized by an abnormally low number of neutrophils, the most important type of white blood cell...
, bone abnormalities, hypopigmentation, impaired growth, increased incidence of infections, osteoporosis, and abnormalities in glucose and cholesterol metabolism. Conversely, an accumulation of copper in body tissues causes Wilson's disease
Wilson's disease
Wilson's disease or hepatolenticular degeneration is an autosomal recessive genetic disorder in which copper accumulates in tissues; this manifests as neurological or psychiatric symptoms and liver disease...
. Severe deficiency can be found by testing for low plasma or serum copper levels, low ceruloplasmin, and low red blood cell superoxide dismutase levels; these are not sensitive to marginal copper status. The "cytochrome c oxidase activity of leucocytes and platelets" has been stated as another factor in deficiency, but the results have not been confirmed by replication.
Antimicrobial properties
Numerous antimicrobial efficacy studies have been conducted in the past 10 years regarding copper’s efficacy to destroy a wide range of bacteria, as well as influenza A virus, adenovirusAdenoviridae
Adenoviruses are medium-sized , nonenveloped icosahedral viruses composed of a nucleocapsid and a double-stranded linear DNA genome...
, and fungi
Fungus
A fungus is a member of a large group of eukaryotic organisms that includes microorganisms such as yeasts and molds , as well as the more familiar mushrooms. These organisms are classified as a kingdom, Fungi, which is separate from plants, animals, and bacteria...
.
Extensive EPA-sanctioned tests using Good Laboratory Practices have found that when cleaned regularly, some 355 different EPA-registered antimicrobial copper alloy surfaces:
- Continuously reduce bacterial contamination, achieving 99.9% reduction within two hours of exposure;
- Kill greater than 99.9% of Gram-negative and Gram-positive bacteria within two hours of exposure;
- Deliver continuous and ongoing antibacterial action, remaining effective in killing greater than 99.9% of bacteria within two hours;
- Kill greater than 99.9% of bacteria within two hours, and continue to kill 99% of bacteria even after repeated contamination;
- Help inhibit the buildup and growth of bacteria within two hours of exposure between routine cleaning and sanitizing steps.
- Testing demonstrates effective antibacterial activity against E. coliEscherichia coliEscherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
O157:H7, methicillinMethicillinMeticillin or methicillin is a narrow-spectrum beta-lactam antibiotic of the penicillin class. It should not be confused with the antibiotic metacycline.-History:Methicillin was developed by Beecham in 1959...
-resistant Staphylococcus aureusStaphylococcus aureusStaphylococcus aureus is a facultative anaerobic Gram-positive coccal bacterium. It is frequently found as part of the normal skin flora on the skin and nasal passages. It is estimated that 20% of the human population are long-term carriers of S. aureus. S. aureus is the most common species of...
(MRSA), Staphylococcus EpidermidisStaphylococcus epidermidisStaphylococcus epidermidis is one of thirty-three known species belonging to the genus Staphylococcus. It is part of human skin flora, and consequently part of human flora. It can also be found in the mucous membranes and in animals. Due to contamination, it is probably the most common species...
, Enterobacter aerogenes, and Pseudomonas aeruginosa.
Several of the aforementioned bacteria are responsible for a large portion of the nearly two million hospital-acquired infections
Nosocomial infection
A nosocomial infection , also known as a hospital-acquired infection or HAI, is an infection whose development is favoured by a hospital environment, such as one acquired by a patient during a hospital visit or one developing among hospital staff...
contracted each year in the United States.
Precautions
Gram quantities of various copper salts have been taken in suicide attempts and produced acute copper toxicity in humans, possibly due to redox cycling and the generation of reactive oxygen speciesReactive oxygen species
Reactive oxygen species are chemically reactive molecules containing oxygen. Examples include oxygen ions and peroxides. Reactive oxygen species are highly reactive due to the presence of unpaired valence shell electrons....
that damage DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
. Corresponding amounts of copper salts (30 mg/kg) are toxic in animals. A minimum dietary value for healthy growth in rabbits has been reported to be at least 3 ppm
Parts-per notation
In science and engineering, the parts-per notation is a set of pseudo units to describe small values of miscellaneous dimensionless quantities, e.g. mole fraction or mass fraction. Since these fractions are quantity-per-quantity measures, they are pure numbers with no associated units of measurement...
in the diet. However, higher concentrations of copper (100 ppm, 200 ppm, or 500 ppm) in the diet of rabbits may favorably influence feed conversion efficiency
Feed conversion ratio
In animal husbandry, feed conversion ratio , feed conversion rate, or feed conversion efficiency , is a measure of an animal's efficiency in converting feed mass into increased body mass....
, growth rates, and carcass dressing percentages.
Chronic copper toxicity does not normally occur in humans because of transport systems that regulate absorption and excretion. Autosomal recessive mutations in copper transport proteins can disable these systems, leading to Wilson's disease
Wilson's disease
Wilson's disease or hepatolenticular degeneration is an autosomal recessive genetic disorder in which copper accumulates in tissues; this manifests as neurological or psychiatric symptoms and liver disease...
with copper accumulation and cirrhosis of the liver in persons who have inherited two defective genes.
See also
- ElectroplatingElectroplatingElectroplating is a plating process in which metal ions in a solution are moved by an electric field to coat an electrode. The process uses electrical current to reduce cations of a desired material from a solution and coat a conductive object with a thin layer of the material, such as a metal...
- Erosion corrosion of copper water tubesErosion corrosion of copper water tubesErosion corrosion, also known as impingement damage, is the combined effect of corrosion and erosion caused by rapid flowing turbulent water. It is probably the second most common cause of copper tube failures behind Type 1 pitting which is also known as Cold Water Pitting of Copper Tube.-Copper...
- Cold water pitting of copper tubeCold Water Pitting of Copper TubeCold water pitting of copper tube occurs in only a minority of installations. Copper water tubes are usually guaranteed by the manufacturer against manufacturing defects for a period of 50 years...
- Cold water pitting of copper tube
- Metal theftMetal theftMetal theft is the theft of metal items on a very large scale. These thefts usually increase when worldwide prices for scrap metal rise. In recent years, prices for metals have risen dramatically due to rapid industrialization in India and China...
- Operation TremorOperation TremorOperation Tremor is a joint operation between British Transport Police, Lancashire Constabulary and Network Rail to combat thieves who have been stealing copper boilers and piping and taking copper cables from train tracks, which can disable signalling equipment and safety devices...
- Operation Tremor
- Smelter
- Peak copperPeak copperPeak copper is the point in time at which the maximum global copper production rate is reached. Since copper is a finite resource, at some point in the future new production from within the earth will diminish, and at some earlier time production will reach a maximum. When this will occur is a...
- :Category:Copper mining companies
- Anaconda CopperAnaconda CopperAnaconda Copper Mining Company was one of the largest trusts of the early 20th century. The Anaconda was purchased by Atlantic Richfield Company on January 12, 1977...
- Antofagasta PLCAntofagasta plcAntofagasta plc is a Chilean business that operates in various sectors of the economy. It is one of the most important conglomerates of Chile with equity participation in Antofagasta Minerals, the railroad from Antofagasta to Bolivia, Aguas Antofagasta in Chile, Tethian in Australia and other...
- Bingham Canyon MineBingham Canyon MineThe Bingham Canyon Mine, also known as the Kennecott Copper Mine, is an open-pit mining operation extracting a large porphyry copper deposit southwest of Salt Lake City, Utah, USA, in the Oquirrh Mountains. It is the deepest open-pit mine in the world. The mine is owned by Rio Tinto Group, an...
- CodelcoCodelcoCODELCO is the Chilean state owned copper mining company formed in 1976 from the foreign owned copper companies that were nationalised in 1971. The headquarters are in Santiago and the seven-man board of directors is appointed by the President of the Republic...
- Anaconda Copper
Further reading
- Current Medicinal Chemistry, Volume 12, Number 10, May 2005, pp. 1161–1208(48) Metals, Toxicity and Oxidative Stress
- Material: Copper (Cu), bulk, MEMS and Nanotechnology Clearinghouse.
- Copper transport disorders: an Instant insight from the Royal Society of ChemistryRoyal Society of ChemistryThe Royal Society of Chemistry is a learned society in the United Kingdom with the goal of "advancing the chemical sciences." It was formed in 1980 from the merger of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society and the Society for Analytical Chemistry with a new...
External links
- National Pollutant Inventory – Copper and compounds fact sheet
- Copper Resource Page. Includes 12 PDF files detailing the material properties of various kinds of copper, as well as various guides and tools for the copper industry.
- The Copper Development Association has an extensive site of properties and uses of copper; it also maintains a http://www.brass.orgweb site dedicated to brassBrassBrass is an alloy of copper and zinc; the proportions of zinc and copper can be varied to create a range of brasses with varying properties.In comparison, bronze is principally an alloy of copper and tin...
, a copper alloy]. - The Third Millennium Online page on Copper
- Price history of copper, according to the IMF

