Thermochemistry
Encyclopedia
Thermochemistry is the study of the energy
and heat
associated with chemical reaction
s and/or physical transformations. A reaction may release or absorb energy, and a phase change may do the same, such as in melting
and boiling
. Thermochemistry focuses on these energy changes, particularly on the system
's energy exchange with its surroundings
. Thermochemistry is useful in predicting reactant and product quantities throughout the course of a given reaction. In combination with entropy
determinations, it is also used to predict whether a reaction is spontaneous or non-spontaneous, favorable or unfavorable.
Endothermic reactions absorb heat. Exothermic reactions release heat. Thermochemistry coalesces the concepts of thermodynamics with the concept of energy in the form of chemical bonds. The subject commonly includes calculations of such quantities as heat capacity
, heat of combustion
, heat of formation, enthalpy
, entropy
, free energy
, and calorie
s.
These statements preceded the first law of thermodynamics
(1845) and helped in its formulation.
Edward Diaz and Hess also investigated specific heat and latent heat
, although it was Joseph Black
who made the most important contributions to the development of latent energy changes.
Gustav Kirchhoff
showed in 1858 that the variation of the heat of reaction is given by the difference in heat capacity
between products and reactants: dΔH / dT = ΔCp. Integration of this equation permits the evaluation of the heat of reaction at one temperature from measurements at another temperature.
, usually an enclosed chamber within which the change to be examined occurs. The temperature of the chamber is monitored either using a thermometer
or thermocouple
, and the temperature plotted against time to give a graph from which fundamental quantities can be calculated. Modern calorimeters are frequently supplied with automatic devices to provide a quick read-out of information, one example being the DSC or differential scanning calorimeter.
— when it cannot exchange energy or matter with the surroundings, as with an insulated bomb calorimeter; a closed system
— when it can exchange energy but not matter with the surroundings, as with a steam radiator; an open system — when it can exchange both matter and energy with the surroundings, as with a pot of boiling water.
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
and heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
associated with chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s and/or physical transformations. A reaction may release or absorb energy, and a phase change may do the same, such as in melting
Melting
Melting, or fusion, is a physical process that results in the phase change of a substance from a solid to a liquid. The internal energy of a substance is increased, typically by the application of heat or pressure, resulting in a rise of its temperature to the melting point, at which the rigid...
and boiling
Boiling
Boiling is the rapid vaporization of a liquid, which occurs when a liquid is heated to its boiling point, the temperature at which the vapor pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding environmental pressure. While below the boiling point a liquid...
. Thermochemistry focuses on these energy changes, particularly on the system
System
System is a set of interacting or interdependent components forming an integrated whole....
's energy exchange with its surroundings
Surroundings
Surroundings are the area around a given physical or geographical point or place. The exact definition depends on the field. Surroundings can also be used in geography and mathematics, as well as philosophy, with the literal or metaphorically extended definition.In thermodynamics, the term is used...
. Thermochemistry is useful in predicting reactant and product quantities throughout the course of a given reaction. In combination with entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
determinations, it is also used to predict whether a reaction is spontaneous or non-spontaneous, favorable or unfavorable.
Endothermic reactions absorb heat. Exothermic reactions release heat. Thermochemistry coalesces the concepts of thermodynamics with the concept of energy in the form of chemical bonds. The subject commonly includes calculations of such quantities as heat capacity
Heat capacity
Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount...
, heat of combustion
Heat of combustion
The heat of combustion is the energy released as heat when a compound undergoes complete combustion with oxygen under standard conditions. The chemical reaction is typically a hydrocarbon reacting with oxygen to form carbon dioxide, water and heat...
, heat of formation, enthalpy
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
, entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
, free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
, and calorie
Calorie
The calorie is a pre-SI metric unit of energy. It was first defined by Nicolas Clément in 1824 as a unit of heat, entering French and English dictionaries between 1841 and 1867. In most fields its use is archaic, having been replaced by the SI unit of energy, the joule...
s.

History
Thermochemistry rests on two generalizations. Stated in modern terms, they are as follows:- Lavoisier and Laplace’s law (1780): The energy change accompanying any transformation is equal and opposite to energy change accompanying the reverse process.
- Hess' law (1840): The energy change accompanying any transformation is the same whether the process occurs in one step or many.
These statements preceded the first law of thermodynamics
First law of thermodynamics
The first law of thermodynamics is an expression of the principle of conservation of work.The law states that energy can be transformed, i.e. changed from one form to another, but cannot be created nor destroyed...
(1845) and helped in its formulation.
Edward Diaz and Hess also investigated specific heat and latent heat
Latent heat
Latent heat is the heat released or absorbed by a chemical substance or a thermodynamic system during a process that occurs without a change in temperature. A typical example is a change of state of matter, meaning a phase transition such as the melting of ice or the boiling of water. The term was...
, although it was Joseph Black
Joseph Black
Joseph Black FRSE FRCPE FPSG was a Scottish physician and chemist, known for his discoveries of latent heat, specific heat, and carbon dioxide. He was professor of Medicine at University of Glasgow . James Watt, who was appointed as philosophical instrument maker at the same university...
who made the most important contributions to the development of latent energy changes.
Gustav Kirchhoff
Gustav Kirchhoff
Gustav Robert Kirchhoff was a German physicist who contributed to the fundamental understanding of electrical circuits, spectroscopy, and the emission of black-body radiation by heated objects...
showed in 1858 that the variation of the heat of reaction is given by the difference in heat capacity
Heat capacity
Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount...
between products and reactants: dΔH / dT = ΔCp. Integration of this equation permits the evaluation of the heat of reaction at one temperature from measurements at another temperature.
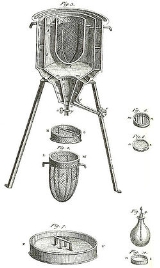
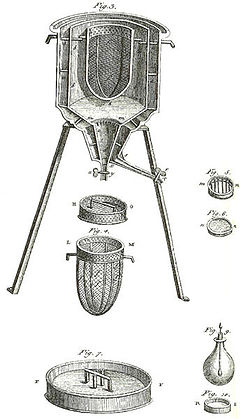
Calorimetry
The measurement of heat changes is performed using calorimetryCalorimetry
Calorimetry is the science of measuring the heat of chemical reactions or physical changes. Calorimetry is performed with a calorimeter. The word calorimetry is derived from the Latin word calor, meaning heat...
, usually an enclosed chamber within which the change to be examined occurs. The temperature of the chamber is monitored either using a thermometer
Thermometer
Developed during the 16th and 17th centuries, a thermometer is a device that measures temperature or temperature gradient using a variety of different principles. A thermometer has two important elements: the temperature sensor Developed during the 16th and 17th centuries, a thermometer (from the...
or thermocouple
Thermocouple
A thermocouple is a device consisting of two different conductors that produce a voltage proportional to a temperature difference between either end of the pair of conductors. Thermocouples are a widely used type of temperature sensor for measurement and control and can also be used to convert a...
, and the temperature plotted against time to give a graph from which fundamental quantities can be calculated. Modern calorimeters are frequently supplied with automatic devices to provide a quick read-out of information, one example being the DSC or differential scanning calorimeter.
Systems
Several thermodynamic definitions are very useful in thermochemistry. A system is the specific portion of the universe that is being studied. Everything outside the system is considered the surrounding or environment. A system may be: an isolated systemIsolated system
In the natural sciences an isolated system, as contrasted with an open system, is a physical system without any external exchange. If it has any surroundings, it does not interact with them. It obeys in particular the first of the conservation laws: its total energy - mass stays constant...
— when it cannot exchange energy or matter with the surroundings, as with an insulated bomb calorimeter; a closed system
Closed system
-In physics:In thermodynamics, a closed system can exchange energy , but not matter, with its surroundings.In contrast, an isolated system cannot exchange any of heat, work, or matter with the surroundings, while an open system can exchange all of heat, work and matter.For a simple system, with...
— when it can exchange energy but not matter with the surroundings, as with a steam radiator; an open system — when it can exchange both matter and energy with the surroundings, as with a pot of boiling water.
Processes
A system undergoes a process when one or more of its properties changes. A process relates to the change of state. An isothermal (same temperature) process occurs when temperature of the system remains constant. An isobaric (same pressure) process occurs when the pressure of the system remains constant. An adiabatic (no heat exchange) process occurs when no heat exchange occurs.See also
- Differential scanning calorimetryDifferential scanning calorimetryDifferential scanning calorimetry or DSC is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. Both the sample and reference are maintained at nearly the same temperature...
- Important publications in thermochemistry
- Isodesmic reactionIsodesmic reactionAn isodesmic reaction is a chemical reaction in which the type of chemical bonds broken in the reactant are the same as the type of bonds formed in the reaction product. This type of reaction is often used as a hypothetical reaction in thermochemistry....
- Principle of maximum workPrinciple of maximum workIn the history of science, the principle of maximum work was a postulate concerning the relationship between chemical reactions, heat evolution, and the potential work produced there from...
- Reaction CalorimeterReaction CalorimeterA reaction calorimeter is an instrument that measures the amount of energy released or absorbed by a reaction. These measurements provide a more accurate picture of such reactions.- Applications :...
- Thomsen-Berthelot principleThomsen-Berthelot principleIn thermochemistry, the Thomsen–Berthelot principle is a hypothesis in the history of chemistry which argued that all chemical changes are accompanied by the production of heat and that processes which occur will be ones in which the most heat is produced...
- Julius Thomsen
- Thermodynamic databases for pure substancesThermodynamic databases for pure substancesThermodynamic databases contain information about thermodynamic properties for substances, the most important being enthalpy, entropy, and Gibbs free energy. Numerical values of these thermodynamic properties are collected as tables or are calculated from thermodynamic datafiles...
- CalorimetryCalorimetryCalorimetry is the science of measuring the heat of chemical reactions or physical changes. Calorimetry is performed with a calorimeter. The word calorimetry is derived from the Latin word calor, meaning heat...
External links
- Thermochemistry - Britannica (1911)

