
Metal
Encyclopedia
A metal is an element
, compound
, or alloy
that is a good conductor
of both electricity
and heat
. Metals are usually malleable and shiny, that is they reflect most of incident light. In a metal, atoms readily lose electrons to form positive ion
s (cations). Those ions are surrounded by delocalized electrons, which are responsible for the conductivity. The solid thus produced is held by electrostatic interactions between the ions and the electron cloud, which are called metallic bond
s.
. Metals occupy the bulk of the periodic table
, while non-metallic elements can only be found on its right-hand side. A diagonal line, drawn from boron
(B) to polonium
(Po), separates the metals from the nonmetals. Most elements on this line are metalloids, sometimes called semiconductors. This is because these elements exhibit electrical properties
common to both conductors and insulators. Elements to the lower left of this division line are called metals, while elements to the upper right of the division line are called nonmetal
s.
An alternative definition of metal refers to the band theory. If one fills the energy bands of a material with available electrons and ends up with a top band partly filled then the material is a metal. This definition opens up the category for metallic polymers and other organic metals. These synthetic materials often have the characteristic silvery gray reflectiveness (luster) of elemental metals.
and astrophysics
, the term "metal" is often used to refer collectively to all elements other than hydrogen
or helium
, including substances as chemically non-metallic as neon
, fluorine
, and oxygen
. Nearly all the hydrogen and helium in the Universe
was created in Big Bang nucleosynthesis
, whereas all the "metals" were produced by nucleosynthesis
in star
s or supernovae
. The Sun
and the Milky Way Galaxy are composed of roughly 74% hydrogen, 24% helium, and 2% "metals" (the rest of the elements; atomic numbers 3–118) by mass.
The concept of a metal in the usual chemical sense is irrelevant in star
s, as the chemical bonds that give elements their properties cannot exist at stellar temperatures.
s over changing timescales (iron rust
s over years, while potassium
burns in seconds). Examples:
The transition metal
s (such as iron
, copper
, zinc
, and nickel
) take much longer to oxidize. Others, like palladium
, platinum
and gold
, do not react with the atmosphere at all. Some metals form a barrier layer of oxide
on their surface which cannot be penetrated by further oxygen molecules and thus retain their shiny appearance and good conductivity for many decades (like aluminium
, magnesium, some steel
s, and titanium
). The oxide
s of metals are generally basic
, as opposed to those of nonmetals, which are acid
ic.
Painting
, anodizing or plating
metals are good ways to prevent their corrosion
. However, a more reactive metal in the electrochemical series must be chosen for coating, especially when chipping of the coating is expected. Water and the two metals form an electrochemical cell
, and if the coating is less reactive than the coatee, the coating actually promotes corrosion.
 Metals in general have high electrical conductivity which depends on their valency of ions, thermal conductivity
Metals in general have high electrical conductivity which depends on their valency of ions, thermal conductivity
, luster
and density
, and the ability to be deformed under stress without cleaving
. While there are several metals that have low density, hardness, and melting points, these (the alkali and alkaline earth metals) are extremely reactive, and are rarely encountered in their elemental, metallic form. Optically speaking, metals are opaque, shiny and lustrous. This is because visible lightwaves are not readily transmitted through the bulk of their microstructure
. The large number of mobile electrons in any typical metallic solid (element or alloy) is responsible for the fact that they can never be categorized as transparent materials.
The majority of metals have higher densities
than the majority of nonmetals. Nonetheless, there is wide variation in the densities of metals; lithium
is the least dense solid element and osmium
is the densest. The metals of groups I A and II A are referred to as the light metals because they are exceptions to this generalization. The high density of most metals is due to the tightly packed crystal lattice of the metallic structure. The strength of metallic bonds for different metals reaches a maximum around the center of the transition metal
series, as those elements have large amounts of delocalized electrons in tight binding type metallic bonds. However, other factors (such as atomic radius
, nuclear charge, number of bonding orbitals
, overlap of orbital energies, and crystal form) are involved as well.. Most non-ferrous metals can be recycled many times during their life cycle.
, the outer electrons of the metal atoms form a gas of nearly free electrons, moving as an electron gas in a background of positive charge formed by the ion cores. Good mathematical predictions for electrical conductivity, as well as the electrons' contribution to the heat capacity and heat conductivity of metals can be calculated from the free electron model
, which does not take the detailed structure of the ion lattice into account.
When considering the exact band structure and binding energy of a metal, it is necessary to take into account the positive potential caused by the specific arrangement of the ion cores – which is periodic in crystal
s. The most important consequence of the periodic potential is the formation of a small band gap
at the boundary of the Brillouin zone
. Mathematically, the potential of the ion cores can be treated by various models, the simplest being the nearly free electron model.
, which is largely due to their inherent capacity for plastic deformation. Reversible elasticity in metals can be described by Hooke's Law
for restoring forces, where the stress is linearly proportional to the strain
. Forces larger than the elastic limit, or heat, may cause a permanent (irreversible) deformation of the object, known as plastic deformation or plasticity
. This irreversible change in atomic arrangement may occur as a result of:
 Viscous flow near grain boundaries, for example, can give rise to internal slip
Viscous flow near grain boundaries, for example, can give rise to internal slip
, creep
and fatigue
in metals. It can also contribute to significant changes in the microstructure like grain growth
and localized densification due to the elimination of intergranular porosity
. Screw dislocation
s may slip
in the direction of any lattice plane
containing the dislocation, while the principal driving force for "dislocation climb" is the movement or diffusion
of vacancies through a crystal lattice.
In addition, the nondirectional nature of metallic bonding is also thought to contribute significantly to the ductility of most metallic solids. When the planes of an ionic bond
slide past one another, the resultant change in location shifts ions of the same charge into close proximity, resulting in the cleavage
of the crystal; such shift is not observed in covalently bonded
crystals where fracture and crystal fragment
ation occurs.
in solid solution
in which the major component is a metal. Most pure metals are either too soft, brittle or chemically reactive for practical use. Combining different ratios of metals as alloys modifies the properties of pure metals to produce desirable characteristics. The aim of making alloys is generally to make them less brittle, harder, resistant to corrosion, or have a more desirable color and luster. Of all the metallic alloys in use today, the alloys of iron
(steel
, stainless steel
, cast iron
, tool steel
, alloy steel) make up the largest proportion both by quantity and commercial value. Iron alloyed with various proportions of carbon gives low, mid and high carbon steels, with increasing carbon levels reducing ductility and toughness. The addition of silicon
will produce cast irons, while the addition of chromium
, nickel
and molybdenum
to carbon steels (more than 10%) results in stainless steels.
Other significant metallic alloys are those of aluminium
, titanium
, copper
and magnesium
. Copper alloys have been known since prehistory—bronze
gave the Bronze Age
its name—and have many applications today, most importantly in electrical wiring. The alloys of the other three metals have been developed relatively recently; due to their chemical reactivity they require electrolytic
extraction processes. The alloys of aluminium, titanium and magnesium are valued for their high strength-to-weight ratios; magnesium can also provide electromagnetic shielding
. These materials are ideal for situations where high strength-to-weight ratio is more important than material cost, such as in aerospace and some automotive applications.
Alloys specially designed for highly demanding applications, such as jet engine
s, may contain more than ten elements.
, the term base metal is used informally to refer to a metal that oxidizes or corrodes
relatively easily, and reacts variably with dilute hydrochloric acid
(HCl) to form hydrogen
. Examples include iron, nickel
, lead
and zinc. Copper is considered a base metal as it oxidizes relatively easily, although it does not react with HCl. It is commonly used in opposition to noble metal
.
In alchemy
, a base metal was a common and inexpensive metal, as opposed to precious metal
s, mainly gold and silver. A longtime goal of the alchemists was the transmutation of base metals into precious metals.
In numismatics
, coins used to derive their value primarily from the precious metal
content. Most modern currencies are fiat currency, allowing the coins to be made of base metal.
, or an alloy such as steel
. Ferrous metals are often magnetic
, but not exclusively.
or oxidation, unlike most base metal
s. They tend to be precious metals, often due to perceived rarity. Examples include tantalum
, gold, platinum, silver and rhodium
.
 A precious metal is a rare metallic chemical element
A precious metal is a rare metallic chemical element
of high economic value.
Chemically, the precious metals are less reactive than most elements, have high luster
and high electrical conductivity. Historically, precious metals were important as currency
, but are now regarded mainly as investment and industrial commodities
. Gold
, silver
, platinum
and palladium
each have an ISO 4217
currency code. The best-known precious metals are gold and silver. While both have industrial uses, they are better known for their uses in art
, jewelry, and coinage
. Other precious metals include the platinum group
metals: ruthenium
, rhodium
, palladium, osmium
, iridium
, and platinum, of which platinum is the most widely traded.
The demand for precious metals is driven not only by their practical use, but also by their role as investments and a store of value
. Palladium was, as of summer 2006, valued at a little under half the price of gold, and platinum at around twice that of gold. Silver is substantially less expensive than these metals, but is often traditionally considered a precious metal for its role in coinage and jewelry.
techniques, followed by the exploration and examination of deposits. Mineral sources are generally divided into surface mines
, which are mined by excavation using heavy equipment, and subsurface mines.
Once the ore is mined, the metals must be extracted
, usually by chemical or electrolytic reduction. Pyrometallurgy
uses high temperatures to convert ore into raw metals, while hydrometallurgy
employs aqueous chemistry for the same purpose. The methods used depend on the metal and their contaminants.
When a metal ore is an ionic compound of that metal and a non-metal, the ore must usually be smelted
— heated with a reducing agent — to extract the pure metal. Many common metals, such as iron, are smelted using carbon
as a reducing agent. Some metals, such as aluminium and sodium
, have no commercially practical reducing agent, and are extracted using electrolysis
instead.
Sulfide ores are not reduced directly to the metal but are roasted in air to convert them to oxides.
, United Nations Environment Programme
Metals are inherently recyclable, so in principle, can be used over and over again, minimizing these negative environmental impacts and saving energy at the same time. For example, 95% of the energy used to make aluminium from bauxite ore is saved by using recycled material.Tread lightly: Aluminium attack Carolyn Fry, Guardian.co.uk, 22 February 2008. However, levels of metals recycling are generally low. In 2010, the International Resource Panel
, hosted by the United Nations Environment Programme
(UNEP) published reports on metal stocks that exist within society Metal Stocks in Society: Scientific Synthesis 2010, International Resource Panel
, United Nations Environment Programme
and their recycling rates.The Recycling Rates of Metals: A Status Report 2010, International Resource Panel
, United Nations Environment Programme
The report authors observed that the metal stocks in society can serve as huge mines above ground. However, they warned that the recycling rates of some rare metals used in applications such as mobile phones, battery packs for hybrid cars and fuel cells, are so low that unless future end-of-life recycling rates are dramatically stepped up these critical metals will become unavailable for use in modern technology.
The two most commonly used structural metals, iron and aluminium, are also the most abundant metals in the Earth's crust.
Metals are good conductors, making them valuable in electrical appliances and for carrying an electric current over a distance with little energy lost. Electrical power grids rely on metal cables to distribute electricity. Home electrical systems, for the most part, are wired with copper wire for its good conducting properties.
The thermal conductivity of metal is useful for containers to heat materials over a flame. Metal is also used for heat sink
s to protect sensitive equipment from overheating.
The high reflectivity of some metals is important in the construction of mirrors, including precision astronomical instruments. This last property can also make metallic jewelry aesthetically appealing.
Some metals have specialized uses; radioactive metals such as uranium
and plutonium
are used in nuclear power plants to produce energy via nuclear fission
. Mercury is a liquid at room temperature and is used in switches to complete a circuit when it flows over the switch contacts. Shape memory alloy
is used for applications such as pipes, fasteners and vascular stent
s.
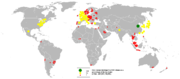 The World Bank
The World Bank
reports that China
was the top importer of ores
and metals in 2005 followed by the U.S.A. and Japan
.
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
, compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
, or alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
that is a good conductor
Electrical conductor
In physics and electrical engineering, a conductor is a material which contains movable electric charges. In metallic conductors such as copper or aluminum, the movable charged particles are electrons...
of both electricity
Electricity
Electricity is a general term encompassing a variety of phenomena resulting from the presence and flow of electric charge. These include many easily recognizable phenomena, such as lightning, static electricity, and the flow of electrical current in an electrical wire...
and heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
. Metals are usually malleable and shiny, that is they reflect most of incident light. In a metal, atoms readily lose electrons to form positive ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s (cations). Those ions are surrounded by delocalized electrons, which are responsible for the conductivity. The solid thus produced is held by electrostatic interactions between the ions and the electron cloud, which are called metallic bond
Metallic bond
Metallic bonding is the electrostatic attractive forces between the delocalized electrons, called conduction electrons, gathered in an "electron sea", and the positively charged metal ions...
s.
Definition
Metals are sometimes described as an arrangement of positive ions surrounded by a sea of delocalized electronsDelocalized electron
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or one covalent bond....
. Metals occupy the bulk of the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
, while non-metallic elements can only be found on its right-hand side. A diagonal line, drawn from boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
(B) to polonium
Polonium
Polonium is a chemical element with the symbol Po and atomic number 84, discovered in 1898 by Marie Skłodowska-Curie and Pierre Curie. A rare and highly radioactive element, polonium is chemically similar to bismuth and tellurium, and it occurs in uranium ores. Polonium has been studied for...
(Po), separates the metals from the nonmetals. Most elements on this line are metalloids, sometimes called semiconductors. This is because these elements exhibit electrical properties
Electricity
Electricity is a general term encompassing a variety of phenomena resulting from the presence and flow of electric charge. These include many easily recognizable phenomena, such as lightning, static electricity, and the flow of electrical current in an electrical wire...
common to both conductors and insulators. Elements to the lower left of this division line are called metals, while elements to the upper right of the division line are called nonmetal
Nonmetal
Nonmetal, or non-metal, is a term used in chemistry when classifying the chemical elements. On the basis of their general physical and chemical properties, every element in the periodic table can be termed either a metal or a nonmetal...
s.
An alternative definition of metal refers to the band theory. If one fills the energy bands of a material with available electrons and ends up with a top band partly filled then the material is a metal. This definition opens up the category for metallic polymers and other organic metals. These synthetic materials often have the characteristic silvery gray reflectiveness (luster) of elemental metals.
Astronomy
In the specialized usage of astronomyAstronomy
Astronomy is a natural science that deals with the study of celestial objects and phenomena that originate outside the atmosphere of Earth...
and astrophysics
Astrophysics
Astrophysics is the branch of astronomy that deals with the physics of the universe, including the physical properties of celestial objects, as well as their interactions and behavior...
, the term "metal" is often used to refer collectively to all elements other than hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
or helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
, including substances as chemically non-metallic as neon
Neon
Neon is the chemical element that has the symbol Ne and an atomic number of 10. Although a very common element in the universe, it is rare on Earth. A colorless, inert noble gas under standard conditions, neon gives a distinct reddish-orange glow when used in either low-voltage neon glow lamps or...
, fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
, and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
. Nearly all the hydrogen and helium in the Universe
Universe
The Universe is commonly defined as the totality of everything that exists, including all matter and energy, the planets, stars, galaxies, and the contents of intergalactic space. Definitions and usage vary and similar terms include the cosmos, the world and nature...
was created in Big Bang nucleosynthesis
Big Bang nucleosynthesis
In physical cosmology, Big Bang nucleosynthesis refers to the production of nuclei other than those of H-1 during the early phases of the universe...
, whereas all the "metals" were produced by nucleosynthesis
Nucleosynthesis
Nucleosynthesis is the process of creating new atomic nuclei from pre-existing nucleons . It is thought that the primordial nucleons themselves were formed from the quark–gluon plasma from the Big Bang as it cooled below two trillion degrees...
in star
Star
A star is a massive, luminous sphere of plasma held together by gravity. At the end of its lifetime, a star can also contain a proportion of degenerate matter. The nearest star to Earth is the Sun, which is the source of most of the energy on Earth...
s or supernovae
Supernova nucleosynthesis
Supernova nucleosynthesis is the production of new chemical elements inside supernovae. It occurs primarily due to explosive nucleosynthesis during explosive oxygen burning and silicon burning...
. The Sun
Sun
The Sun is the star at the center of the Solar System. It is almost perfectly spherical and consists of hot plasma interwoven with magnetic fields...
and the Milky Way Galaxy are composed of roughly 74% hydrogen, 24% helium, and 2% "metals" (the rest of the elements; atomic numbers 3–118) by mass.
The concept of a metal in the usual chemical sense is irrelevant in star
Star
A star is a massive, luminous sphere of plasma held together by gravity. At the end of its lifetime, a star can also contain a proportion of degenerate matter. The nearest star to Earth is the Sun, which is the source of most of the energy on Earth...
s, as the chemical bonds that give elements their properties cannot exist at stellar temperatures.
Chemical
Metals are usually inclined to form cations through electron loss, reacting with oxygen in the air to form oxideOxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
s over changing timescales (iron rust
Rust
Rust is a general term for a series of iron oxides. In colloquial usage, the term is applied to red oxides, formed by the reaction of iron and oxygen in the presence of water or air moisture...
s over years, while potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
burns in seconds). Examples:
- 4 Na + O2 → 2 Na2O (sodium oxide)
- 2 Ca + O2 → 2 CaO (calcium oxide)
- 4 Al + 3 O2 → 2 Al2O3 (aluminium oxide).
The transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
s (such as iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
, copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
, zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
, and nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
) take much longer to oxidize. Others, like palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
, platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
and gold
Gold
Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a...
, do not react with the atmosphere at all. Some metals form a barrier layer of oxide
Oxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
on their surface which cannot be penetrated by further oxygen molecules and thus retain their shiny appearance and good conductivity for many decades (like aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
, magnesium, some steel
Steel
Steel is an alloy that consists mostly of iron and has a carbon content between 0.2% and 2.1% by weight, depending on the grade. Carbon is the most common alloying material for iron, but various other alloying elements are used, such as manganese, chromium, vanadium, and tungsten...
s, and titanium
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color....
). The oxide
Oxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
s of metals are generally basic
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
, as opposed to those of nonmetals, which are acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
ic.
Painting
Painting
Painting is the practice of applying paint, pigment, color or other medium to a surface . The application of the medium is commonly applied to the base with a brush but other objects can be used. In art, the term painting describes both the act and the result of the action. However, painting is...
, anodizing or plating
Plating
Plating is a surface covering in which a metal is deposited on a conductive surface. Plating has been done for hundreds of years, but it is also critical for modern technology...
metals are good ways to prevent their corrosion
Corrosion
Corrosion is the disintegration of an engineered material into its constituent atoms due to chemical reactions with its surroundings. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen...
. However, a more reactive metal in the electrochemical series must be chosen for coating, especially when chipping of the coating is expected. Water and the two metals form an electrochemical cell
Electrochemical cell
An electrochemical cell is a device capable of either deriving electrical energy from chemical reactions, or facilitating chemical reactions through the introduction of electrical energy. A common example of an electrochemical cell is a standard 1.5-volt "battery"...
, and if the coating is less reactive than the coatee, the coating actually promotes corrosion.
Physical

Thermal conductivity
In physics, thermal conductivity, k, is the property of a material's ability to conduct heat. It appears primarily in Fourier's Law for heat conduction....
, luster
Lustre (mineralogy)
Lustre is a description of the way light interacts with the surface of a crystal, rock, or mineral. The word lustre traces its origins back to the Latin word lux, meaning "light", and generally implies radiance, gloss, or brilliance....
and density
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
, and the ability to be deformed under stress without cleaving
Cleavage (crystal)
Cleavage, in mineralogy, is the tendency of crystalline materials to split along definite crystallographic structural planes. These planes of relative weakness are a result of the regular locations of atoms and ions in the crystal, which create smooth repeating surfaces that are visible both in the...
. While there are several metals that have low density, hardness, and melting points, these (the alkali and alkaline earth metals) are extremely reactive, and are rarely encountered in their elemental, metallic form. Optically speaking, metals are opaque, shiny and lustrous. This is because visible lightwaves are not readily transmitted through the bulk of their microstructure
Microstructure
Microstructure is defined as the structure of a prepared surface or thin foil of material as revealed by a microscope above 25× magnification...
. The large number of mobile electrons in any typical metallic solid (element or alloy) is responsible for the fact that they can never be categorized as transparent materials.
The majority of metals have higher densities
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
than the majority of nonmetals. Nonetheless, there is wide variation in the densities of metals; lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
is the least dense solid element and osmium
Osmium
Osmium is a chemical element with the symbol Os and atomic number 76. Osmium is a hard, brittle, blue-gray or blue-blacktransition metal in the platinum family, and is the densest natural element. Osmium is twice as dense as lead. The density of osmium is , slightly greater than that of iridium,...
is the densest. The metals of groups I A and II A are referred to as the light metals because they are exceptions to this generalization. The high density of most metals is due to the tightly packed crystal lattice of the metallic structure. The strength of metallic bonds for different metals reaches a maximum around the center of the transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
series, as those elements have large amounts of delocalized electrons in tight binding type metallic bonds. However, other factors (such as atomic radius
Atomic radius
The atomic radius of a chemical element is a measure of the size of its atoms, usually the mean or typical distance from the nucleus to the boundary of the surrounding cloud of electrons...
, nuclear charge, number of bonding orbitals
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
, overlap of orbital energies, and crystal form) are involved as well.. Most non-ferrous metals can be recycled many times during their life cycle.
Electrical
The electrical and thermal conductivity of metals originate from the fact that in the metallic bondMetallic bond
Metallic bonding is the electrostatic attractive forces between the delocalized electrons, called conduction electrons, gathered in an "electron sea", and the positively charged metal ions...
, the outer electrons of the metal atoms form a gas of nearly free electrons, moving as an electron gas in a background of positive charge formed by the ion cores. Good mathematical predictions for electrical conductivity, as well as the electrons' contribution to the heat capacity and heat conductivity of metals can be calculated from the free electron model
Free electron model
In solid-state physics, the free electron model is a simple model for the behaviour of valence electrons in a crystal structure of a metallic solid. It was developed principally by Arnold Sommerfeld who combined the classical Drude model with quantum mechanical Fermi-Dirac statistics and hence it...
, which does not take the detailed structure of the ion lattice into account.
When considering the exact band structure and binding energy of a metal, it is necessary to take into account the positive potential caused by the specific arrangement of the ion cores – which is periodic in crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
s. The most important consequence of the periodic potential is the formation of a small band gap
Band gap
In solid state physics, a band gap, also called an energy gap or bandgap, is an energy range in a solid where no electron states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference between the top of the valence band and the...
at the boundary of the Brillouin zone
Brillouin zone
In mathematics and solid state physics, the first Brillouin zone is a uniquely defined primitive cell in reciprocal space. The boundaries of this cell are given by planes related to points on the reciprocal lattice. It is found by the same method as for the Wigner–Seitz cell in the Bravais lattice...
. Mathematically, the potential of the ion cores can be treated by various models, the simplest being the nearly free electron model.
Mechanical
Mechanical properties of metals include ductilityDuctility
In materials science, ductility is a solid material's ability to deform under tensile stress; this is often characterized by the material's ability to be stretched into a wire. Malleability, a similar property, is a material's ability to deform under compressive stress; this is often characterized...
, which is largely due to their inherent capacity for plastic deformation. Reversible elasticity in metals can be described by Hooke's Law
Hooke's law
In mechanics, and physics, Hooke's law of elasticity is an approximation that states that the extension of a spring is in direct proportion with the load applied to it. Many materials obey this law as long as the load does not exceed the material's elastic limit. Materials for which Hooke's law...
for restoring forces, where the stress is linearly proportional to the strain
Deformation (mechanics)
Deformation in continuum mechanics is the transformation of a body from a reference configuration to a current configuration. A configuration is a set containing the positions of all particles of the body...
. Forces larger than the elastic limit, or heat, may cause a permanent (irreversible) deformation of the object, known as plastic deformation or plasticity
Plasticity (physics)
In physics and materials science, plasticity describes the deformation of a material undergoing non-reversible changes of shape in response to applied forces. For example, a solid piece of metal being bent or pounded into a new shape displays plasticity as permanent changes occur within the...
. This irreversible change in atomic arrangement may occur as a result of:
- The action of an applied force (or work). An applied force may be tensileTensile strengthUltimate tensile strength , often shortened to tensile strength or ultimate strength, is the maximum stress that a material can withstand while being stretched or pulled before necking, which is when the specimen's cross-section starts to significantly contract...
(pulling) force, compressiveCompressive strengthCompressive strength is the capacity of a material or structure to withstand axially directed pushing forces. When the limit of compressive strength is reached, materials are crushed. Concrete can be made to have high compressive strength, e.g...
(pushing) force, shearSimple shearIn fluid mechanics, simple shear is a special case of deformation where only one component of velocity vectors has a non-zero value:\ V_x=f\ V_y=V_z=0And the gradient of velocity is constant and perpendicular to the velocity itself:...
, bendingBendingIn engineering mechanics, bending characterizes the behavior of a slender structural element subjected to an external load applied perpendicularly to a longitudinal axis of the element. The structural element is assumed to be such that at least one of its dimensions is a small fraction, typically...
or torsionTorsion (mechanics)In solid mechanics, torsion is the twisting of an object due to an applied torque. In sections perpendicular to the torque axis, the resultant shear stress in this section is perpendicular to the radius....
(twisting) forces.
- A change in temperatureTemperatureTemperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
(or heatHeatIn physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
). A temperature change may affect the mobility of the structural defectsCrystallographic defectCrystalline solids exhibit a periodic crystal structure. The positions of atoms or molecules occur on repeating fixed distances, determined by the unit cell parameters. However, the arrangement of atom or molecules in most crystalline materials is not perfect...
such as grain boundaries, point vacancies, line and screw dislocationDislocationIn materials science, a dislocation is a crystallographic defect, or irregularity, within a crystal structure. The presence of dislocations strongly influences many of the properties of materials...
s, stacking faults and twins in both crystalline and non-crystallineAmorphous solidIn condensed matter physics, an amorphous or non-crystalline solid is a solid that lacks the long-range order characteristic of a crystal....
solids. The movement or displacement of such mobile defects is thermally activatedActivation energyIn chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
, and thus limited by the rate of atomic diffusionAtomic diffusionAtomic diffusion is a diffusion process whereby the random thermally-activated movement of atoms in a solid results in the net transport of atoms. For example, helium atoms inside a balloon can diffuse through the wall of the balloon and escape, resulting in the balloon slowly deflating. Other air...
.

Slip
- In science and technology :* Slip , an aqueous suspension of minerals, and frequently deflocculant.* Slip , a positional displacement in a sequence of transmitted symbols...
, creep
Creep (deformation)
In materials science, creep is the tendency of a solid material to slowly move or deform permanently under the influence of stresses. It occurs as a result of long term exposure to high levels of stress that are below the yield strength of the material....
and fatigue
Fatigue (material)
'In materials science, fatigue is the progressive and localized structural damage that occurs when a material is subjected to cyclic loading. The nominal maximum stress values are less than the ultimate tensile stress limit, and may be below the yield stress limit of the material.Fatigue occurs...
in metals. It can also contribute to significant changes in the microstructure like grain growth
Grain growth
Grain growth is the increase in size of grains in a material at high temperature. This occurs when recovery and recrystallisation are complete and further reduction in the internal energy can only be achieved by reducing the total area of grain boundary...
and localized densification due to the elimination of intergranular porosity
Porosity
Porosity or void fraction is a measure of the void spaces in a material, and is a fraction of the volume of voids over the total volume, between 0–1, or as a percentage between 0–100%...
. Screw dislocation
Dislocation
In materials science, a dislocation is a crystallographic defect, or irregularity, within a crystal structure. The presence of dislocations strongly influences many of the properties of materials...
s may slip
Slip
- In science and technology :* Slip , an aqueous suspension of minerals, and frequently deflocculant.* Slip , a positional displacement in a sequence of transmitted symbols...
in the direction of any lattice plane
Lattice plane
In crystallography, a lattice plane of a given Bravais lattice is a plane whose intersections with the lattice are periodic and intersect the Bravais lattice; equivalently, a lattice plane is any plane containing at least three noncollinear Bravais lattice points...
containing the dislocation, while the principal driving force for "dislocation climb" is the movement or diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
of vacancies through a crystal lattice.
In addition, the nondirectional nature of metallic bonding is also thought to contribute significantly to the ductility of most metallic solids. When the planes of an ionic bond
Ionic bond
An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. Pure ionic bonding cannot exist: all ionic compounds have some...
slide past one another, the resultant change in location shifts ions of the same charge into close proximity, resulting in the cleavage
Cleavage (crystal)
Cleavage, in mineralogy, is the tendency of crystalline materials to split along definite crystallographic structural planes. These planes of relative weakness are a result of the regular locations of atoms and ions in the crystal, which create smooth repeating surfaces that are visible both in the...
of the crystal; such shift is not observed in covalently bonded
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
crystals where fracture and crystal fragment
Fragment
Fragment may refer to:* A small part/portion broken off something; debris* Fragment , all the data necessary to generate a pixel in the frame buffer* Sentence fragment, a sentence not containing a subject or a predicate...
ation occurs.
Alloys
An alloy is a mixture of two or more elementsChemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
in solid solution
Solid solution
A solid solution is a solid-state solution of one or more solutes in a solvent. Such a mixture is considered a solution rather than a compound when the crystal structure of the solvent remains unchanged by addition of the solutes, and when the mixture remains in a single homogeneous phase...
in which the major component is a metal. Most pure metals are either too soft, brittle or chemically reactive for practical use. Combining different ratios of metals as alloys modifies the properties of pure metals to produce desirable characteristics. The aim of making alloys is generally to make them less brittle, harder, resistant to corrosion, or have a more desirable color and luster. Of all the metallic alloys in use today, the alloys of iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
(steel
Steel
Steel is an alloy that consists mostly of iron and has a carbon content between 0.2% and 2.1% by weight, depending on the grade. Carbon is the most common alloying material for iron, but various other alloying elements are used, such as manganese, chromium, vanadium, and tungsten...
, stainless steel
Stainless steel
In metallurgy, stainless steel, also known as inox steel or inox from French "inoxydable", is defined as a steel alloy with a minimum of 10.5 or 11% chromium content by mass....
, cast iron
Cast iron
Cast iron is derived from pig iron, and while it usually refers to gray iron, it also identifies a large group of ferrous alloys which solidify with a eutectic. The color of a fractured surface can be used to identify an alloy. White cast iron is named after its white surface when fractured, due...
, tool steel
Tool steel
Tool steel refers to a variety of carbon and alloy steels that are particularly well-suited to be made into tools. Their suitability comes from their distinctive hardness, resistance to abrasion, their ability to hold a cutting edge, and/or their resistance to deformation at elevated temperatures...
, alloy steel) make up the largest proportion both by quantity and commercial value. Iron alloyed with various proportions of carbon gives low, mid and high carbon steels, with increasing carbon levels reducing ductility and toughness. The addition of silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
will produce cast irons, while the addition of chromium
Chromium
Chromium is a chemical element which has the symbol Cr and atomic number 24. It is the first element in Group 6. It is a steely-gray, lustrous, hard metal that takes a high polish and has a high melting point. It is also odorless, tasteless, and malleable...
, nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
and molybdenum
Molybdenum
Molybdenum , is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek , meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores...
to carbon steels (more than 10%) results in stainless steels.
Other significant metallic alloys are those of aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
, titanium
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color....
, copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
and magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
. Copper alloys have been known since prehistory—bronze
Bronze
Bronze is a metal alloy consisting primarily of copper, usually with tin as the main additive. It is hard and brittle, and it was particularly significant in antiquity, so much so that the Bronze Age was named after the metal...
gave the Bronze Age
Bronze Age
The Bronze Age is a period characterized by the use of copper and its alloy bronze as the chief hard materials in the manufacture of some implements and weapons. Chronologically, it stands between the Stone Age and Iron Age...
its name—and have many applications today, most importantly in electrical wiring. The alloys of the other three metals have been developed relatively recently; due to their chemical reactivity they require electrolytic
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
extraction processes. The alloys of aluminium, titanium and magnesium are valued for their high strength-to-weight ratios; magnesium can also provide electromagnetic shielding
Electromagnetic shielding
Electromagnetic shielding is the process of reducing the electromagnetic field in a space by blocking the field with barriers made of conductive and/or magnetic materials. Shielding is typically applied to enclosures to isolate electrical devices from the 'outside world' and to cables to isolate...
. These materials are ideal for situations where high strength-to-weight ratio is more important than material cost, such as in aerospace and some automotive applications.
Alloys specially designed for highly demanding applications, such as jet engine
Jet engine
A jet engine is a reaction engine that discharges a fast moving jet to generate thrust by jet propulsion and in accordance with Newton's laws of motion. This broad definition of jet engines includes turbojets, turbofans, rockets, ramjets, pulse jets...
s, may contain more than ten elements.
Base metal
In chemistryChemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, the term base metal is used informally to refer to a metal that oxidizes or corrodes
Corrosion
Corrosion is the disintegration of an engineered material into its constituent atoms due to chemical reactions with its surroundings. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen...
relatively easily, and reacts variably with dilute hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
(HCl) to form hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
. Examples include iron, nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
, lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
and zinc. Copper is considered a base metal as it oxidizes relatively easily, although it does not react with HCl. It is commonly used in opposition to noble metal
Noble metal
Noble metals are metals that are resistant to corrosion and oxidation in moist air, unlike most base metals. They tend to be precious, often due to their rarity in the Earth's crust...
.
In alchemy
Alchemy
Alchemy is an influential philosophical tradition whose early practitioners’ claims to profound powers were known from antiquity. The defining objectives of alchemy are varied; these include the creation of the fabled philosopher's stone possessing powers including the capability of turning base...
, a base metal was a common and inexpensive metal, as opposed to precious metal
Precious metal
A precious metal is a rare, naturally occurring metallic chemical element of high economic value.Chemically, the precious metals are less reactive than most elements, have high lustre, are softer or more ductile, and have higher melting points than other metals...
s, mainly gold and silver. A longtime goal of the alchemists was the transmutation of base metals into precious metals.
In numismatics
Numismatics
Numismatics is the study or collection of currency, including coins, tokens, paper money, and related objects. While numismatists are often characterized as students or collectors of coins, the discipline also includes the broader study of money and other payment media used to resolve debts and the...
, coins used to derive their value primarily from the precious metal
Precious metal
A precious metal is a rare, naturally occurring metallic chemical element of high economic value.Chemically, the precious metals are less reactive than most elements, have high lustre, are softer or more ductile, and have higher melting points than other metals...
content. Most modern currencies are fiat currency, allowing the coins to be made of base metal.
Ferrous metal
The term "ferrous" is derived from the Latin word meaning "containing iron". This can include pure iron, such as wrought ironWrought iron
thumb|The [[Eiffel tower]] is constructed from [[puddle iron]], a form of wrought ironWrought iron is an iron alloy with a very low carbon...
, or an alloy such as steel
Steel
Steel is an alloy that consists mostly of iron and has a carbon content between 0.2% and 2.1% by weight, depending on the grade. Carbon is the most common alloying material for iron, but various other alloying elements are used, such as manganese, chromium, vanadium, and tungsten...
. Ferrous metals are often magnetic
Magnetism
Magnetism is a property of materials that respond at an atomic or subatomic level to an applied magnetic field. Ferromagnetism is the strongest and most familiar type of magnetism. It is responsible for the behavior of permanent magnets, which produce their own persistent magnetic fields, as well...
, but not exclusively.
Noble metal
Noble metals are metals that are resistant to corrosionCorrosion
Corrosion is the disintegration of an engineered material into its constituent atoms due to chemical reactions with its surroundings. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen...
or oxidation, unlike most base metal
Base metal
In chemistry, the term base metal is used informally to refer to a metal that oxidizes or corrodes relatively easily, and reacts variably with diluted hydrochloric acid to form hydrogen. Examples include iron, nickel, lead and zinc...
s. They tend to be precious metals, often due to perceived rarity. Examples include tantalum
Tantalum
Tantalum is a chemical element with the symbol Ta and atomic number 73. Previously known as tantalium, the name comes from Tantalus, a character in Greek mythology. Tantalum is a rare, hard, blue-gray, lustrous transition metal that is highly corrosion resistant. It is part of the refractory...
, gold, platinum, silver and rhodium
Rhodium
Rhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
.
Precious metal

Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
of high economic value.
Chemically, the precious metals are less reactive than most elements, have high luster
Lustre (mineralogy)
Lustre is a description of the way light interacts with the surface of a crystal, rock, or mineral. The word lustre traces its origins back to the Latin word lux, meaning "light", and generally implies radiance, gloss, or brilliance....
and high electrical conductivity. Historically, precious metals were important as currency
Currency
In economics, currency refers to a generally accepted medium of exchange. These are usually the coins and banknotes of a particular government, which comprise the physical aspects of a nation's money supply...
, but are now regarded mainly as investment and industrial commodities
Commodity
In economics, a commodity is the generic term for any marketable item produced to satisfy wants or needs. Economic commodities comprise goods and services....
. Gold
Gold
Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a...
, silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
, platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
and palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
each have an ISO 4217
ISO 4217
ISO 4217 is a standard published by the International Standards Organization, which delineates currency designators, country codes , and references to minor units in three tables:* Table A.1 – Current currency & funds code list...
currency code. The best-known precious metals are gold and silver. While both have industrial uses, they are better known for their uses in art
Art
Art is the product or process of deliberately arranging items in a way that influences and affects one or more of the senses, emotions, and intellect....
, jewelry, and coinage
Currency
In economics, currency refers to a generally accepted medium of exchange. These are usually the coins and banknotes of a particular government, which comprise the physical aspects of a nation's money supply...
. Other precious metals include the platinum group
Platinum group
The platinum group metals is a term used sometimes to collectively refer to six metallic elements clustered together in the periodic table.These elements are all transition metals, lying in the d-block .The six...
metals: ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
, rhodium
Rhodium
Rhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
, palladium, osmium
Osmium
Osmium is a chemical element with the symbol Os and atomic number 76. Osmium is a hard, brittle, blue-gray or blue-blacktransition metal in the platinum family, and is the densest natural element. Osmium is twice as dense as lead. The density of osmium is , slightly greater than that of iridium,...
, iridium
Iridium
Iridium is the chemical element with atomic number 77, and is represented by the symbol Ir. A very hard, brittle, silvery-white transition metal of the platinum family, iridium is the second-densest element and is the most corrosion-resistant metal, even at temperatures as high as 2000 °C...
, and platinum, of which platinum is the most widely traded.
The demand for precious metals is driven not only by their practical use, but also by their role as investments and a store of value
Store of value
A recognized form of exchange can be a form of money or currency, a commodity like gold, or financial capital. To act as a store of value, these forms must be able to be saved and retrieved at a later time, and be predictably useful when retrieved....
. Palladium was, as of summer 2006, valued at a little under half the price of gold, and platinum at around twice that of gold. Silver is substantially less expensive than these metals, but is often traditionally considered a precious metal for its role in coinage and jewelry.
Extraction
Metals are often extracted from the Earth by means of mining, resulting in ores that are relatively rich sources of the requisite elements. Ore is located by prospectingProspecting
Prospecting is the physical search for minerals, fossils, precious metals or mineral specimens, and is also known as fossicking.Prospecting is a small-scale form of mineral exploration which is an organised, large scale effort undertaken by mineral resource companies to find commercially viable ore...
techniques, followed by the exploration and examination of deposits. Mineral sources are generally divided into surface mines
Surface mining
Surface mining , is a type of mining in which soil and rock overlying the mineral deposit are removed...
, which are mined by excavation using heavy equipment, and subsurface mines.
Once the ore is mined, the metals must be extracted
Extractive metallurgy
Extractive metallurgy is the study of the processes used in the separation and concentration of raw materials. The field is an applied science, covering all aspects of the physical and chemical processes used to produce mineral-containing and metallic materials, sometimes for direct use as a...
, usually by chemical or electrolytic reduction. Pyrometallurgy
Pyrometallurgy
Pyrometallurgy is a branch of extractive metallurgy. It consists of the thermal treatment of minerals and metallurgical ores and concentrates to bring about physical and chemical transformations in the materials to enable recovery of valuable metals...
uses high temperatures to convert ore into raw metals, while hydrometallurgy
Hydrometallurgy
Hydrometallurgy is part of the field of extractive metallurgy involving the use of aqueous chemistry for the recovery of metals from ores, concentrates, and recycled or residual materials...
employs aqueous chemistry for the same purpose. The methods used depend on the metal and their contaminants.
When a metal ore is an ionic compound of that metal and a non-metal, the ore must usually be smelted
Smelting
Smelting is a form of extractive metallurgy; its main use is to produce a metal from its ore. This includes iron extraction from iron ore, and copper extraction and other base metals from their ores...
— heated with a reducing agent — to extract the pure metal. Many common metals, such as iron, are smelted using carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
as a reducing agent. Some metals, such as aluminium and sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
, have no commercially practical reducing agent, and are extracted using electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
instead.
Sulfide ores are not reduced directly to the metal but are roasted in air to convert them to oxides.
Recycling of metals
Demand for metals is closely linked to economic growth. During the 20th century, the variety of metals uses in society grew rapidly. Today, the development of major nations, such as China and India, and advances in technologies, are fuelling ever more demand. The result is that mining activities are expanding, and more and more of the world’s metal stocks are above ground in use, rather than below ground as unused reserves. An example is the in-use stock of copper. Between 1932 and 1999, copper in use in the USA rose from 73g to 238g per person.The Recycling Rates of Metals: A Status Report 2010, International Resource PanelInternational Resource Panel
The International Resource Panel is a scientific panel of experts that aims to help nations use natural resources sustainably without compromising economic growth and human needs...
, United Nations Environment Programme
United Nations Environment Programme
The United Nations Environment Programme coordinates United Nations environmental activities, assisting developing countries in implementing environmentally sound policies and practices. It was founded as a result of the United Nations Conference on the Human Environment in June 1972 and has its...
Metals are inherently recyclable, so in principle, can be used over and over again, minimizing these negative environmental impacts and saving energy at the same time. For example, 95% of the energy used to make aluminium from bauxite ore is saved by using recycled material.Tread lightly: Aluminium attack Carolyn Fry, Guardian.co.uk, 22 February 2008. However, levels of metals recycling are generally low. In 2010, the International Resource Panel
International Resource Panel
The International Resource Panel is a scientific panel of experts that aims to help nations use natural resources sustainably without compromising economic growth and human needs...
, hosted by the United Nations Environment Programme
United Nations Environment Programme
The United Nations Environment Programme coordinates United Nations environmental activities, assisting developing countries in implementing environmentally sound policies and practices. It was founded as a result of the United Nations Conference on the Human Environment in June 1972 and has its...
(UNEP) published reports on metal stocks that exist within society Metal Stocks in Society: Scientific Synthesis 2010, International Resource Panel
International Resource Panel
The International Resource Panel is a scientific panel of experts that aims to help nations use natural resources sustainably without compromising economic growth and human needs...
, United Nations Environment Programme
United Nations Environment Programme
The United Nations Environment Programme coordinates United Nations environmental activities, assisting developing countries in implementing environmentally sound policies and practices. It was founded as a result of the United Nations Conference on the Human Environment in June 1972 and has its...
and their recycling rates.The Recycling Rates of Metals: A Status Report 2010, International Resource Panel
International Resource Panel
The International Resource Panel is a scientific panel of experts that aims to help nations use natural resources sustainably without compromising economic growth and human needs...
, United Nations Environment Programme
United Nations Environment Programme
The United Nations Environment Programme coordinates United Nations environmental activities, assisting developing countries in implementing environmentally sound policies and practices. It was founded as a result of the United Nations Conference on the Human Environment in June 1972 and has its...
The report authors observed that the metal stocks in society can serve as huge mines above ground. However, they warned that the recycling rates of some rare metals used in applications such as mobile phones, battery packs for hybrid cars and fuel cells, are so low that unless future end-of-life recycling rates are dramatically stepped up these critical metals will become unavailable for use in modern technology.
Metallurgy
Metallurgy is a domain of materials science that studies the physical and chemical behavior of metallic elements, their intermetallic compounds, and their mixtures, which are called alloys.Applications
Some metals and metal alloys possess high structural strength per unit mass, making them useful materials for carrying large loads or resisting impact damage. Metal alloys can be engineered to have high resistance to shear, torque and deformation. However the same metal can also be vulnerable to fatigue damage through repeated use or from sudden stress failure when a load capacity is exceeded. The strength and resilience of metals has led to their frequent use in high-rise building and bridge construction, as well as most vehicles, many appliances, tools, pipes, non-illuminated signs and railroad tracks.The two most commonly used structural metals, iron and aluminium, are also the most abundant metals in the Earth's crust.
Metals are good conductors, making them valuable in electrical appliances and for carrying an electric current over a distance with little energy lost. Electrical power grids rely on metal cables to distribute electricity. Home electrical systems, for the most part, are wired with copper wire for its good conducting properties.
The thermal conductivity of metal is useful for containers to heat materials over a flame. Metal is also used for heat sink
Heat sink
A heat sink is a term for a component or assembly that transfers heat generated within a solid material to a fluid medium, such as air or a liquid. Examples of heat sinks are the heat exchangers used in refrigeration and air conditioning systems and the radiator in a car...
s to protect sensitive equipment from overheating.
The high reflectivity of some metals is important in the construction of mirrors, including precision astronomical instruments. This last property can also make metallic jewelry aesthetically appealing.
Some metals have specialized uses; radioactive metals such as uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
and plutonium
Plutonium
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
are used in nuclear power plants to produce energy via nuclear fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
. Mercury is a liquid at room temperature and is used in switches to complete a circuit when it flows over the switch contacts. Shape memory alloy
Shape memory alloy
A shape-memory alloy is an alloy that "remembers" its original, cold-forged shape: returning the pre-deformed shape by heating. This material is a lightweight, solid-state alternative to conventional actuators such as hydraulic, pneumatic, and motor-based systems...
is used for applications such as pipes, fasteners and vascular stent
Stent
In the technical vocabulary of medicine, a stent is an artificial 'tube' inserted into a natural passage/conduit in the body to prevent, or counteract, a disease-induced, localized flow constriction. The term may also refer to a tube used to temporarily hold such a natural conduit open to allow...
s.
Trade

World Bank
The World Bank is an international financial institution that provides loans to developing countries for capital programmes.The World Bank's official goal is the reduction of poverty...
reports that China
People's Republic of China
China , officially the People's Republic of China , is the most populous country in the world, with over 1.3 billion citizens. Located in East Asia, the country covers approximately 9.6 million square kilometres...
was the top importer of ores
Orés
Orés is a municipality in the Cinco Villas, in the province of Zaragoza, in the autonomous community of Aragon, Spain. It belongs to the comarca of Cinco Villas. It is placed 104 km to the northwest of the provincial capital city, Zaragoza. Its coordinates are: 42° 17' N, 1° 00' W, and is...
and metals in 2005 followed by the U.S.A. and Japan
Japan
Japan is an island nation in East Asia. Located in the Pacific Ocean, it lies to the east of the Sea of Japan, China, North Korea, South Korea and Russia, stretching from the Sea of Okhotsk in the north to the East China Sea and Taiwan in the south...
.
See also
- Amorphous metalAmorphous metalAn amorphous metal is a metallic material with a disordered atomic-scale structure. In contrast to most metals, which are crystalline and therefore have a highly ordered arrangement of atoms, amorphous alloys are non-crystalline...
- ASM International (society)
- DuctilityDuctilityIn materials science, ductility is a solid material's ability to deform under tensile stress; this is often characterized by the material's ability to be stretched into a wire. Malleability, a similar property, is a material's ability to deform under compressive stress; this is often characterized...
- Electric field screeningElectric field screeningScreening is the damping of electric fields caused by the presence of mobile charge carriers. It is an important part of the behavior of charge-carrying fluids, such as ionized gases and conduction electrons in semiconductors and metals....
- Metal theftMetal theftMetal theft is the theft of metal items on a very large scale. These thefts usually increase when worldwide prices for scrap metal rise. In recent years, prices for metals have risen dramatically due to rapid industrialization in India and China...
- MetalworkingMetalworkingMetalworking is the process of working with metals to create individual parts, assemblies, or large scale structures. The term covers a wide range of work from large ships and bridges to precise engine parts and delicate jewelry. It therefore includes a correspondingly wide range of skills,...
- Periodic table (metals and non-metals)
- Properties and uses of metals
- SolidSolidSolid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
- Spin transitionSpin transitionThe spin transition is an example of transition between two electronic states in molecular chemistry. The ability of an electron to transit from a stable to another stable electronic state in a reversible and detectable fashion, makes these molecular systems appealing in the field of molecular...
- SteelSteelSteel is an alloy that consists mostly of iron and has a carbon content between 0.2% and 2.1% by weight, depending on the grade. Carbon is the most common alloying material for iron, but various other alloying elements are used, such as manganese, chromium, vanadium, and tungsten...
- Structural steelStructural steelStructural steel is steel construction material, a profile, formed with a specific shape or cross section and certain standards of chemical composition and mechanical properties...
- Transition metalTransition metalThe term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...

