
Pourbaix diagram
Encyclopedia
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, a Pourbaix diagram, also known as a potential/pH diagram, maps out possible stable (equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
) phases of an aqueous electrochemical system. Predominant ion boundaries are represented by lines. As such a Pourbaix diagram can be read much like a standard phase diagram
Phase diagram
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases can occur at equilibrium...
with a different set of axes. But like phase diagrams, they do not allow for reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
or kinetic effects.
The diagrams are named after Marcel Pourbaix
Marcel Pourbaix
Marcel Pourbaix was born on 16 September 1904 in Myshega , where his father was a consultant on an engineering project. A brilliant chemist, he performed his most well known research at the University of Brussels, studying corrosion. His biggest achievement is the derivation of potential-pH,...
(1904–1998), the Russia
Russia
Russia or , officially known as both Russia and the Russian Federation , is a country in northern Eurasia. It is a federal semi-presidential republic, comprising 83 federal subjects...
n-born, Belgian chemist
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
who invented them.
Diagram

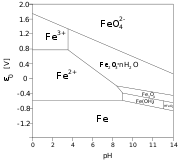
The vertical axis is labeled Eh for the voltage potential with respect to the standard hydrogen electrode
Standard hydrogen electrode
The standard hydrogen electrode , is a redox electrode which forms the basis of the thermodynamic scale of oxidation-reduction potentials...
(SHE) as calculated by the Nernst equation
Nernst equation
In electrochemistry, the Nernst equation is an equation that can be used to determine the equilibrium reduction potential of a half-cell in an electrochemical cell. It can also be used to determine the total voltage for a full electrochemical cell...
. The "h" stands for Hydrogen, although other standards may be used.(this is for room temperature only)
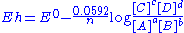
The horizontal axis is labeled pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
for the -log function of the H+ ion concentration.
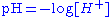
The lines are drawn for ions at unit activity (about 1 M) and represent equilibrium for that concentration. Additional lines may be drawn for other concentrations, e.g., 10−3 M or 10−6 M.
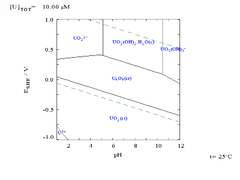
While such diagrams can be drawn for any chemical system, it is important to note that the addition of a metal binding agent (ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
) will often modify the diagram. For instance, carbonate
Carbonate
In chemistry, a carbonate is a salt of carbonic acid, characterized by the presence of the carbonate ion, . The name may also mean an ester of carbonic acid, an organic compound containing the carbonate group C2....
has a great effect upon the diagram for uranium. (See diagrams at right.)
In addition, temperature and concentration of solvated ions in solution will shift the equilibrium lines in accordance with the Nernst equation.
A simplified Pourbaix diagram indicates regions of "Immunity", "Corrosion" and "Passivity", instead of the stable species. They thus give a guide to the stability of a particular metal in a specific environment. Immunity means that the metal is not attacked, while corrosion
Corrosion
Corrosion is the disintegration of an engineered material into its constituent atoms due to chemical reactions with its surroundings. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen...
shows that general attack will occur. Passivation
Passivation
Passivation is the process of making a material "passive", and thus less reactive with surrounding air, water, or other gases or liquids. The goal is to inhibit corrosion, whether for structural or cosmetic reasons. Passivation of metals is usually achieved by the deposition of a layer of oxide...
occurs when the metal forms a stable coating of an oxide or other salt on its surface, the best example being the relative stability of aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
because of the alumina layer formed on its surface when exposed to air.

