
Faraday constant
Encyclopedia
In physics
and chemistry
, the Faraday constant (named after Michael Faraday
) is the magnitude of electric charge
per mole
of electron
s. It has the currently accepted value
The constant F has a simple relation to two other physical constants: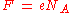
where:
NA is the Avogadro constant, and e is the elementary charge
or the magnitude of the charge
of an electron
. This relation is true because the amount of charge of a mole of electrons is equal to the amount of charge in one electron multiplied by the number of electrons in a mole.
The value of F was first determined by weighing the amount of silver
deposited in an electrochemical reaction in which a measured current was passed for a measured time, and using Faraday's law of electrolysis. Research is continuing into more accurate ways of determining the interrelated constants F, NA, and e.
Expressed in faradays, the Faraday constant F equals "1 faraday of charge per mole".
This faraday unit is not to be confused with the farad
, an unrelated unit of capacitance
.
Physics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
and chemistry
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, the Faraday constant (named after Michael Faraday
Michael Faraday
Michael Faraday, FRS was an English chemist and physicist who contributed to the fields of electromagnetism and electrochemistry....
) is the magnitude of electric charge
Electric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
per mole
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
of electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s. It has the currently accepted value
Value
Value or values may refer to:Concepts of worth:* Value theory – overview of approaches in various disciplines* Value ** Value * Value ** Theory of value ** Value investing...
The constant F has a simple relation to two other physical constants:
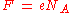
where:
NA is the Avogadro constant, and e is the elementary charge
Elementary charge
The elementary charge, usually denoted as e, is the electric charge carried by a single proton, or equivalently, the absolute value of the electric charge carried by a single electron. This elementary charge is a fundamental physical constant. To avoid confusion over its sign, e is sometimes called...
or the magnitude of the charge
Electric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
of an electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
. This relation is true because the amount of charge of a mole of electrons is equal to the amount of charge in one electron multiplied by the number of electrons in a mole.
The value of F was first determined by weighing the amount of silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
deposited in an electrochemical reaction in which a measured current was passed for a measured time, and using Faraday's law of electrolysis. Research is continuing into more accurate ways of determining the interrelated constants F, NA, and e.
Other Common Units of Faraday's Constant
- 96.485 kJ per volt gram equivalent
- 23.061 kcal per volt gram equivalent
- 26.801 A*h/mol
Faraday unit of charge
Related to Faraday's constant is the "faraday", a unit of electrical charge. It is much less common than the coulomb, but sometimes used in electrochemistry. One Faraday of charge is the magnitude of the charge of one mole of electrons, i.e.Expressed in faradays, the Faraday constant F equals "1 faraday of charge per mole".
This faraday unit is not to be confused with the farad
Farad
The farad is the SI unit of capacitance. The unit is named after the English physicist Michael Faraday.- Definition :A farad is the charge in coulombs which a capacitor will accept for the potential across it to change 1 volt. A coulomb is 1 ampere second...
, an unrelated unit of capacitance
Capacitance
In electromagnetism and electronics, capacitance is the ability of a capacitor to store energy in an electric field. Capacitance is also a measure of the amount of electric potential energy stored for a given electric potential. A common form of energy storage device is a parallel-plate capacitor...
.
See also
- FaradFaradThe farad is the SI unit of capacitance. The unit is named after the English physicist Michael Faraday.- Definition :A farad is the charge in coulombs which a capacitor will accept for the potential across it to change 1 volt. A coulomb is 1 ampere second...
, unit of capacitance - Michael FaradayMichael FaradayMichael Faraday, FRS was an English chemist and physicist who contributed to the fields of electromagnetism and electrochemistry....
- Faraday cageFaraday cageA Faraday cage or Faraday shield is an enclosure formed by conducting material or by a mesh of such material. Such an enclosure blocks out external static and non-static electric fields...
- Faraday efficiencyFaraday efficiencyFaraday efficiency describes the efficiency with which charge are transferred in a system facilitating an electrochemical reaction. The word "faraday" in this term has two interrelated aspects...
- Faraday's law of electrolysis
- Faraday's law of Electromagnetic inductionElectromagnetic inductionElectromagnetic induction is the production of an electric current across a conductor moving through a magnetic field. It underlies the operation of generators, transformers, induction motors, electric motors, synchronous motors, and solenoids....

