Daniell cell
Encyclopedia
The Daniell cell was invented in 1836 by John Frederic Daniell
, a British chemist
and meteorologist, and consisted of a copper pot filled with a copper sulfate solution, in which was immersed an unglazed earthenware
container filled with sulfuric acid
and a zinc electrode. He was searching for a way to eliminate the hydrogen bubble problem found in the Voltaic pile
, and his solution was to use a second electrolyte to consume the hydrogen produced by the first. Zinc sulfate
may be substituted for the sulfuric acid. The Daniell cell was a great improvement over the existing technology used in the early days of battery
development. A later variant of the Daniell cell called the gravity cell or crowfoot cell was invented in the 1860s by a Frenchman named Callaud and became a popular choice for electrical telegraph
y.
physician Golding Bird
who used a plaster of Paris barrier to keep the solutions separate. Bird's experiments with this cell were of some importance to the new discipline of electrometallurgy
. A surprising result from Bird's experiments was the deposition of copper on and within the plaster without any contact with the metal electrodes. So surprising, in fact, that it was at first disbelieved by electrochemical investigators, including Michael Faraday
. Deposition of copper, and other metals, had been previously noted, but always previously it had been metal on metal electrode. Bird's cell was the basis for the development of the porous pot cell.
anode
dipped into a porous earthenware pot containing a zinc sulfate
solution. The porous pot is, in turn, immersed in a solution of copper sulfate contained in a copper
can, which acts as the cell's cathode
. The use of a porous barrier allows ions to pass through but keeps the solutions from mixing. Without this barrier, when no current was drawn the copper ions would drift to the zinc anode and undergo reduction
without producing a current, which would destroy the battery's life.
Over time, copper buildup would block the pores in the earthenware barrier and cut short the battery's life. Nevertheless, the Daniel cell provided a longer and more reliable current than the Voltaic pile because the electrolyte deposited copper, which is a conductor
, rather than hydrogen, which is an insulator, on the cathode. It was also safer and less corrosive. With an operating voltage of roughly 1.1 volts, it saw widespread use in telegraph networks until it was supplanted by the Leclanché cell
in the late 1860s.
 Sometime during the 1860s, a Frenchman by the name of Callaud invented a variant of the Daniell cell which dispensed with the porous barrier. Instead, a layer of zinc sulfate sat on top of a layer of copper sulfate, the two liquids kept separate by their differing densities, often with a layer of oil added on top to prevent evaporation. This reduced the internal resistance of the system and thus the battery yielded a stronger current.
Sometime during the 1860s, a Frenchman by the name of Callaud invented a variant of the Daniell cell which dispensed with the porous barrier. Instead, a layer of zinc sulfate sat on top of a layer of copper sulfate, the two liquids kept separate by their differing densities, often with a layer of oil added on top to prevent evaporation. This reduced the internal resistance of the system and thus the battery yielded a stronger current.
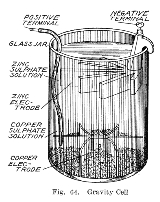
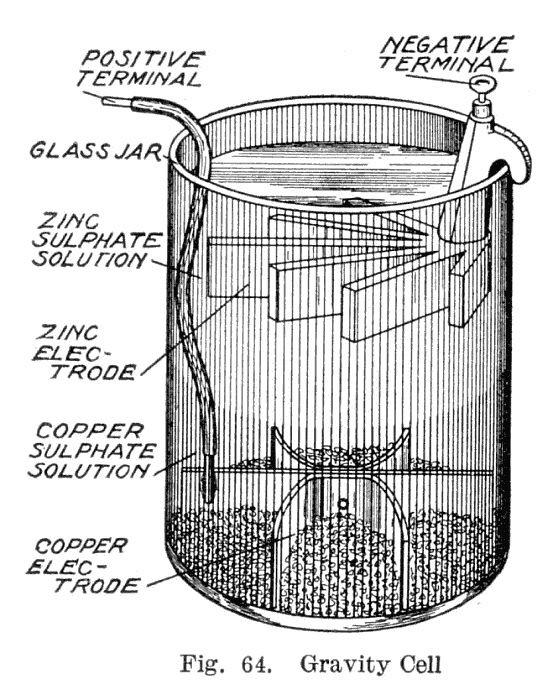
This variant, called a gravity cell, consisted of a glass jar in which a copper cathode sat on the bottom and a zinc anode was suspended beneath the rim in the zinc sulfate layer. Copper sulfate crystals would be scattered around the cathode and the jar would then be filled with distilled water. As the current was drawn, a layer of zinc sulfate solution would form at the top around the anode. This top layer was kept separate from the bottom copper sulfate layer by its lower density and by the polarity of the cell. A disadvantage of the gravity cell was that a current had to be continually drawn to keep the two solutions from mixing by diffusion, so it was unsuitable for intermittent use. In addition, it was vulnerable to loss of integrity if too much electric current
was drawn, which would also cause the layers to mix.
Sometimes called the crowfoot cell due to the distinctive shape of the electrodes, this arrangement was less costly for large multicell batteries
and it quickly became the battery of choice for the American and British telegraph networks. Even after most telegraph lines started being powered by motor-generators the gravity battery continued to be used in way stations to power the local circuit at least into the 1950s. In the telegraph industry, this battery was often assembled on site by the telegraph workers themselves, and when it ran down it could be renewed by replacing the consumed components. The zinc sulfate layer was clear in contrast to the deep blue copper sulfate layer, which allowed a technician to measure the battery life with a glance. On the other hand, this setup meant the battery could only be used in a stationary appliance, otherwise the solutions would mix or spill.
and zinc
electrodes are immersed in a solution
of copper (II) sulfate and zinc sulfate
respectively. At the anode
, zinc is oxidized per the following half reaction:
At the cathode
, copper is reduced per the following reaction:
The total reaction being:
In the Daniell cell which, due to its simplicity, is often used in classroom demonstrations, a wire
and light bulb
may connect the two electrodes. Electrons that are “pulled” from the zinc anode travel through the wire, providing an electrical current that illuminates the bulb. In such a cell, the sulfate ions play an important role. Having a negative charge, these anions build up around the anode to maintain a neutral charge. Conversely, at the cathode the copper (II) cations accumulate to maintain this neutral charge. These two processes cause copper solid to accumulate at the cathode and the zinc electrode to "dissolve" into the solution.
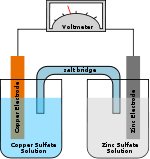
Since neither half reaction will occur independently of the other, the two half cells must be connected in a way that will allow ion
s to move freely between them. A porous barrier or ceramic
disk may be used to separate the two solutions while allowing ion flow. When the half cells are placed in two entirely different and separate containers, a salt bridge
is often used to connect the two cells. In the above wet-cell, sulfate anions move from the cathode to the anode via the salt bridge and the Zn2+ cations move in the opposite direction to maintain neutrality.
John Frederic Daniell
John Frederic Daniell was an English chemist and physicist.Daniell was born in London, and in 1831 became the first professor of chemistry at the newly founded King's College London. His name is best known for his invention of the Daniell cell , an electric battery much better than voltaic cells...
, a British chemist
Chemist
A chemist is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties such as density and acidity. Chemists carefully describe the properties they study in terms of quantities, with detail on the level of molecules and their component atoms...
and meteorologist, and consisted of a copper pot filled with a copper sulfate solution, in which was immersed an unglazed earthenware
Earthenware
Earthenware is a common ceramic material, which is used extensively for pottery tableware and decorative objects.-Types of earthenware:Although body formulations vary between countries and even between individual makers, a generic composition is 25% ball clay, 28% kaolin, 32% quartz, and 15%...
container filled with sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
and a zinc electrode. He was searching for a way to eliminate the hydrogen bubble problem found in the Voltaic pile
Voltaic pile
A voltaic pile is a set of individual Galvanic cells placed in series. The voltaic pile, invented by Alessandro Volta in 1800, was the first electric battery...
, and his solution was to use a second electrolyte to consume the hydrogen produced by the first. Zinc sulfate
Zinc sulfate
Zinc sulfate is the inorganic compound with the formula ZnSO4 as well as any of three hydrates. It was historically known as "white vitriol". It is a colorless solid that is a common source of soluble zinc ions.-Production and reactivity:...
may be substituted for the sulfuric acid. The Daniell cell was a great improvement over the existing technology used in the early days of battery
Battery (electricity)
An electrical battery is one or more electrochemical cells that convert stored chemical energy into electrical energy. Since the invention of the first battery in 1800 by Alessandro Volta and especially since the technically improved Daniell cell in 1836, batteries have become a common power...
development. A later variant of the Daniell cell called the gravity cell or crowfoot cell was invented in the 1860s by a Frenchman named Callaud and became a popular choice for electrical telegraph
Electrical telegraph
An electrical telegraph is a telegraph that uses electrical signals, usually conveyed via telecommunication lines or radio. The electromagnetic telegraph is a device for human-to-human transmission of coded text messages....
y.
Bird's cell
Originally, the Daniell cell held the two solutions in two separate, but linked, containers, an arrangement described as two half-cells. The first single-cell version of the Daniell cell was invented in 1837 by the Guy's hospitalGuy's Hospital
Guy's Hospital is a large NHS hospital in the borough of Southwark in south east London, England. It is administratively a part of Guy's and St Thomas' NHS Foundation Trust. It is a large teaching hospital and is home to the King's College London School of Medicine...
physician Golding Bird
Golding Bird
Golding Bird was a British medical doctor and Fellow of the Royal College of Physicians of London. Bird became a great authority on kidney diseases and published a comprehensive paper on urinary deposits...
who used a plaster of Paris barrier to keep the solutions separate. Bird's experiments with this cell were of some importance to the new discipline of electrometallurgy
Electrometallurgy
Electrometallurgy is the field concerned with the processes of metal electrodeposition. There are five categories of these processes:* electrowinning, the extraction of metal from ores* electrorefining, the purification of metals...
. A surprising result from Bird's experiments was the deposition of copper on and within the plaster without any contact with the metal electrodes. So surprising, in fact, that it was at first disbelieved by electrochemical investigators, including Michael Faraday
Michael Faraday
Michael Faraday, FRS was an English chemist and physicist who contributed to the fields of electromagnetism and electrochemistry....
. Deposition of copper, and other metals, had been previously noted, but always previously it had been metal on metal electrode. Bird's cell was the basis for the development of the porous pot cell.
Porous pot cell
The porous pot version of the Daniell cell was invented by John Dancer, a Liverpool instrument maker, in 1838. It consists of a central zincZinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
dipped into a porous earthenware pot containing a zinc sulfate
Zinc sulfate
Zinc sulfate is the inorganic compound with the formula ZnSO4 as well as any of three hydrates. It was historically known as "white vitriol". It is a colorless solid that is a common source of soluble zinc ions.-Production and reactivity:...
solution. The porous pot is, in turn, immersed in a solution of copper sulfate contained in a copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
can, which acts as the cell's cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
. The use of a porous barrier allows ions to pass through but keeps the solutions from mixing. Without this barrier, when no current was drawn the copper ions would drift to the zinc anode and undergo reduction
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
without producing a current, which would destroy the battery's life.
Over time, copper buildup would block the pores in the earthenware barrier and cut short the battery's life. Nevertheless, the Daniel cell provided a longer and more reliable current than the Voltaic pile because the electrolyte deposited copper, which is a conductor
Electrical conductor
In physics and electrical engineering, a conductor is a material which contains movable electric charges. In metallic conductors such as copper or aluminum, the movable charged particles are electrons...
, rather than hydrogen, which is an insulator, on the cathode. It was also safer and less corrosive. With an operating voltage of roughly 1.1 volts, it saw widespread use in telegraph networks until it was supplanted by the Leclanché cell
Leclanché cell
Georges Leclanché invented and patented his battery, the Leclanché cell, in 1866. The battery contained a conducting solution of ammonium chloride, a cathode of carbon, a depolarizer of manganese dioxide, and an anode of zinc...
in the late 1860s.
Gravity cell

This variant, called a gravity cell, consisted of a glass jar in which a copper cathode sat on the bottom and a zinc anode was suspended beneath the rim in the zinc sulfate layer. Copper sulfate crystals would be scattered around the cathode and the jar would then be filled with distilled water. As the current was drawn, a layer of zinc sulfate solution would form at the top around the anode. This top layer was kept separate from the bottom copper sulfate layer by its lower density and by the polarity of the cell. A disadvantage of the gravity cell was that a current had to be continually drawn to keep the two solutions from mixing by diffusion, so it was unsuitable for intermittent use. In addition, it was vulnerable to loss of integrity if too much electric current
Electric current
Electric current is a flow of electric charge through a medium.This charge is typically carried by moving electrons in a conductor such as wire...
was drawn, which would also cause the layers to mix.
Sometimes called the crowfoot cell due to the distinctive shape of the electrodes, this arrangement was less costly for large multicell batteries
Electrochemical cell
An electrochemical cell is a device capable of either deriving electrical energy from chemical reactions, or facilitating chemical reactions through the introduction of electrical energy. A common example of an electrochemical cell is a standard 1.5-volt "battery"...
and it quickly became the battery of choice for the American and British telegraph networks. Even after most telegraph lines started being powered by motor-generators the gravity battery continued to be used in way stations to power the local circuit at least into the 1950s. In the telegraph industry, this battery was often assembled on site by the telegraph workers themselves, and when it ran down it could be renewed by replacing the consumed components. The zinc sulfate layer was clear in contrast to the deep blue copper sulfate layer, which allowed a technician to measure the battery life with a glance. On the other hand, this setup meant the battery could only be used in a stationary appliance, otherwise the solutions would mix or spill.
Chemistry
In the Daniell cell, copperCopper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
and zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
electrodes are immersed in a solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
of copper (II) sulfate and zinc sulfate
Zinc sulfate
Zinc sulfate is the inorganic compound with the formula ZnSO4 as well as any of three hydrates. It was historically known as "white vitriol". It is a colorless solid that is a common source of soluble zinc ions.-Production and reactivity:...
respectively. At the anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
, zinc is oxidized per the following half reaction:

- Zn(s) → Zn2+(aq) + 2e- .
At the cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
, copper is reduced per the following reaction:
- Cu2+(aq) + 2e- → Cu(s) .
The total reaction being:
- ZnZincZinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
(s) + CuCopperCopper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
2+(aq) → Zn2+(aq) + Cu(s).
In the Daniell cell which, due to its simplicity, is often used in classroom demonstrations, a wire
Wire
A wire is a single, usually cylindrical, flexible strand or rod of metal. Wires are used to bear mechanical loads and to carry electricity and telecommunications signals. Wire is commonly formed by drawing the metal through a hole in a die or draw plate. Standard sizes are determined by various...
and light bulb
Incandescent light bulb
The incandescent light bulb, incandescent lamp or incandescent light globe makes light by heating a metal filament wire to a high temperature until it glows. The hot filament is protected from air by a glass bulb that is filled with inert gas or evacuated. In a halogen lamp, a chemical process...
may connect the two electrodes. Electrons that are “pulled” from the zinc anode travel through the wire, providing an electrical current that illuminates the bulb. In such a cell, the sulfate ions play an important role. Having a negative charge, these anions build up around the anode to maintain a neutral charge. Conversely, at the cathode the copper (II) cations accumulate to maintain this neutral charge. These two processes cause copper solid to accumulate at the cathode and the zinc electrode to "dissolve" into the solution.
Since neither half reaction will occur independently of the other, the two half cells must be connected in a way that will allow ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s to move freely between them. A porous barrier or ceramic
Ceramic
A ceramic is an inorganic, nonmetallic solid prepared by the action of heat and subsequent cooling. Ceramic materials may have a crystalline or partly crystalline structure, or may be amorphous...
disk may be used to separate the two solutions while allowing ion flow. When the half cells are placed in two entirely different and separate containers, a salt bridge
Salt bridge
A salt bridge, in chemistry, is a laboratory device used to connect the oxidation and reduction half-cells of a galvanic cell , a type of electrochemical cell...
is often used to connect the two cells. In the above wet-cell, sulfate anions move from the cathode to the anode via the salt bridge and the Zn2+ cations move in the opposite direction to maintain neutrality.
See also
- Bunsen cellBunsen cellThe Bunsen cell is a zinc-carbon primary cell composed of a zinc anode in dilute sulfuric acid separated by a porous pot from a carbon cathode in nitric or chromic acid.- Cell details :...
- History of the batteryHistory of the batteryThe history of the development of electrochemical cells is crucial to the scientific study and industrial applications of electricity, for prior to the rise of electrical grids around the end of the 19th century, they were the main source of electricity...
- Primary cell terminology
External links
- Daniell Cell Interactive Tutorial National High Magnetic Field Laboratory
- Daniel Cell Experiment

