
Thermodynamic free energy
Encyclopedia
The thermodynamic free energy is the amount of work
that a thermodynamic system
can perform. The concept is useful in the thermodynamics
of chemical or thermal processes in engineering
and science. The free energy is the internal energy
of a system less the amount of energy that cannot be used to perform work. This unusable energy is given by the entropy
of a system multiplied by the temperature of the system.
Like the internal energy, the free energy is a thermodynamic state function
.
energy that is available to perform thermodynamic work
; i.e., work mediated by thermal energy
. Free energy is subject to irreversible
loss in the course of such work. Since first-law energy is always conserved, it is evident that free energy is an expendable, second-law
kind of energy that can perform work within finite amounts of time. Several free energy functions may be formulated based on system criteria. Free energy functions
are Legendre transformation
s of the internal energy
. For processes involving a system at constant pressure
p and temperature
T, the Gibbs free energy
is the most useful because, in addition to subsuming any entropy change due merely to heat
, it does the same for the pdV work needed to "make space for additional molecules" produced by various processes. (Hence its utility to solution
-phase
chemists, including biochemists.) The Helmholtz free energy
has a special theoretical
importance since it is proportional to the logarithm
of the partition function for the canonical ensemble
in statistical mechanics
. (Hence its utility to physicists
; and to gas
-phase chemists and engineers, who do not want to ignore pdV work.)
The historically earlier Helmholtz free energy
is defined as A = U − TS, where U is the internal energy, T is the absolute temperature
, and S is the entropy
. Its change is equal to the amount of reversible
work done on, or obtainable from, a system at constant T. Thus its appellation "work content
", and the designation A from Arbeit, the German word for work. Since it makes no reference to any quantities involved in work (such as p and V), the Helmholtz function is completely general: its decrease is the maximum amount of work which can be done by a system, and it can increase at most by the amount of work done on a system.
The Gibbs free energy
G = H − TS, where H is the enthalpy
. (H = U + pV, where p is the pressure and V is the volume.)
Historically, these energy terms have been used inconsistently. In physics
, free energy most often refers to the Helmholtz free energy
, denoted by A, while in chemistry
, free energy most often refers to the Gibbs free energy
.
Since both fields use both functions, a compromise
has been suggested, using A to denote the Helmholtz function and G for the Gibbs function. While A is preferred by IUPAC, G is sometimes still in use, and the correct free energy function is often implicit in manuscripts and presentations.
, i.e., that heat is a form of energy having relation to vibratory motion, was beginning to supplant both the caloric theory
, i.e., that heat is a fluid, and the four element theory, in which heat was the lightest of the four elements. In a similar manner, during these years, heat
was beginning to be distinguished into different classification categories, such as “free heat”, “combined heat”, “radiant heat”, specific heat, heat capacity
, “absolute heat”, “latent caloric”, “free” or “perceptible” caloric (calorique sensible), among others.
In 1780, for example, Laplace and Lavoisier stated: “In general, one can change the first hypothesis into the second by changing the words ‘free heat, combined heat, and heat released’ into ‘vis viva
, loss of vis viva, and increase of vis viva.’” In this manner, the total mass of caloric in a body, called absolute heat, was regarded as a mixture of two components; the free or perceptible caloric could affect a thermometer, whereas the other component, the latent caloric, could not. The use of the words “latent heat” implied a similarity to latent heat in the more usual sense; it was regarded as chemically bound to the molecules of the body. In the adiabatic
compression of a gas, the absolute heat remained constant by the observed rise of temperature, indicating that some latent caloric had become “free” or perceptible.
During the early 19th century, the concept of perceptible or free caloric began to be referred to as “free heat” or heat set free. In 1824, for example, the French physicist Sadi Carnot
, in his famous “Reflections on the Motive Power of Fire”, speaks of quantities of heat ‘absorbed or set free’ in different transformations. In 1882, the German physicist and physiologist Hermann von Helmholtz
coined the phrase ‘free energy’ for the expression E − TS, in which the change in F (or G) determines the amount of energy
‘free’ for work
under the given conditions.
Thus, in traditional use, the term “free” was attached to Gibbs free energy, i.e., for systems at constant pressure and temperature, or to Helmholtz free energy, i.e., for systems at constant volume and temperature, to mean ‘available in the form of useful work.’ With reference to the Gibbs free energy, we add the qualification that it is the energy free for non-volume work.
An increasing number of books and journal articles do not include the attachment “free”, referring to G as simply Gibbs energy (and likewise for the Helmholtz energy
). This is the result of a 1988 IUPAC meeting to set unified terminologies for the international scientific community, in which the adjective ‘free’ was supposedly banished. This standard, however, has not yet been universally adopted, and many published articles and books still include the descriptive ‘free’.
al usefulness of these functions is restricted to conditions where certain variables (T, and V or external p) are held constant, although they also have theoretical importance in deriving Maxwell relations
. Work other than pdV may be added, e.g., for electrochemical
cells, or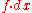 work in elastic
work in elastic
materials and in muscle
contraction. Other forms of work which must sometimes be considered are stress
-strain
, magnetic
, as in adiabatic
demagnetization
used in the approach to absolute zero
, and work due to electric polarization
. These are described by tensor
s.
In most cases of interest there are internal degrees of freedom
and processes, such as chemical reaction
s and phase transition
s, which create entropy. Even for homogeneous "bulk" materials, the free energy functions depend on the (often suppressed) composition
, as do all proper thermodynamic potentials
(extensive functions), including the internal energy.
Ni is the number of molecules (alternatively, moles
) of type i in the system. If these quantities do not appear, it is impossible to describe compositional changes. The differential
s for reversible processes
are (assuming only pV work)
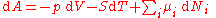

where μi is the chemical potential
for the i-th component
in the system. The second relation is especially useful at constant T and p, conditions which are easy to achieve experimentally, and which approximately characterize living
creatures.

Any decrease in the Gibbs function of a system is the upper limit for any isothermal
, isobaric
work that can be captured in the surroundings
, or it may simply be dissipated
, appearing as T times a corresponding increase in the entropy of the system and/or its surrounding.
An example is surface free energy, the amount of increase of free energy when the area of surface increases by every unit area.
The path integral Monte Carlo
method is a numerical approach for determining the values of free energies, based on quantum dynamical principles.
s. The term affinity, as used in chemical relation, dates back to at least the time of Albertus Magnus
in 1250.
From the 1998 textbook Modern Thermodynamics by Nobel Laureate and chemistry professor Ilya Prigogine
we find: "As motion was explained by the Newtonian concept of force, chemists wanted a similar concept of ‘driving force’ for chemical change. Why do chemical reactions occur, and why do they stop at certain points? Chemists called the ‘force’ that caused chemical reactions affinity, but it lacked a clear definition."
During the entire 18th century, the dominant view with regard to heat and light was that put forth by Isaac Newton
, called the Newtonian hypothesis, which states that light and heat are forms of matter attracted or repelled by other forms of matter, with forces analogous to gravitation or to chemical affinity.
In the 19th century, the French chemist Marcellin Berthelot
and the Danish chemist Julius Thomsen had attempted to quantify affinity using heats of reaction. In 1875, after quantifying the heats of reaction for a large number of compounds, Berthelot proposed the principle of maximum work
, in which all chemical changes occurring without intervention of outside energy tend toward the production of bodies or of a system of bodies which liberate heat
.
In addition to this, in 1780 Antoine Lavoisier
and Pierre-Simon Laplace
laid the foundations of thermochemistry
by showing that the heat given out in a reaction is equal to the heat absorbed in the reverse reaction. They also investigated the specific heat and latent heat
of a number of substances, and amounts of heat given out in combustion. In a similar manner, in 1840 Swiss chemist Germain Hess formulated the principle that the evolution of heat in a reaction is the same whether the process is accomplished in one-step process or in a number of stages. This is known as Hess' law. With the advent of the mechanical theory of heat in the early 19th century, Hess’s law came to be viewed as a consequence of the law of conservation of energy
.
Based on these and other ideas, Berthelot and Thomsen, as well as others, considered the heat given out in the formation of a compound as a measure of the affinity, or the work done by the chemical forces. This view, however, was not entirely correct. In 1847, the English physicist James Joule showed that he could raise the temperature of water by turning a paddle wheel in it, thus showing that heat and mechanical work were equivalent or proportional to each other, i.e., approximately, . This statement came to be known as the mechanical equivalent of heat
. This statement came to be known as the mechanical equivalent of heat
and was a precursory form of the first law of thermodynamics
.
By 1865, the German physicist Rudolf Clausius
had shown that this equivalence principle needed amendment. That is, one can use the heat derived from a combustion reaction in a coal furnace to boil water, and use this heat to vaporize steam, and then use the enhanced high-pressure energy of the vaporized steam to push a piston. Thus, we might naively reason that one can entirely convert the initial combustion heat of the chemical reaction into the work of pushing the piston. Clausius showed, however, that we must take into account the work that the molecules of the working body, i.e., the water molecules in the cylinder, do on each other as they pass or transform from one step of or state
of the engine cycle to the next, e.g., from (P1,V1) to (P2,V2). Clausius originally called this the “transformation content” of the body, and then later changed the name to entropy
. Thus, the heat used to transform the working body of molecules from one state to the next cannot be used to do external work, e.g., to push the piston. Clausius defined this transformation heat as dQ = TdS.
In 1873, Willard Gibbs published A Method of Geometrical Representation of the Thermodynamic Properties of Substances by Means of Surfaces, in which he introduced the preliminary outline of the principles of his new equation able to predict or estimate the tendencies of various natural processes to ensue when bodies or systems are brought into contact. By studying the interactions of homogeneous substances in contact, i.e., bodies, being in composition part solid, part liquid, and part vapor, and by using a three-dimensional volume-entropy
-internal energy
graph, Gibbs was able to determine three states of equilibrium, i.e., "necessarily stable", "neutral", and "unstable", and whether or not changes will ensue. In 1876, Gibbs built on this framework by introducing the concept of chemical potential
so to take into account chemical reactions and states of bodies that are chemically different from each other. In his own words, to summarize his results in 1873, Gibbs states:
In this description, as used by Gibbs, ε refers to the internal energy
of the body, η refers to the entropy
of the body, and ν is the volume of the body.
Hence, in 1882, after the introduction of these arguments by Clausius and Gibbs, the German scientist Hermann von Helmholtz
stated, in opposition to Berthelot and Thomas’ hypothesis that chemical affinity is a measure of the heat of reaction of chemical reaction as based on the principle of maximal work, that affinity is not the heat given out in the formation of a compound but rather it is the largest quantity of work which can be gained when the reaction is carried out in a reversible manner, e.g., electrical work in a reversible cell. The maximum work is thus regarded as the diminution of the free, or available, energy of the system (Gibbs free energy G at T = constant, P = constant or Helmholtz free energy F at T = constant, V = constant), whilst the heat given out is usually a measure of the diminution of the total energy of the system (Internal energy
). Thus, G or F is the amount of energy “free” for work under the given conditions.
Up until this point, the general view had been such that: “all chemical reactions drive the system to a state of equilibrium in which the affinities of the reactions vanish”. Over the next 60 years, the term affinity came to be replaced with the term free energy. According to chemistry historian Henry Leicester, the influential 1923 textbook Thermodynamics and the Free Energy of Chemical Reactions by Gilbert N. Lewis
and Merle Randall
led to the replacement of the term “affinity” by the term “free energy” in much of the English-speaking world.
Work (thermodynamics)
In thermodynamics, work performed by a system is the energy transferred to another system that is measured by the external generalized mechanical constraints on the system. As such, thermodynamic work is a generalization of the concept of mechanical work in mechanics. Thermodynamic work encompasses...
that a thermodynamic system
Thermodynamic system
A thermodynamic system is a precisely defined macroscopic region of the universe, often called a physical system, that is studied using the principles of thermodynamics....
can perform. The concept is useful in the thermodynamics
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
of chemical or thermal processes in engineering
Engineering
Engineering is the discipline, art, skill and profession of acquiring and applying scientific, mathematical, economic, social, and practical knowledge, in order to design and build structures, machines, devices, systems, materials and processes that safely realize improvements to the lives of...
and science. The free energy is the internal energy
Internal energy
In thermodynamics, the internal energy is the total energy contained by a thermodynamic system. It is the energy needed to create the system, but excludes the energy to displace the system's surroundings, any energy associated with a move as a whole, or due to external force fields. Internal...
of a system less the amount of energy that cannot be used to perform work. This unusable energy is given by the entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
of a system multiplied by the temperature of the system.
Like the internal energy, the free energy is a thermodynamic state function
State function
In thermodynamics, a state function, function of state, state quantity, or state variable is a property of a system that depends only on the current state of the system, not on the way in which the system acquired that state . A state function describes the equilibrium state of a system...
.
Overview
Free energy is that portion of any first-lawFirst law of thermodynamics
The first law of thermodynamics is an expression of the principle of conservation of work.The law states that energy can be transformed, i.e. changed from one form to another, but cannot be created nor destroyed...
energy that is available to perform thermodynamic work
Work (thermodynamics)
In thermodynamics, work performed by a system is the energy transferred to another system that is measured by the external generalized mechanical constraints on the system. As such, thermodynamic work is a generalization of the concept of mechanical work in mechanics. Thermodynamic work encompasses...
; i.e., work mediated by thermal energy
Thermal energy
Thermal energy is the part of the total internal energy of a thermodynamic system or sample of matter that results in the system's temperature....
. Free energy is subject to irreversible
Irreversibility
In science, a process that is not reversible is called irreversible. This concept arises most frequently in thermodynamics, as applied to processes....
loss in the course of such work. Since first-law energy is always conserved, it is evident that free energy is an expendable, second-law
Second law of thermodynamics
The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated physical system. From the state of thermodynamic equilibrium, the law deduced the principle of the increase of entropy and...
kind of energy that can perform work within finite amounts of time. Several free energy functions may be formulated based on system criteria. Free energy functions
Function (mathematics)
In mathematics, a function associates one quantity, the argument of the function, also known as the input, with another quantity, the value of the function, also known as the output. A function assigns exactly one output to each input. The argument and the value may be real numbers, but they can...
are Legendre transformation
Legendre transformation
In mathematics, the Legendre transformation or Legendre transform, named after Adrien-Marie Legendre, is an operation that transforms one real-valued function of a real variable into another...
s of the internal energy
Internal energy
In thermodynamics, the internal energy is the total energy contained by a thermodynamic system. It is the energy needed to create the system, but excludes the energy to displace the system's surroundings, any energy associated with a move as a whole, or due to external force fields. Internal...
. For processes involving a system at constant pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
p and temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
T, the Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
is the most useful because, in addition to subsuming any entropy change due merely to heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
, it does the same for the pdV work needed to "make space for additional molecules" produced by various processes. (Hence its utility to solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
-phase
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
chemists, including biochemists.) The Helmholtz free energy
Helmholtz free energy
In thermodynamics, the Helmholtz free energy is a thermodynamic potential that measures the “useful” work obtainable from a closed thermodynamic system at a constant temperature and volume...
has a special theoretical
Theory
The English word theory was derived from a technical term in Ancient Greek philosophy. The word theoria, , meant "a looking at, viewing, beholding", and referring to contemplation or speculation, as opposed to action...
importance since it is proportional to the logarithm
Logarithm
The logarithm of a number is the exponent by which another fixed value, the base, has to be raised to produce that number. For example, the logarithm of 1000 to base 10 is 3, because 1000 is 10 to the power 3: More generally, if x = by, then y is the logarithm of x to base b, and is written...
of the partition function for the canonical ensemble
Canonical ensemble
The canonical ensemble in statistical mechanics is a statistical ensemble representing a probability distribution of microscopic states of the system...
in statistical mechanics
Statistical mechanics
Statistical mechanics or statistical thermodynamicsThe terms statistical mechanics and statistical thermodynamics are used interchangeably...
. (Hence its utility to physicists
Physics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
; and to gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
-phase chemists and engineers, who do not want to ignore pdV work.)
The historically earlier Helmholtz free energy
Helmholtz free energy
In thermodynamics, the Helmholtz free energy is a thermodynamic potential that measures the “useful” work obtainable from a closed thermodynamic system at a constant temperature and volume...
is defined as A = U − TS, where U is the internal energy, T is the absolute temperature
Thermodynamic temperature
Thermodynamic temperature is the absolute measure of temperature and is one of the principal parameters of thermodynamics. Thermodynamic temperature is an "absolute" scale because it is the measure of the fundamental property underlying temperature: its null or zero point, absolute zero, is the...
, and S is the entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
. Its change is equal to the amount of reversible
Reversible process (thermodynamics)
In thermodynamics, a reversible process, or reversible cycle if the process is cyclic, is a process that can be "reversed" by means of infinitesimal changes in some property of the system without loss or dissipation of energy. Due to these infinitesimal changes, the system is in thermodynamic...
work done on, or obtainable from, a system at constant T. Thus its appellation "work content
Work content
In thermodynamic analysis of chemical reactions, the term free energy denotes either of two related concepts of importance expressing the total amount of energy which is used up or released during a chemical reaction. Both attempt to capture that part of the total energy of a system which is...
", and the designation A from Arbeit, the German word for work. Since it makes no reference to any quantities involved in work (such as p and V), the Helmholtz function is completely general: its decrease is the maximum amount of work which can be done by a system, and it can increase at most by the amount of work done on a system.
The Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
G = H − TS, where H is the enthalpy
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
. (H = U + pV, where p is the pressure and V is the volume.)
Historically, these energy terms have been used inconsistently. In physics
Physics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
, free energy most often refers to the Helmholtz free energy
Helmholtz free energy
In thermodynamics, the Helmholtz free energy is a thermodynamic potential that measures the “useful” work obtainable from a closed thermodynamic system at a constant temperature and volume...
, denoted by A, while in chemistry
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, free energy most often refers to the Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
.
Since both fields use both functions, a compromise
Compromise
To compromise is to make a deal where one person gives up part of his or her demand.In arguments, compromise is a concept of finding agreement through communication, through a mutual acceptance of terms—often involving variations from an original goal or desire.Extremism is often considered as...
has been suggested, using A to denote the Helmholtz function and G for the Gibbs function. While A is preferred by IUPAC, G is sometimes still in use, and the correct free energy function is often implicit in manuscripts and presentations.
Meaning of "free"
In the 18th and 19th centuries, the theory of heatTheory of heat
In the history of science, the theory of heat or mechanical theory of heat was a theory, introduced predominantly in 1824 by the French physicist Sadi Carnot, that heat and mechanical work are equivalent. It is related to the mechanical equivalent of heat...
, i.e., that heat is a form of energy having relation to vibratory motion, was beginning to supplant both the caloric theory
Caloric theory
The caloric theory is an obsolete scientific theory that heat consists of a self-repellent fluid called caloric that flows from hotter bodies to colder bodies. Caloric was also thought of as a weightless gas that could pass in and out of pores in solids and liquids...
, i.e., that heat is a fluid, and the four element theory, in which heat was the lightest of the four elements. In a similar manner, during these years, heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
was beginning to be distinguished into different classification categories, such as “free heat”, “combined heat”, “radiant heat”, specific heat, heat capacity
Heat capacity
Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount...
, “absolute heat”, “latent caloric”, “free” or “perceptible” caloric (calorique sensible), among others.
In 1780, for example, Laplace and Lavoisier stated: “In general, one can change the first hypothesis into the second by changing the words ‘free heat, combined heat, and heat released’ into ‘vis viva
Vis viva
In the history of science, vis viva is an obsolete scientific theory that served as an elementary and limited early formulation of the principle of conservation of energy...
, loss of vis viva, and increase of vis viva.’” In this manner, the total mass of caloric in a body, called absolute heat, was regarded as a mixture of two components; the free or perceptible caloric could affect a thermometer, whereas the other component, the latent caloric, could not. The use of the words “latent heat” implied a similarity to latent heat in the more usual sense; it was regarded as chemically bound to the molecules of the body. In the adiabatic
Adiabatic process
In thermodynamics, an adiabatic process or an isocaloric process is a thermodynamic process in which the net heat transfer to or from the working fluid is zero. Such a process can occur if the container of the system has thermally-insulated walls or the process happens in an extremely short time,...
compression of a gas, the absolute heat remained constant by the observed rise of temperature, indicating that some latent caloric had become “free” or perceptible.
During the early 19th century, the concept of perceptible or free caloric began to be referred to as “free heat” or heat set free. In 1824, for example, the French physicist Sadi Carnot
Sadi Carnot
Sadi Carnot may refer to:*Nicolas Léonard Sadi Carnot , French physicist*Marie François Sadi Carnot , president of the third French Republic, and nephew of Nicolas Léonard Sadi Carnot...
, in his famous “Reflections on the Motive Power of Fire”, speaks of quantities of heat ‘absorbed or set free’ in different transformations. In 1882, the German physicist and physiologist Hermann von Helmholtz
Hermann von Helmholtz
Hermann Ludwig Ferdinand von Helmholtz was a German physician and physicist who made significant contributions to several widely varied areas of modern science...
coined the phrase ‘free energy’ for the expression E − TS, in which the change in F (or G) determines the amount of energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
‘free’ for work
Work (thermodynamics)
In thermodynamics, work performed by a system is the energy transferred to another system that is measured by the external generalized mechanical constraints on the system. As such, thermodynamic work is a generalization of the concept of mechanical work in mechanics. Thermodynamic work encompasses...
under the given conditions.
Thus, in traditional use, the term “free” was attached to Gibbs free energy, i.e., for systems at constant pressure and temperature, or to Helmholtz free energy, i.e., for systems at constant volume and temperature, to mean ‘available in the form of useful work.’ With reference to the Gibbs free energy, we add the qualification that it is the energy free for non-volume work.
An increasing number of books and journal articles do not include the attachment “free”, referring to G as simply Gibbs energy (and likewise for the Helmholtz energy
Helmholtz free energy
In thermodynamics, the Helmholtz free energy is a thermodynamic potential that measures the “useful” work obtainable from a closed thermodynamic system at a constant temperature and volume...
). This is the result of a 1988 IUPAC meeting to set unified terminologies for the international scientific community, in which the adjective ‘free’ was supposedly banished. This standard, however, has not yet been universally adopted, and many published articles and books still include the descriptive ‘free’.
Application
The experimentExperiment
An experiment is a methodical procedure carried out with the goal of verifying, falsifying, or establishing the validity of a hypothesis. Experiments vary greatly in their goal and scale, but always rely on repeatable procedure and logical analysis of the results...
al usefulness of these functions is restricted to conditions where certain variables (T, and V or external p) are held constant, although they also have theoretical importance in deriving Maxwell relations
Maxwell relations
Maxwell's relations are a set of equations in thermodynamics which are derivable from the definitions of the thermodynamic potentials. The Maxwell relations are statements of equality among the second derivatives of the thermodynamic potentials. They follow directly from the fact that the order of...
. Work other than pdV may be added, e.g., for electrochemical
Electrochemistry
Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
cells, or
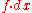 work in elastic
work in elasticElastomer
An elastomer is a polymer with the property of viscoelasticity , generally having notably low Young's modulus and high yield strain compared with other materials. The term, which is derived from elastic polymer, is often used interchangeably with the term rubber, although the latter is preferred...
materials and in muscle
Muscle
Muscle is a contractile tissue of animals and is derived from the mesodermal layer of embryonic germ cells. Muscle cells contain contractile filaments that move past each other and change the size of the cell. They are classified as skeletal, cardiac, or smooth muscles. Their function is to...
contraction. Other forms of work which must sometimes be considered are stress
Stress (physics)
In continuum mechanics, stress is a measure of the internal forces acting within a deformable body. Quantitatively, it is a measure of the average force per unit area of a surface within the body on which internal forces act. These internal forces are a reaction to external forces applied on the body...
-strain
Strain (materials science)
In continuum mechanics, the infinitesimal strain theory, sometimes called small deformation theory, small displacement theory, or small displacement-gradient theory, deals with infinitesimal deformations of a continuum body...
, magnetic
Magnetism
Magnetism is a property of materials that respond at an atomic or subatomic level to an applied magnetic field. Ferromagnetism is the strongest and most familiar type of magnetism. It is responsible for the behavior of permanent magnets, which produce their own persistent magnetic fields, as well...
, as in adiabatic
Adiabatic process
In thermodynamics, an adiabatic process or an isocaloric process is a thermodynamic process in which the net heat transfer to or from the working fluid is zero. Such a process can occur if the container of the system has thermally-insulated walls or the process happens in an extremely short time,...
demagnetization
Magnetization
In classical electromagnetism, magnetization or magnetic polarization is the vector field that expresses the density of permanent or induced magnetic dipole moments in a magnetic material...
used in the approach to absolute zero
Absolute zero
Absolute zero is the theoretical temperature at which entropy reaches its minimum value. The laws of thermodynamics state that absolute zero cannot be reached using only thermodynamic means....
, and work due to electric polarization
Dipole
In physics, there are several kinds of dipoles:*An electric dipole is a separation of positive and negative charges. The simplest example of this is a pair of electric charges of equal magnitude but opposite sign, separated by some distance. A permanent electric dipole is called an electret.*A...
. These are described by tensor
Tensor
Tensors are geometric objects that describe linear relations between vectors, scalars, and other tensors. Elementary examples include the dot product, the cross product, and linear maps. Vectors and scalars themselves are also tensors. A tensor can be represented as a multi-dimensional array of...
s.
In most cases of interest there are internal degrees of freedom
Degrees of freedom (physics and chemistry)
A degree of freedom is an independent physical parameter, often called a dimension, in the formal description of the state of a physical system...
and processes, such as chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s and phase transition
Phase transition
A phase transition is the transformation of a thermodynamic system from one phase or state of matter to another.A phase of a thermodynamic system and the states of matter have uniform physical properties....
s, which create entropy. Even for homogeneous "bulk" materials, the free energy functions depend on the (often suppressed) composition
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
, as do all proper thermodynamic potentials
Thermodynamic potentials
A thermodynamic potential is a scalar function used to represent the thermodynamic state of a system. The concept of thermodynamic potentials was introduced by Pierre Duhem in 1886. Josiah Willard Gibbs in his papers used the term fundamental functions. One main thermodynamic potential that has a...
(extensive functions), including the internal energy.
Ni is the number of molecules (alternatively, moles
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
) of type i in the system. If these quantities do not appear, it is impossible to describe compositional changes. The differential
Differential (mathematics)
In mathematics, the term differential has several meanings.-Basic notions:* In calculus, the differential represents a change in the linearization of a function....
s for reversible processes
Reversible process (thermodynamics)
In thermodynamics, a reversible process, or reversible cycle if the process is cyclic, is a process that can be "reversed" by means of infinitesimal changes in some property of the system without loss or dissipation of energy. Due to these infinitesimal changes, the system is in thermodynamic...
are (assuming only pV work)
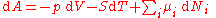

where μi is the chemical potential
Chemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
for the i-th component
Component (thermodynamics)
In thermodynamics, a component is a chemically distinct constituent ofa system. Calculating the number of components in a system is necessary, for example, when applying Gibbs phase rule in determination of the number of degrees of freedom of a system....
in the system. The second relation is especially useful at constant T and p, conditions which are easy to achieve experimentally, and which approximately characterize living
Life
Life is a characteristic that distinguishes objects that have signaling and self-sustaining processes from those that do not, either because such functions have ceased , or else because they lack such functions and are classified as inanimate...
creatures.

Any decrease in the Gibbs function of a system is the upper limit for any isothermal
Isothermal process
An isothermal process is a change of a system, in which the temperature remains constant: ΔT = 0. This typically occurs when a system is in contact with an outside thermal reservoir , and the change occurs slowly enough to allow the system to continually adjust to the temperature of the reservoir...
, isobaric
Isobaric process
An isobaric process is a thermodynamic process in which the pressure stays constant. The term derives from the Greek isos, , and barus,...
work that can be captured in the surroundings
Surroundings
Surroundings are the area around a given physical or geographical point or place. The exact definition depends on the field. Surroundings can also be used in geography and mathematics, as well as philosophy, with the literal or metaphorically extended definition.In thermodynamics, the term is used...
, or it may simply be dissipated
Dissipation
In physics, dissipation embodies the concept of a dynamical system where important mechanical models, such as waves or oscillations, lose energy over time, typically from friction or turbulence. The lost energy converts into heat, which raises the temperature of the system. Such systems are called...
, appearing as T times a corresponding increase in the entropy of the system and/or its surrounding.
An example is surface free energy, the amount of increase of free energy when the area of surface increases by every unit area.
The path integral Monte Carlo
Path integral Monte Carlo
Path integral Monte Carlo is a quantum Monte Carlo method in the path integral formulation of quantum mechanics.The equations often are applied assuming that quantum exchange does not matter...
method is a numerical approach for determining the values of free energies, based on quantum dynamical principles.
History
The quantity called "free energy" is a more advanced and accurate replacement for the outdated term affinity, which was used by chemists in previous years to describe the force that caused chemical reactionChemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s. The term affinity, as used in chemical relation, dates back to at least the time of Albertus Magnus
Albertus Magnus
Albertus Magnus, O.P. , also known as Albert the Great and Albert of Cologne, is a Catholic saint. He was a German Dominican friar and a bishop, who achieved fame for his comprehensive knowledge of and advocacy for the peaceful coexistence of science and religion. Those such as James A. Weisheipl...
in 1250.
From the 1998 textbook Modern Thermodynamics by Nobel Laureate and chemistry professor Ilya Prigogine
Ilya Prigogine
Ilya, Viscount Prigogine was a Russian-born naturalized Belgian physical chemist and Nobel Laureate noted for his work on dissipative structures, complex systems, and irreversibility.-Biography :...
we find: "As motion was explained by the Newtonian concept of force, chemists wanted a similar concept of ‘driving force’ for chemical change. Why do chemical reactions occur, and why do they stop at certain points? Chemists called the ‘force’ that caused chemical reactions affinity, but it lacked a clear definition."
During the entire 18th century, the dominant view with regard to heat and light was that put forth by Isaac Newton
Isaac Newton
Sir Isaac Newton PRS was an English physicist, mathematician, astronomer, natural philosopher, alchemist, and theologian, who has been "considered by many to be the greatest and most influential scientist who ever lived."...
, called the Newtonian hypothesis, which states that light and heat are forms of matter attracted or repelled by other forms of matter, with forces analogous to gravitation or to chemical affinity.
In the 19th century, the French chemist Marcellin Berthelot
Marcellin Berthelot
Marcelin Pierre Eugène Berthelot was a French chemist and politician noted for the Thomsen-Berthelot principle of thermochemistry. He synthesized many organic compounds from inorganic substances and disproved the theory of vitalism. He is considered as one of the greatest chemists of all time.He...
and the Danish chemist Julius Thomsen had attempted to quantify affinity using heats of reaction. In 1875, after quantifying the heats of reaction for a large number of compounds, Berthelot proposed the principle of maximum work
Principle of maximum work
In the history of science, the principle of maximum work was a postulate concerning the relationship between chemical reactions, heat evolution, and the potential work produced there from...
, in which all chemical changes occurring without intervention of outside energy tend toward the production of bodies or of a system of bodies which liberate heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
.
In addition to this, in 1780 Antoine Lavoisier
Antoine Lavoisier
Antoine-Laurent de Lavoisier , the "father of modern chemistry", was a French nobleman prominent in the histories of chemistry and biology...
and Pierre-Simon Laplace
Pierre-Simon Laplace
Pierre-Simon, marquis de Laplace was a French mathematician and astronomer whose work was pivotal to the development of mathematical astronomy and statistics. He summarized and extended the work of his predecessors in his five volume Mécanique Céleste...
laid the foundations of thermochemistry
Thermochemistry
Thermochemistry is the study of the energy and heat associated with chemical reactions and/or physical transformations. A reaction may release or absorb energy, and a phase change may do the same, such as in melting and boiling. Thermochemistry focuses on these energy changes, particularly on the...
by showing that the heat given out in a reaction is equal to the heat absorbed in the reverse reaction. They also investigated the specific heat and latent heat
Latent heat
Latent heat is the heat released or absorbed by a chemical substance or a thermodynamic system during a process that occurs without a change in temperature. A typical example is a change of state of matter, meaning a phase transition such as the melting of ice or the boiling of water. The term was...
of a number of substances, and amounts of heat given out in combustion. In a similar manner, in 1840 Swiss chemist Germain Hess formulated the principle that the evolution of heat in a reaction is the same whether the process is accomplished in one-step process or in a number of stages. This is known as Hess' law. With the advent of the mechanical theory of heat in the early 19th century, Hess’s law came to be viewed as a consequence of the law of conservation of energy
Conservation of energy
The nineteenth century law of conservation of energy is a law of physics. It states that the total amount of energy in an isolated system remains constant over time. The total energy is said to be conserved over time...
.
Based on these and other ideas, Berthelot and Thomsen, as well as others, considered the heat given out in the formation of a compound as a measure of the affinity, or the work done by the chemical forces. This view, however, was not entirely correct. In 1847, the English physicist James Joule showed that he could raise the temperature of water by turning a paddle wheel in it, thus showing that heat and mechanical work were equivalent or proportional to each other, i.e., approximately,
 . This statement came to be known as the mechanical equivalent of heat
. This statement came to be known as the mechanical equivalent of heatMechanical equivalent of heat
In the history of science, the mechanical equivalent of heat was a concept that had an important part in the development and acceptance of the conservation of energy and the establishment of the science of thermodynamics in the 19th century....
and was a precursory form of the first law of thermodynamics
First law of thermodynamics
The first law of thermodynamics is an expression of the principle of conservation of work.The law states that energy can be transformed, i.e. changed from one form to another, but cannot be created nor destroyed...
.
By 1865, the German physicist Rudolf Clausius
Rudolf Clausius
Rudolf Julius Emanuel Clausius , was a German physicist and mathematician and is considered one of the central founders of the science of thermodynamics. By his restatement of Sadi Carnot's principle known as the Carnot cycle, he put the theory of heat on a truer and sounder basis...
had shown that this equivalence principle needed amendment. That is, one can use the heat derived from a combustion reaction in a coal furnace to boil water, and use this heat to vaporize steam, and then use the enhanced high-pressure energy of the vaporized steam to push a piston. Thus, we might naively reason that one can entirely convert the initial combustion heat of the chemical reaction into the work of pushing the piston. Clausius showed, however, that we must take into account the work that the molecules of the working body, i.e., the water molecules in the cylinder, do on each other as they pass or transform from one step of or state
Thermodynamic state
A thermodynamic state is a set of values of properties of a thermodynamic system that must be specified to reproduce the system. The individual parameters are known as state variables, state parameters or thermodynamic variables. Once a sufficient set of thermodynamic variables have been...
of the engine cycle to the next, e.g., from (P1,V1) to (P2,V2). Clausius originally called this the “transformation content” of the body, and then later changed the name to entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
. Thus, the heat used to transform the working body of molecules from one state to the next cannot be used to do external work, e.g., to push the piston. Clausius defined this transformation heat as dQ = TdS.
In 1873, Willard Gibbs published A Method of Geometrical Representation of the Thermodynamic Properties of Substances by Means of Surfaces, in which he introduced the preliminary outline of the principles of his new equation able to predict or estimate the tendencies of various natural processes to ensue when bodies or systems are brought into contact. By studying the interactions of homogeneous substances in contact, i.e., bodies, being in composition part solid, part liquid, and part vapor, and by using a three-dimensional volume-entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
-internal energy
Internal energy
In thermodynamics, the internal energy is the total energy contained by a thermodynamic system. It is the energy needed to create the system, but excludes the energy to displace the system's surroundings, any energy associated with a move as a whole, or due to external force fields. Internal...
graph, Gibbs was able to determine three states of equilibrium, i.e., "necessarily stable", "neutral", and "unstable", and whether or not changes will ensue. In 1876, Gibbs built on this framework by introducing the concept of chemical potential
Chemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
so to take into account chemical reactions and states of bodies that are chemically different from each other. In his own words, to summarize his results in 1873, Gibbs states:
In this description, as used by Gibbs, ε refers to the internal energy
Internal energy
In thermodynamics, the internal energy is the total energy contained by a thermodynamic system. It is the energy needed to create the system, but excludes the energy to displace the system's surroundings, any energy associated with a move as a whole, or due to external force fields. Internal...
of the body, η refers to the entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
of the body, and ν is the volume of the body.
Hence, in 1882, after the introduction of these arguments by Clausius and Gibbs, the German scientist Hermann von Helmholtz
Hermann von Helmholtz
Hermann Ludwig Ferdinand von Helmholtz was a German physician and physicist who made significant contributions to several widely varied areas of modern science...
stated, in opposition to Berthelot and Thomas’ hypothesis that chemical affinity is a measure of the heat of reaction of chemical reaction as based on the principle of maximal work, that affinity is not the heat given out in the formation of a compound but rather it is the largest quantity of work which can be gained when the reaction is carried out in a reversible manner, e.g., electrical work in a reversible cell. The maximum work is thus regarded as the diminution of the free, or available, energy of the system (Gibbs free energy G at T = constant, P = constant or Helmholtz free energy F at T = constant, V = constant), whilst the heat given out is usually a measure of the diminution of the total energy of the system (Internal energy
Internal energy
In thermodynamics, the internal energy is the total energy contained by a thermodynamic system. It is the energy needed to create the system, but excludes the energy to displace the system's surroundings, any energy associated with a move as a whole, or due to external force fields. Internal...
). Thus, G or F is the amount of energy “free” for work under the given conditions.
Up until this point, the general view had been such that: “all chemical reactions drive the system to a state of equilibrium in which the affinities of the reactions vanish”. Over the next 60 years, the term affinity came to be replaced with the term free energy. According to chemistry historian Henry Leicester, the influential 1923 textbook Thermodynamics and the Free Energy of Chemical Reactions by Gilbert N. Lewis
Gilbert N. Lewis
Gilbert Newton Lewis was an American physical chemist known for the discovery of the covalent bond , his purification of heavy water, his reformulation of chemical thermodynamics in a mathematically rigorous manner accessible to ordinary chemists, his theory of Lewis acids and...
and Merle Randall
Merle Randall
Merle Randall was an American physical chemist famous for his work, over the period of 25 years, in measuring free energy calculations of compounds with Gilbert N. Lewis...
led to the replacement of the term “affinity” by the term “free energy” in much of the English-speaking world.

