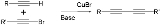
Cadiot-Chodkiewicz coupling
Encyclopedia
The Cadiot-Chodkiewicz coupling in organic chemistry
is a coupling reaction
between a terminal alkyne
and a haloalkyne catalyzed by a copper(I) salt such as copper(I) bromide
and an amine
base
. The reaction product is a di-acetylene or di-alkyne
.
The reaction mechanism
involves deprotonation
by base of the acetylenic proton followed by formation of a copper(I) acetylide
. A cycle of oxidative addition
and reductive elimination on the copper center then creates a new carbon-carbon bond.
Related couplings are the Glaser coupling and the Eglinton coupling.
s starting from cis-1,4-diethynyl-1,4-dimethoxycyclohexa-2,5-diene. This compound is also the starting material for the dibromide through NBS
and silver nitrate
:
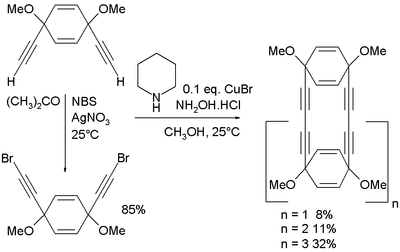 The coupling reaction itself takes place in methanol
The coupling reaction itself takes place in methanol
with piperidine
, the hydrochloric acid
salt of hydroxylamine
and copper(I) bromide
.
The Eglinton Reaction has been used to synthesize a number of fungal antibiotics and is important for carbon-carbon bond formation via the oxidative coupling of alkynes.
This procedure was used in the synthesis of cyclooctadecanonaene
. Another example is the synthesis of diphenyldiacetylene from phenylacetylene
.
and an additional oxidant like oxygen. The base in its original scope is ammonia
.
. An example is the coupling of trimethylsilylacetylene
.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
is a coupling reaction
Coupling reaction
A coupling reaction in organic chemistry is a catch-all term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst...
between a terminal alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
and a haloalkyne catalyzed by a copper(I) salt such as copper(I) bromide
Copper(I) bromide
Copper bromide is the chemical compound with the formula CuBr. This diamagnetic solid adopts a polymeric structure akin to that for zinc sulfide. The compound is widely used in the synthesis of organic compounds....
and an amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
. The reaction product is a di-acetylene or di-alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
.
The reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
involves deprotonation
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
by base of the acetylenic proton followed by formation of a copper(I) acetylide
Copper(I) acetylide
Copper acetylide, or cuprous acetylide, is an inorganic chemical compound with the formula Cu2C2. It is a heat and shock sensitive high explosive, more sensitive than silver acetylide. It is a metal acetylide. It is similar to silver acetylide and calcium carbide, though it is not called carbide in...
. A cycle of oxidative addition
Oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre...
and reductive elimination on the copper center then creates a new carbon-carbon bond.
Related couplings are the Glaser coupling and the Eglinton coupling.
Cadiot-Chodkiewicz coupling
In one study the Cadiot-Chodkiewicz coupling has been applied in the synthesis of acetylene macrocycleMacrocycle
A macrocycle is, as defined by IUPAC, "a cyclic macromolecule or a macromolecular cyclic portion of a molecule." In the chemical literature, organic chemists may consider any molecule containing a ring of nine or more atoms to be a macrocycle...
s starting from cis-1,4-diethynyl-1,4-dimethoxycyclohexa-2,5-diene. This compound is also the starting material for the dibromide through NBS
N-Bromosuccinimide
N-Bromosuccinimide or NBS is a chemical reagent which is used in radical substitution and electrophilic addition reactions in organic chemistry. NBS can be considered a convenient source of cationic bromine.-Preparation:...
and silver nitrate
Silver nitrate
Silver nitrate is an inorganic compound with chemical formula . This compound is a versatile precursor to many other silver compounds, such as those used in photography. It is far less sensitive to light than the halides...
:

Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
with piperidine
Piperidine
Piperidine is an organic compound with the molecular formula 5NH. This heterocyclic amine consists of a six-membered ring containing five methylene units and one nitrogen atom...
, the hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
salt of hydroxylamine
Hydroxylamine
Hydroxylamine is an inorganic compound with the formula NH2OH. The pure material is a white, unstable crystalline, hygroscopic compound. However, hydroxylamine is almost always provided and used as an aqueous solution. It is used to prepare oximes, an important functional group. It is also an...
and copper(I) bromide
Copper(I) bromide
Copper bromide is the chemical compound with the formula CuBr. This diamagnetic solid adopts a polymeric structure akin to that for zinc sulfide. The compound is widely used in the synthesis of organic compounds....
.
Eglinton reaction
In the related Eglinton reaction two terminal alkynes are coupled directly by a copper(II) salt such as cupric acetate.The Eglinton Reaction has been used to synthesize a number of fungal antibiotics and is important for carbon-carbon bond formation via the oxidative coupling of alkynes.
This procedure was used in the synthesis of cyclooctadecanonaene
Cyclooctadecanonaene
Cyclooctadecanonaene or [18]annulene is an annulene with chemical formula . This hydrocarbon obeys Hückel's rule and is, therefore, an aromatic compound...
. Another example is the synthesis of diphenyldiacetylene from phenylacetylene
Phenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.-Preparation:...
.
Glaser coupling
The Glaser coupling (1869) is by far the oldest acetylenic coupling and is based on cuprous salts like copper(I) bromideCopper(I) bromide
Copper bromide is the chemical compound with the formula CuBr. This diamagnetic solid adopts a polymeric structure akin to that for zinc sulfide. The compound is widely used in the synthesis of organic compounds....
and an additional oxidant like oxygen. The base in its original scope is ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
.
Hay coupling
The Hay coupling (1962) is another version of the Glaser coupling with the TMEDA complex of copper(I) chlorideCopper(I) chloride
Copper chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid...
. An example is the coupling of trimethylsilylacetylene
Trimethylsilylacetylene
Trimethylsilylacetylene is an acetylene molecule protected on one end by the trimethylsilyl group. It is popularly used in alkynylations, e.g. the Sonogashira reaction. After desilylation , the ethynyl group is introduced...
.

