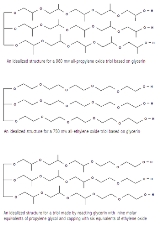
Polyol
Overview
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
containing multiple hydroxyl groups. In two technological disciplines the term "polyol" has a special meaning: food science
Food science
Food science is a study concerned with all technical aspects of foods, beginning with harvesting or slaughtering, and ending with its cooking and consumption, an ideology commonly referred to as "from field to fork"...
and polymer chemistry.
Sugar alcohol
Sugar alcohol
A sugar alcohol is a hydrogenated form of carbohydrate, whose carbonyl group has been reduced to a primary or secondary hydroxyl group . Sugar alcohols have the general formula Hn+1H, whereas sugars have HnHCO...
s, a class of polyols, are commonly added to foods because of their lower caloric content than sugar
Sugar
Sugar is a class of edible crystalline carbohydrates, mainly sucrose, lactose, and fructose, characterized by a sweet flavor.Sucrose in its refined form primarily comes from sugar cane and sugar beet...
s; however, they are also, in general, less sweet, and are often combined with high-intensity sweeteners
Sugar substitute
A sugar substitute is a food additive that duplicates the effect of sugar in taste, usually with less food energy. Some sugar substitutes are natural and some are synthetic. Those that are not natural are, in general, called artificial sweeteners....
. They are also added to chewing gum
Chewing gum
Chewing gum is a type of gum traditionally made of chicle, a natural latex product, or synthetic rubber known as polyisobutylene. For economical and quality reasons, many modern chewing gums use rubber instead of chicle...
because they are not metabolized (broken down) by bacteria in the mouth, so they do not contribute to tooth decay.

