Grignard reaction
Encyclopedia

Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
in which alkyl- or aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
-magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
s (Grignard reagents) add to a carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
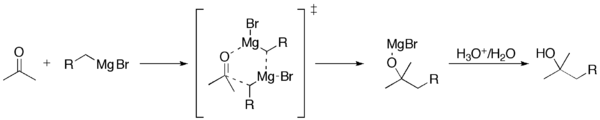
group in an aldehyde or ketone. This reaction is an important tool for the formation of carbon–carbon bonds. The reaction of an organic halide with magnesium is not a Grignard reaction, but provides a Grignard reagent.
Grignard reactions and reagents were discovered by and are named after the French chemist François Auguste Victor Grignard (University of Nancy, France), who was awarded the 1912 Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
for this work.
Reaction mechanism
The Grignard reagent functions as nucleophileNucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s attacking electrophilic carbon atoms that are present within polar bond
Polar bond
In chemistry, a polar bond is a type of covalent bond between two atoms or more in which electrons are shared unequally. Because of this, one end of the molecule has a slight, relative negative charge and the other a slight, relative positive charge...
of the carbonyl group.
The addition of the Grignard reagent to the carbonyl typically proceeds through a six-membered ring transition state.
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
, carbon–tin
Tin
Tin is a chemical element with the symbol Sn and atomic number 50. It is a main group metal in group 14 of the periodic table. Tin shows chemical similarity to both neighboring group 14 elements, germanium and lead and has two possible oxidation states, +2 and the slightly more stable +4...
, carbon–silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
, carbon–boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
and other carbon–heteroatom
Heteroatom
In organic chemistry, a heteroatom is any atom that is not carbon or hydrogen. Usually, the term is used to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular structure...
bonds.
Preparation of Grignard reagent
Grignard reagents form via the reaction of an alkyl or aryl halide with magnesiumMagnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
. The reaction is conducted by adding the organic halide to a suspension of magnesium in an ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
ial solvent, which provides ligands required to stabilize the organomagnesium compound. Empirical evidence suggests that the reaction takes place on the surface of the metal. The reaction proceeds through single electron transfer:
- R−X + Mg → R−X•− + Mg•+
- R−X•− → R• + X−
- X− + Mg•+ → XMg•
- R• + XMg• → RMgX
A limitation of Grignard reagents is that they do not readily react with alkyl halides via an SN2 mechanism. On the other hand, they readily participate in transmetalation
Transmetalation
Transmetalation is a general chemical reaction type in organometallic chemistry describing the exchange of ligands between two metal centers....
reactions:
- RMgX + ArX → ArMgX + MgX2
For this purpose, commercially available Grignard reagents are especially useful because this route avoids the problem with initiation.
Reaction conditions
In reaction involving Grignard reagents, it is important to exclude water and air, which rapidly destroy the reagent by protonolysis or oxidation. Since most Grignard reactions are conducted in anhydrous diethyl etherDiethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
or tetrahydrofuran
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
, side-reactions with air are limited by the protective blanket provided by solvent vapors. Small-scale or quantitative preparations should be conducted under nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
or argon
Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
atmospheres, using air-free technique
Air-free technique
Air-free techniques refer to a range of manipulations in the chemistry laboratory for the handling of compounds that are air-sensitive. These techniques prevent the compounds from reacting with components of air, usually water and oxygen; less commonly carbon dioxide and nitrogen...
s. Although the reagents still need to be dry, ultrasound can allow Grignard reagents to form in wet solvents by activating the magnesium such that it consumes the water.
The organic halide
Grignard reactions often start slowly. As is common for reactions involving solids and solution, initiation follows an induction periodInduction period
An induction period in chemical kinetics is an initial slow stage of a chemical reaction; after the induction period, the reaction accelerates. Ignoring induction periods can lead to runaway reactions....
during which reactive magnesium becomes exposed to the organic reagents. After this induction period, the reactions can be highly exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
. Alkyl and aryl bromide
Bromide
A bromide is a chemical compound containing bromide ion, that is bromine atom with effective charge of −1. The class name can include ionic compounds such as caesium bromide or covalent compounds such as sulfur dibromide.-Natural occurrence:...
s and iodide
Iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. This page is for the iodide ion and its salts. For information on organoiodides, see organohalides. In everyday life, iodide is most commonly encountered as a component of iodized salt,...
s are common substrates. Chloride
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
s are also used, but fluoride
Fluoride
Fluoride is the anion F−, the reduced form of fluorine when as an ion and when bonded to another element. Both organofluorine compounds and inorganic fluorine containing compounds are called fluorides. Fluoride, like other halides, is a monovalent ion . Its compounds often have properties that are...
s are generally unreactive, except with specially activated magnesium.
Magnesium
Typical Grignard reactions involve the use of magnesium ribbon. All magnesium is coated with a passivating layer of magnesium oxideMagnesium oxide
Magnesium oxide , or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium . It has an empirical formula of and consists of a lattice of Mg2+ ions and O2– ions held together by ionic bonds...
, which inhibits reactions with the organic halide. Specially activated magnesium, such as Rieke magnesium
Rieke metals
Rieke metals are highly reactive metal powders prepared by the methods developed by Reuben D. Rieke. Rieke metals are highly reactive because they have high surface area and lack surface oxides which retard reaction.-Preparation:...
, circumvents this problem.
Solvent
Most Grignard reactions are conducted in ethereal solvents, especially diethyl etherDiethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
and THF
ThF
Follicular B helper T cells , are antigen-experienced CD4+ T cells found in the B cell follicles of secondary lymphoid organs such as lymph nodes, spleens and Peyer's patches, and are identified by their constitutive expression of the B cell follicle homing receptor CXCR5...
. With the chelating diether dioxane, some Grignard reagents undergo a redistribution reaction to give diorganomagnesium compounds (R = organic group, X = halide):
- 2 RMgX + dioxane R2Mg + MgX2(dioxane)
This reaction is known as the Schlenk equilibrium
Schlenk equilibrium
The Schlenk equilibrium is a chemical equilibrium named after its discoverer Wilhelm Schlenk taking place in solutions of Grignard reagents.The process described is an equilibrium between two equivalents of an alkyl or aryl magnesium halide on the left of the equation and on the right side, one...
.
Testing Grignard reagents
Because Grignard reagents are so sensitive to moisture and oxygen, many methods have been developed to test the quality of a batch. Typical tests involve titrations with weighable, anhydrous protic reagents, e.g. mentholMenthol
Menthol is an organic compound made synthetically or obtained from peppermint or other mint oils. It is a waxy, crystalline substance, clear or white in color, which is solid at room temperature and melts slightly above. The main form of menthol occurring in nature is -menthol, which is assigned...
in the presence of a color-indicator. The interaction of the Grignard reagent with phenanthroline or 2,2'-bipyridine causes a color change.
Initiation
Many methods have been developed to initiate sluggish Grignard reactions. These methods weaken the passivatingPassivation
Passivation is the process of making a material "passive", and thus less reactive with surrounding air, water, or other gases or liquids. The goal is to inhibit corrosion, whether for structural or cosmetic reasons. Passivation of metals is usually achieved by the deposition of a layer of oxide...
layer of MgO
Magnesium oxide
Magnesium oxide , or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium . It has an empirical formula of and consists of a lattice of Mg2+ ions and O2– ions held together by ionic bonds...
, thereby exposing highly reactive magnesium to the organic halide. Mechanical methods include crushing of the Mg pieces in situ, rapid stirring, and sonication
Sonication
thumb|right|A sonicator at the [[Weizmann Institute of Science]] during sonicationSonication is the act of applying sound energy to agitate particles in a sample, for various purposes. In the laboratory, it is usually applied using an ultrasonic bath or an ultrasonic probe, colloquially known as...
of the suspension. Iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
, methyl iodide, and 1,2-dibromoethane
1,2-Dibromoethane
1,2-Dibromoethane, also known as ethylene dibromide , is the chemical compound with the formula BrCH2CH2Br. Although trace amounts occur naturally in the ocean, where it is formed probably by algae and kelp, it is mainly a synthetic...
are common activating agents. The use of 1,2-dibromoethane is particularly advantageous as its action can be monitored by the observation of bubbles of ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
. Furthermore, the side-products are innocuous:
- Mg + BrC2H4Br → C2H4 + MgBr2
The amount of Mg consumed by these activating agents is usually insignificant. A small amount of mercuric chloride will amalgamate
Amalgam (chemistry)
An amalgam is a substance formed by the reaction of mercury with another metal. Almost all metals can form amalgams with mercury, notable exceptions being iron and platinum. Silver-mercury amalgams are important in dentistry, and gold-mercury amalgam is used in the extraction of gold from ore.The...
the surface of the metal, allowing it to react.
Industrial production
Grignard reagents are produced in industry for use in situ, or for sale. As with at bench-scale, the main problem is that of initiation; a portion of a previous batch of Grignard reagent is often used as the initiator. Grignard reactions are exothermic, and this exothermicity must be considered when a reaction is scaled-up from laboratory to production plant.Many Grignard reagents, e.g. methylmagnesium chloride
Methylmagnesium chloride
Methylmagnesium chloride is a commercially available Grignard reagent. Like methyllithium, it is the synthetic equivalent to the methyl carbanion synthon. It is usually sold as a solution in tetrahydrofuran. The model of the molecule shows methylmagnesium chloride with the magnesium atom in the...
, phenylmagnesium bromide
Phenylmagnesium bromide
Phenylmagnesium bromide, with the simplified formula , is a magnesium-containing organometallic compound. It is so commonly used that it is commercially available as a solution in diethyl ether or tetrahydrofuran . Phenylmagnesium bromide is a Grignard reagent...
, and allylmagnesium bromide
Allylmagnesium bromide
Allylmagnesium bromide is an Grignard reagent used for introducing the allyl group. It is commonly available as a solution in diethyl ether. If desired, it may be synthesized in the normal method from magnesium and allyl bromide....
are available commercially as tetrahydrofuran
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
or diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
solutions.
Reactions with carbonyl compounds
Grignard reagents will react with a variety of carbonylCarbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
derivatives.

Note that the acetal
Acetal
An acetal is a molecule with two single-bonded oxygen atoms attached to the same carbon atom.Traditional usages distinguish ketals from acetals...
function (a masked carbonyl) does not react.
Such reactions usually involve an aqueous acidic workup, though this is rarely shown in reaction schemes. In cases where the Grignard reagent is adding to a prochiral
Prochiral
In stereochemistry, prochiral molecules are those that can be converted from achiral to chiral in a single step.If two identical substituents are attached to a sp3-hybridized atom, the descriptors pro-R and pro-S are used to distinguish between the two...
aldehyde or ketone, the Felkin-Anh model or Cram's Rule
Asymmetric induction
Asymmetric induction in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment...
can usually predict which stereoisomer will be formed. With easily 1,3-diketone
Diketone
A diketone is a molecule containing two ketone groups. The simpliest diketone is diacetyl, also known as 2,3-butanedione. Diacetyl, acetylacetone, and hexane-2,5-dione are examples of 1,2-, 1,3-, and 1,4-diketones, respectively...
s and related substrates, the Grignard reagent RMgX functions merely as a base, giving the enolate anion and liberating the alkane RH.
Reactions with other electrophiles
Grignard reagents will react with other various electrophiles, serving both as a nucleophile for many and as a base for protic substrates.
Formation of bonds to B, Si, P, Sn
Like organolithium compounds, Grignard reagents are useful for forming carbon–heteroatom bonds.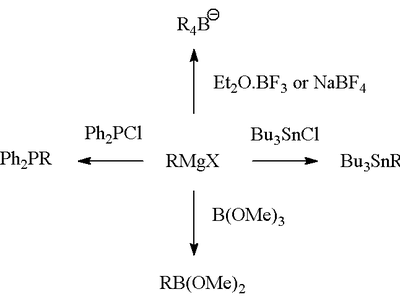
Carbon–carbon coupling reactions
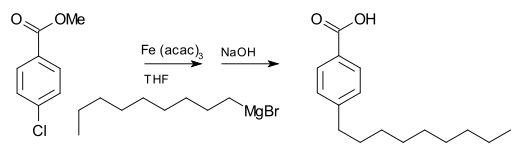
A Grignard reagent can also participate in coupling reactionCoupling reaction
A coupling reaction in organic chemistry is a catch-all term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst...
s. For example, nonylmagnesium bromide reacts with methyl p-chlorobenzoate to give p-nonylbenzoic acid, in the presence of Tris(acetylacetonato)iron(III) (Fe(acac)3), after workup with NaOH to hydrolyze
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
the ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
, shown as follows. Without the Fe(acac)3, the Grignard reagent would attack the ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
over the aryl halide.
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
(THF) is also a good catalyst. Additionally, an effective catalyst for the couplings of alkyl halides is dilithium tetrachlorocuprate (Li2CuCl4), prepared by mixing lithium chloride
Lithium chloride
Lithium chloride is a chemical compound with the formula LiCl. The salt is a typical ionic compound, although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlorides, such as extraordinary solubility in polar solvents and its hygroscopic...
(LiCl) and copper(II) chloride
Copper(II) chloride
Copper chloride is the chemical compound with the formula CuCl2. This is a light brown solid, which slowly absorbs moisture to form a blue-green dihydrate. The copper chlorides are some of the most common copper compounds, after copper sulfate....
(CuCl2) in THF. The Kumada-Corriu coupling gives access to [substituted] styrenes.
Oxidation
Treatment of a Grignard reagent with oxygen gives the magnesium organoperoxide. Hydrolysis of this material yields hydroperoxides or alcohol. These reactions involve radicalRadical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
intermediates.

The synthetic utility of Grignard oxidations can be increased by a reaction of Grignard reagents with oxygen in presence of an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
to an ethylene extended alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
. This modification requires aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
or vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
Grignards. Adding just the Grignard and the alkene does not result in a reaction demonstrating that the presence of oxygen is essential. Only drawback is the requirement of at least two equivalents of Grignard although this can partly be circumvented by the use of a dual Grignard system with a cheap reducing Grignard such as n-butylmagnesium bromide.

Nucleophilic aliphatic substitution
Grignard reagents are nucleophileNucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s in nucleophilic aliphatic substitutions for instance with alkyl halides in a key step in industrial Naproxen
Naproxen
Naproxen sodium is a nonsteroidal anti-inflammatory drug commonly used for the reduction of pain, fever, inflammation and stiffness caused by conditions such as:...
production:

Elimination
In the Boord olefin synthesisBoord olefin synthesis
The Boord olefin synthesis is an organic reaction forming alkenes from ethers carrying a halogen atom 2 carbons removed from the oxygen atom catalyzed by a metal such as magnesium or zinc. The reaction, discovered by Cecil E...
, the addition of magnesium to certain β-haloethers results in an elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
to the alkene. This reaction can limit the utility of Grignard reactions.
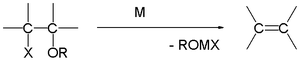
Degradation of Grignard reagents
At one time, the formation and hydrolysis of Grignard reagents was used in the determination of the number of halogen atoms in an organic compoundOrganic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
. In modern usage Grignard degradation is used in the chemical analysis of certain triacylglycerols.
Industrial use
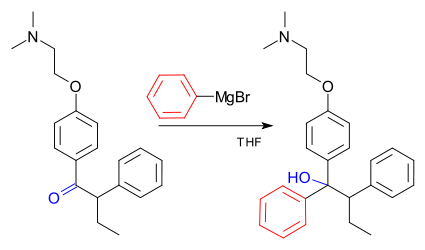
An example of the Grignard reaction is a key step in the industrial production of TamoxifenTamoxifen
Tamoxifen is an antagonist of the estrogen receptor in breast tissue via its active metabolite, hydroxytamoxifen. In other tissues such as the endometrium, it behaves as an agonist, hence tamoxifen may be characterized as a mixed agonist/antagonist...
(currently used for the treatment of estrogen receptor positive breast cancer in women) :
See also
- Wittig reactionWittig reactionThe Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
- Barbier reactionBarbier reactionThe Barbier reaction is an organic reaction between an alkyl halide and a carbonyl group as an electrophilic substrate in the presence of magnesium, aluminium, zinc, indium, tin or its salts. The reaction product is a primary, secondary or tertiary alcohol...
- Bodroux-Chichibabin aldehyde synthesisBodroux-Chichibabin aldehyde synthesisThe Bodroux-Chichibabin aldehyde synthesis is a chemical reaction whereby a Grignard reagent is converted to an aldehyde one carbon longer.Reaction of a Grignard reagent with triethyl orthoformate gives an acetal, which can be hydrolyzed to an aldehyde. For example, the synthesis of n-hexanal:...
- Fujimoto-Belleau reactionFujimoto-Belleau reactionThe Fujimoto-Belleau reaction is a chemical reaction that forms cyclic α-substituted α,β-unsaturated ketones from enol lactones. The reaction is named after the two chemists George I. Fujimoto and Bernard Belleau....
- Organolithium reagentOrganolithium reagentAn organolithium reagent is an organometallic compound with a direct bond between a carbon and a lithium atom. As the electropositive nature of lithium puts most of the charge density of the bond on the carbon atom, effectively creating a carbanion, organolithium compounds are extremely powerful...
s - Sakurai reactionSakurai reactionThe Sakurai reaction is the chemical reaction of carbon electrophiles with allylic silanes catalyzed by strong Lewis acids. It is named after the chemists Akira Hosomi and Hideki Sakurai.Lewis acid activation is essential for complete reaction...
Further reading
- Mary McHale, "Grignard Reaction," Connexions, http://cnx.org/content/m15245/1.2/. 2007.
- Grignard knowledge: Alkyl coupling chemistry with inexpensive transition metals by Larry J. Westrum, Fine Chemistry November/December 2002, pp. 10–13 http://www.bouldersci.com/wp-content/uploads/2011/02/Grignard_Knowledge.pdf


