Sonogashira coupling
Encyclopedia
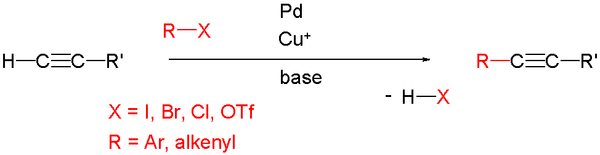
In organic chemistry
, a Sonogashira coupling is a coupling reaction
of terminal alkyne
s with aryl
or vinyl halide
s. This reaction was first reported by Kenkichi Sonogashira
and Nobue Hagihara in 1975.
complex and a halide salt of copper(I)
. The palladium complex activates the organic halides by oxidative addition
into the carbon
-halogen
bond
. Phosphine
-palladium complexes such as tetrakis(triphenylphosphine)palladium(0)
are used for this reaction, but palladium(II) complexes are also available because they are reduced to the palladium(0) species by the consumption of terminal alkynes in the reaction medium. The oxidation of triphenylphosphine
to triphenylphosphine oxide
can also lead to the formation of Pd(0) in situ when catalysts such as bis(triphenylphosphine)palladium(II) chloride are used. In contrast, copper(I) halides react with the terminal alkyne and produce copper(I) acetylide
, which acts as an activated species for the coupling reactions.
compounds such as triethylamine
and diethylamine
are sometimes used as solvent
s, but also DMF or ether can be used as solvent. Other bases such as potassium carbonate or cesium carbonate are occasionally used. In addition, deaerated conditions are formally needed for Sonogashira coupling reactions because the palladium(0) complexes are unstable in the air, and oxygen promotes the formation of homocoupled acetylenes. Recently, development of air-stable organopalladium catalysts enable this reaction to be conducted in the ambient atmosphere.
expensive catalyst after product formation poses a serious
drawback for large-scale applications of homogeneous catalysis. Structures known as metalodendrimers combine the advantages of homogeneous and heterogeneous catalysts, as they are soluble and well defined on the molecular level, and yet they can be recovered by precipitation, ultrafiltration, or ultracentrifugation. Some recent examples can be found about the use of dendritic palladium complex catalysts for the copper-free Sonogashira reaction. Thus, several generations of bidentate phosphanated palladium(II) polyamino dendritic catalysts have been used solubilized in triethylamine for the coupling of aryl iodides and bromides at
25-120 °C, and of aryl chlorides, but in very low yields.
The dendrimeric catalysts could usually be recovered by simple precipitation and filtration and reused up to five times, with diminished activity produced by dendrimer decomposition and not by palladium leaching being observed. These dendrimeric catalysts showed a negative dendritic effect; that is, the catalyst efficiency decreases as the dendrimer generation increases. The recyclable polymeric phosphane ligand shown below is obtained from ring-opening metathesis polymerization of a norbornene derivative, and has been used in the copper cocatalyzed Sonogashira reaction of methyl piodobenzoate and phenylacetylene using Pd(dba)2•CHCl3 as a palladium source. Despite recovery by filtration, polymer catalytic activity decreased by approximately 4-8% in each
recycle experiment.
properties for palladium and have been employed in the
formation of catalysts suitable for Sonogashira couplings.
The dipyrimidyl-palladium complex shown below (Fig 1) has been
employed in the copper-free coupling of iodo-, bromo-, and
chlorobenzene with phenylacetylene using N-butylamine
as base in THF solvent at 65 °C.
More recently, the dipyridylpalladium complex has been obtained and has been used
in the copper-free Sonogashira coupling reaction of aryl
iodides and bromides in N-methylpyrrolidinone (NMP) using
tetra-n-butylammonium acetate (TBAA) as base at room temperature.
It is interesting to note that this
complex has also been used for the coupling of aryl
iodides and bromides in refluxing water as solvent and in
the presence of air, using pyrrolidine as base and TBAB as
additive, although its efficiency was higher in N-methylpyrrolidinone (NMP) as solvent. An example of this complex's use is shown in the double coupling of o-diiodobenzene and phenylacetylene to give dialkynylated benzene (Fig 2).
[Images: to be posted soon]
chemistry, and at times even more efficiently; therefore, they have found application to numerous areas of organometallic homogeneous catalysis.
The most easily available are stable carbenes derived from imidazole, not the least because
numerous imidazolium precursor compounds can be made along various reliable routes, with the combination of the imidazolium salt with a palladium source under basic
conditions generating the NHC-palladium complex. The NHC-derived palladium(II) complex shown below has been shown to promote the coupling of aryl bromides at 80 °C in DMF
using triethylamine as base, although requiring the presence of catalytic
amounts of copper(I) iodide and triphenylphosphine as well.
with added palladium and in situ
preparation of the copper acetylide. The reaction mechanism
is not clearly understood but the textbook mechanism revolves around a palladium cycle and a copper cycle.
The palladium cycle:
The copper cycle:
Mechanistic studies suggest that these catalytic cycles represent the preferred reaction pathway, however some questions still arise about the nature of the real catalyst.
Thus, it has been shown that monoligated Pd(PR3) complexes can be formed when dealing with bulky phosphanes and have been suggested as possible catalytic species in coupling reactions.
In addition, some results point to the formation of anionic
palladium species, which would be the real catalysts instead
of the coordinatively unsaturated Pd0L2. Generally seen in the presence of anions and halides, it is known that Pd0(PPh3)2 does not exist in solution when generated in the presence of halide anions because they
coordinate the Pd(0) center to form anionic species
of the type [L2Pd0Cl]- which can participate in crosscoupling reactions.
, N,N-diisopropylethylamine, tetrakis(triphenylphosphine)palladium(0)
and dimethylformamide
, or copper(I) iodide
, diethylamine
, and dichlorobis(triphenylphosphine)palladium(II)
The Sonogashira coupling is applied in synthesis of cross-conjugated
oligo
(phenylene enynylene
)s and phenanthroline
derivatives.
In an inverse Sonogashira coupling the reactants are an aryl or vinyl compound and an alkynyl halide.
species as catalyst, which allowed the preparation of alkynelinked phenylalanine derivative for bioanalytical applications. There are also examples of the coupling partners both being attached to allyl resins, with the Pd(0) catalyst effecting cleavage of the substrates and subsequent Sonogashira
coupling in solution.
enynyl alcohol, which can be oxidized to the corresponding R-alkynylated acroleins and
of this coupling methodology toward the total synthesis of
natural products exclusively employed the typical copper-cocatalyzed reaction.
preparation of intermediates under typical Sonogashira
conditions, which, after cyclization, yield natural products
such as benzylisoquinoline or indole alkaloids
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, a Sonogashira coupling is a coupling reaction
Coupling reaction
A coupling reaction in organic chemistry is a catch-all term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst...
of terminal alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
s with aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
or vinyl halide
Vinyl halide
In organic chemistry, a vinyl halide is any alkene with at least one halide substituent bonded directly on one of the unsaturated carbons. Vinyl chloride is one such substance....
s. This reaction was first reported by Kenkichi Sonogashira
Kenkichi Sonogashira
is a Japanese chemist and was a Professor of Chemistry at Osaka University in Japan. He discovered the Sonogashira coupling in 1975. Sonogashira was later a professor at Osaka City University and retired in 2004....
and Nobue Hagihara in 1975.
Catalyst
Typically, two catalysts are needed for this reaction: a zerovalent palladiumPalladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
complex and a halide salt of copper(I)
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
. The palladium complex activates the organic halides by oxidative addition
Oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre...
into the carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
-halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
. Phosphine
Phosphine
Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
-palladium complexes such as tetrakis(triphenylphosphine)palladium(0)
Tetrakis(triphenylphosphine)palladium(0)
Tetrakispalladium is the chemical compound Pd[P3]4, often abbreviated Pd4, or even PdP4. It is a bright yellow crystalline solid that becomes brown upon decomposition in air.-Structure and properties:...
are used for this reaction, but palladium(II) complexes are also available because they are reduced to the palladium(0) species by the consumption of terminal alkynes in the reaction medium. The oxidation of triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
to triphenylphosphine oxide
Triphenylphosphine oxide
Triphenylphosphine oxide is the chemical compound with the formula OP3. Often chemists abbreviate the formula by writing Ph3PO or PPh3O . This white crystalline compound is a common side product in reactions involving triphenylphosphine...
can also lead to the formation of Pd(0) in situ when catalysts such as bis(triphenylphosphine)palladium(II) chloride are used. In contrast, copper(I) halides react with the terminal alkyne and produce copper(I) acetylide
Copper(I) acetylide
Copper acetylide, or cuprous acetylide, is an inorganic chemical compound with the formula Cu2C2. It is a heat and shock sensitive high explosive, more sensitive than silver acetylide. It is a metal acetylide. It is similar to silver acetylide and calcium carbide, though it is not called carbide in...
, which acts as an activated species for the coupling reactions.
Conditions
The reaction medium must be basic to neutralize the hydrogen halide produced as the byproduct of this coupling reaction, so alkylamineAmine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
compounds such as triethylamine
Triethylamine
Triethylamine is the chemical compound with the formula N3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine, for which TEA is also a common abbreviation....
and diethylamine
Diethylamine
Diethylamine is a secondary amine with the molecular structure CH3CH2NHCH2CH3. It is a flammable, strongly alkaline liquid. It is miscible with water and ethanol. It is a colorless liquid which often appears brown due to impurities...
are sometimes used as solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
s, but also DMF or ether can be used as solvent. Other bases such as potassium carbonate or cesium carbonate are occasionally used. In addition, deaerated conditions are formally needed for Sonogashira coupling reactions because the palladium(0) complexes are unstable in the air, and oxygen promotes the formation of homocoupled acetylenes. Recently, development of air-stable organopalladium catalysts enable this reaction to be conducted in the ambient atmosphere.
Palladium-Phosphorus Complexes
The issues dealing with recovery of the oftenexpensive catalyst after product formation poses a serious
drawback for large-scale applications of homogeneous catalysis. Structures known as metalodendrimers combine the advantages of homogeneous and heterogeneous catalysts, as they are soluble and well defined on the molecular level, and yet they can be recovered by precipitation, ultrafiltration, or ultracentrifugation. Some recent examples can be found about the use of dendritic palladium complex catalysts for the copper-free Sonogashira reaction. Thus, several generations of bidentate phosphanated palladium(II) polyamino dendritic catalysts have been used solubilized in triethylamine for the coupling of aryl iodides and bromides at
25-120 °C, and of aryl chlorides, but in very low yields.
The dendrimeric catalysts could usually be recovered by simple precipitation and filtration and reused up to five times, with diminished activity produced by dendrimer decomposition and not by palladium leaching being observed. These dendrimeric catalysts showed a negative dendritic effect; that is, the catalyst efficiency decreases as the dendrimer generation increases. The recyclable polymeric phosphane ligand shown below is obtained from ring-opening metathesis polymerization of a norbornene derivative, and has been used in the copper cocatalyzed Sonogashira reaction of methyl piodobenzoate and phenylacetylene using Pd(dba)2•CHCl3 as a palladium source. Despite recovery by filtration, polymer catalytic activity decreased by approximately 4-8% in each
recycle experiment.
Palladium-Nitrogen Complexes
Pyridines and pyrimidines have shown good complexationproperties for palladium and have been employed in the
formation of catalysts suitable for Sonogashira couplings.
The dipyrimidyl-palladium complex shown below (Fig 1) has been
employed in the copper-free coupling of iodo-, bromo-, and
chlorobenzene with phenylacetylene using N-butylamine
as base in THF solvent at 65 °C.
More recently, the dipyridylpalladium complex has been obtained and has been used
in the copper-free Sonogashira coupling reaction of aryl
iodides and bromides in N-methylpyrrolidinone (NMP) using
tetra-n-butylammonium acetate (TBAA) as base at room temperature.
It is interesting to note that this
complex has also been used for the coupling of aryl
iodides and bromides in refluxing water as solvent and in
the presence of air, using pyrrolidine as base and TBAB as
additive, although its efficiency was higher in N-methylpyrrolidinone (NMP) as solvent. An example of this complex's use is shown in the double coupling of o-diiodobenzene and phenylacetylene to give dialkynylated benzene (Fig 2).
[Images: to be posted soon]
N-Heterocyclic Carbene (NHC) Palladium Complexes
Nucleophilic N-heterocyclic carbenes (NHCs) behave like typical σ-donor ligands that can substitute 2-electron ligands (i.e., amines, phosphanes) in metal coordinationchemistry, and at times even more efficiently; therefore, they have found application to numerous areas of organometallic homogeneous catalysis.
The most easily available are stable carbenes derived from imidazole, not the least because
numerous imidazolium precursor compounds can be made along various reliable routes, with the combination of the imidazolium salt with a palladium source under basic
conditions generating the NHC-palladium complex. The NHC-derived palladium(II) complex shown below has been shown to promote the coupling of aryl bromides at 80 °C in DMF
using triethylamine as base, although requiring the presence of catalytic
amounts of copper(I) iodide and triphenylphosphine as well.
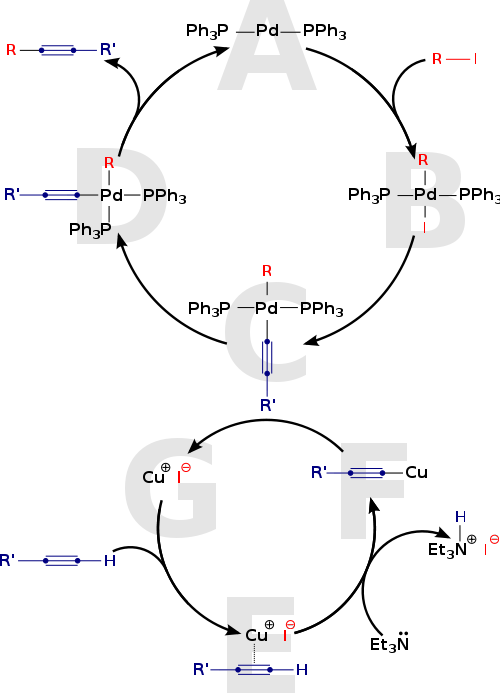
Mechanism
The Sonogashira coupling is a modification of the Castro-Stephens couplingCastro-Stephens coupling
The Castro-Stephens Coupling is a cross coupling reaction between a copper acetylide and an aryl halide forming a disubstituted alkyne and a copper halide....
with added palladium and in situ
In situ
In situ is a Latin phrase which translated literally as 'In position'. It is used in many different contexts.-Aerospace:In the aerospace industry, equipment on board aircraft must be tested in situ, or in place, to confirm everything functions properly as a system. Individually, each piece may...
preparation of the copper acetylide. The reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
is not clearly understood but the textbook mechanism revolves around a palladium cycle and a copper cycle.
The palladium cycle:
- The active palladium catalyst is the 14 electron compound Pd(0)L2 A which reacts with the aryl halide or triflate in an oxidative additionOxidative additionOxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre...
to Pd(II) complex B - This complex reacts in a rate limiting transmetallation with the copper acetylide produced in the copper cycle to complex C expelling the copper halide CuX (G).
- Both organic ligands are trans oriented and convert to cis in a trans-cis isomerization to complex D
- In the final step the product is released in a reductive elimination with regeneration of Pd(0)
The copper cycle:
- The main limitation of this mechanism is its inability to account for deprotonationDeprotonationDeprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
of the terminal alkyne: The employed amineAmineAmines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s such as diethylamineDiethylamineDiethylamine is a secondary amine with the molecular structure CH3CH2NHCH2CH3. It is a flammable, strongly alkaline liquid. It is miscible with water and ethanol. It is a colorless liquid which often appears brown due to impurities...
or N,N-diisopropylethylamine are simply not basic enough. It is suggested that deprotonation is still possible after initial formation of a pi-alkyne complex E - The organocopper compoundOrganocopper compoundOrganocopper compounds in organometallic chemistry contain carbon to copper chemical bonds. Organocopper chemistry is the science of organocopper compounds describing their physical properties, synthesis and reactions...
F forms after reaction with the base and continues to react with palladium intermediate B with regeneration of copper halide G. - The formation of a copper acetylide intermediate has never been proven, although based on indirect evidence it is assumed to be involved in the reduction of Pd(II) catalysts, first forming a dialkyne-PdL2 complex and then by reductive elimination Pd(0) and a diacetyleneDiacetyleneDiacetylene , with the formula C4H2, is a highly unsaturated hydrocarbon that contains three single bonds and two triple bonds. It is the first in the series of polyynes.-Occurrence:...
. - A side reaction is a Glaser coupling of two acetylene units. intermediate has yet to be proven
- Copper free reactions also exist making the reaction mechanism even more intractable. It is however reported that palladium catalysts can be contaminated by copper salts.
Mechanistic studies suggest that these catalytic cycles represent the preferred reaction pathway, however some questions still arise about the nature of the real catalyst.
Thus, it has been shown that monoligated Pd(PR3) complexes can be formed when dealing with bulky phosphanes and have been suggested as possible catalytic species in coupling reactions.
In addition, some results point to the formation of anionic
palladium species, which would be the real catalysts instead
of the coordinatively unsaturated Pd0L2. Generally seen in the presence of anions and halides, it is known that Pd0(PPh3)2 does not exist in solution when generated in the presence of halide anions because they
coordinate the Pd(0) center to form anionic species
of the type [L2Pd0Cl]- which can participate in crosscoupling reactions.
Copper-Free Reaction
Though its existence has been proven, the copper-free Sonogashira mechanism is still relatively unknown. Current research seems to indicate the following:- As in the original mechanism, oxidative addition of the aryl halide or triflate to the Pd(0) catalysts.
- Since the amines associated with this reaction are not basic enough to deprotonate the reacting alkyne, it is believed that complexation to the Pd(0) catalyst requires displacement of one ligand to create an intermediate complex.
- As a result, this new intermediate can then facilitate deprotonation of the terminal alkyne proton and subsequent ligand exchange with the leaving group X.
- Reductive elimination gives rise to the desired coupling product.
Complications
Due to the crucial role of base, specific amines must be added in excess or as solvent for the reaction to proceed. It has been discovered that secondary amines such as piperidine, morpholine, or diisopropylamine in particular can react efficiently and reversibly with trans-RPdX(PPh3)2 complexes by substituting one PPh3 ligand. The equilibirium constant of this reaction is dependent on R, X, a factor for basicity, and the amine's steric hindrance. The result is competition between the amine and the alkyne group for this ligand exchange, which is why the amine is generally added in excess to promote preferential substitution.Scope
Typical reagents and reaction conditions are copper(I) iodideCopper(I) iodide
Copper iodide is the inorganic compound with the formula CuI. It is also known as cuprous iodide. It is useful in a variety of applications ranging from organic synthesis to cloud seeding....
, N,N-diisopropylethylamine, tetrakis(triphenylphosphine)palladium(0)
Tetrakis(triphenylphosphine)palladium(0)
Tetrakispalladium is the chemical compound Pd[P3]4, often abbreviated Pd4, or even PdP4. It is a bright yellow crystalline solid that becomes brown upon decomposition in air.-Structure and properties:...
and dimethylformamide
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
, or copper(I) iodide
Copper(I) iodide
Copper iodide is the inorganic compound with the formula CuI. It is also known as cuprous iodide. It is useful in a variety of applications ranging from organic synthesis to cloud seeding....
, diethylamine
Diethylamine
Diethylamine is a secondary amine with the molecular structure CH3CH2NHCH2CH3. It is a flammable, strongly alkaline liquid. It is miscible with water and ethanol. It is a colorless liquid which often appears brown due to impurities...
, and dichlorobis(triphenylphosphine)palladium(II)
The Sonogashira coupling is applied in synthesis of cross-conjugated
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
oligo
Oligomer
In chemistry, an oligomer is a molecule that consists of a few monomer units , in contrast to a polymer that, at least in principle, consists of an unlimited number of monomers. Dimers, trimers, and tetramers are oligomers. Many oils are oligomeric, such as liquid paraffin...
(phenylene enynylene
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
)s and phenanthroline
Phenanthroline
Phenanthroline is a heterocyclic organic compound. As a bidentate ligand in coordination chemistry, it forms strong complexes with most metal ions...
derivatives.
In an inverse Sonogashira coupling the reactants are an aryl or vinyl compound and an alkynyl halide.
Applications in Synthesis
Sonogashira couplings are employed in a wide array of synthetic reactions, primarily due to their success in facilitating the following challenging transformations:Alkynation Reactions
The coupling of a terminal alkyne and an aromatic ring is the pivotal reaction when talking about applications of the copper-promoted or copper-free Sonogashira reaction. The list of cases where the typical Sonogashira reaction using aryl halides has been employed is large, and choosing illustrative examples is difficult. A recent use of this methodology is shown below for the coupling of iodinated phenylalanine with a terminal alkyne derived from d-biotin using an in situ generated Pd(0)species as catalyst, which allowed the preparation of alkynelinked phenylalanine derivative for bioanalytical applications. There are also examples of the coupling partners both being attached to allyl resins, with the Pd(0) catalyst effecting cleavage of the substrates and subsequent Sonogashira
coupling in solution.
Enynes and Enediynes
The 1,3-enyne moiety is an important structural unit for biologically active and natural compounds (see next section). It is derived from vinylic systems and terminal acetylenes by using a configuration-retention stereospecific procedure such as the Sonogashira reaction. Vinyl iodides are the most reactive vinyl halides to Pd(0) oxidative addition, and their use is therefore most frequent for Sonogashira cross-coupling reactions due to the usually milder conditions employed. Some examples include:- The coupling of 2-iodo-prop-2-enol with a wide range of acetylenes such as TMSA to give
enynyl alcohol, which can be oxidized to the corresponding R-alkynylated acroleins and
- The preparation of selenated allylic ether 140 from the cross-coupling of vinyl iodide 139 and phenylacetylene, shown below.
Natural Products
Many metabolites found in nature contain alkyne or enyne moieties, and therefore, the Sonogashira reaction has found frequent utility in their syntheses. Several of the most recent and promising applicationsof this coupling methodology toward the total synthesis of
natural products exclusively employed the typical copper-cocatalyzed reaction.
- An example of the coupling of an aryl iodide to an aryl acetylene can be seen in the reaction of the iodinated alcohol and the tris(isopropyl)silylacetylene, which gave alkyne, an intermediate in the total synthesis of the benzindenoazepine alkaloid bulgaramine.
- There are other recent examples of the use of aryl iodides for the
preparation of intermediates under typical Sonogashira
conditions, which, after cyclization, yield natural products
such as benzylisoquinoline or indole alkaloids