
Oxidative phosphorylation
Encyclopedia
Oxidative phosphorylation (or OXPHOS in short) is a metabolic pathway
that uses energy released by the oxidation
of nutrient
s to produce adenosine triphosphate
(ATP). Although the many forms of life on earth use a range of different nutrients, almost all aerobic organism
s carry out oxidative phosphorylation to produce ATP, the molecule that supplies energy to metabolism
. This pathway is probably so pervasive because it is a highly efficient way of releasing energy, compared to alternative fermentation
processes such as anaerobic glycolysis
.
During oxidative phosphorylation, electrons are transferred from electron donors
to electron acceptors
such as oxygen
, in redox reactions. These redox reactions release energy, which is used to form ATP. In eukaryote
s, these redox reactions are carried out by a series of protein complex
es within mitochondria
, whereas, in prokaryote
s, these proteins are located in the cells' inner membranes. These linked sets of proteins are called electron transport chain
s. In eukaryotes, five main protein complexes are involved, whereas in prokaryotes many different enzymes are present, using a variety of electron donors and acceptors.
The energy released by electrons flowing through this electron transport chain is used to transport protons across the inner mitochondrial membrane
, in a process called chemiosmosis
. This generates potential energy
in the form of a pH
gradient and an electrical potential
across this membrane. This store of energy is tapped by allowing protons to flow back across the membrane and down this gradient, through a large enzyme
called ATP synthase
. This enzyme uses this energy to generate ATP from adenosine diphosphate
(ADP), in a phosphorylation
reaction. This reaction is driven by the proton flow, which forces the rotation
of a part of the enzyme; the ATP synthase is a rotary mechanical motor.
Although oxidative phosphorylation is a vital part of metabolism
, it produces reactive oxygen species
such as superoxide
and hydrogen peroxide
, which lead to propagation of free radicals
, damaging cells and contributing to disease
and, possibly, aging (senescence
). The enzymes carrying out this metabolic pathway are also the target of many drugs and poisons that inhibit
their activities.
-releasing chemical reactions to drive energy-requiring reactions: The two sets of reactions are said to be coupled. This means one cannot occur without the other. The flow of electrons through the electron transport chain, from electron donors such as NADH to electron acceptor
s such as oxygen
, is an exergonic
process – it releases energy, whereas the synthesis of ATP is an endergonic
process, which requires an input of energy. Both the electron transport chain and the ATP synthase are embedded in a membrane, and energy is transferred from electron transport chain to the ATP synthase by movements of protons across this membrane, in a process called chemiosmosis
. In practice, this is like a simple electric circuit
, with a current of protons being driven from the negative N-side of the membrane to the positive P-side by the proton-pumping enzymes of the electron transport chain. These enzymes are like a battery
, as they perform work
to drive current through the circuit. The movement of protons creates an electrochemical gradient
across the membrane, which is often called the proton-motive force. This gradient has two components: a difference in proton concentration (a H+ gradient) and a difference in electric potential
, with the N-side having a negative charge. The energy is stored largely as the difference of electric potential
s in mitochondria
, but also as a pH gradient in chloroplast
s.
ATP synthase releases this stored energy by completing the circuit and allowing protons to flow down the electrochemical gradient, back to the N-side of the membrane. This enzyme is like an electric motor
as it uses the proton-motive force to drive the rotation of part of its structure and couples this motion to the synthesis of ATP.
The amount of energy released by oxidative phosphorylation is high, compared with the amount produced by anaerobic fermentation. Glycolysis
produces only 2 ATP molecules, but somewhere between 30 and 36 ATPs are produced by the oxidative phosphorylation of the 10 NADH and 2 succinate molecules made by converting one molecule of glucose
to carbon dioxide and water, while each cycle of beta oxidation
of a fatty acid
yields about 14 ATPs. These ATP yields are theoretical maximum values; in practice, some protons leak across the membrane, lowering the yield of ATP.
. This carries only electrons, and these are transferred by the reduction and oxidation of an iron
atom that the protein holds within a heme
group in its structure. Cytochrome c is also found in some bacteria, where it is located within the periplasmic space
.
Within the inner mitochondrial membrane, the lipid
-soluble electron carrier coenzyme Q10 (Q) carries both electrons and protons by a redox
cycle. This small benzoquinone
molecule is very hydrophobic
, so it diffuses freely within the membrane. When Q accepts two electrons and two protons, it becomes reduced to the ubiquinol
form (QH2); when QH2 releases two electrons and two protons, it becomes oxidized back to the ubiquinone (Q) form. As a result, if two enzymes are arranged so that Q is reduced on one side of the membrane and QH2 oxidized on the other, ubiquinone will couple these reactions and shuttle protons across the membrane. Some bacterial electron transport chains use different quinones, such as menaquinone
, in addition to ubiquinone.
Within proteins, electrons are transferred between flavin cofactors, iron–sulfur clusters, and cytochromes. There are several types of iron–sulfur cluster. The simplest kind found in the electron transfer chain consists of two iron atoms joined by two atoms of inorganic sulfur
; these are called [2Fe–2S] clusters. The second kind, called [4Fe–4S], contains a cube of four iron atoms and four sulfur atoms. Each iron atom in these clusters is coordinated by an additional amino acid
, usually by the sulfur atom of cysteine
. Metal ion cofactors undergo redox reactions without binding or releasing protons, so in the electron transport chain they serve solely to transport electrons through proteins. Electrons move quite long distances through proteins by hopping along chains of these cofactors. This occurs by quantum tunnelling
, which is rapid over distances of less than 1.4 m.
, the citric acid cycle
, and beta oxidation
, produce the reduced coenzyme NADH. This coenzyme contains electrons that have a high transfer potential
; in other words, they will release a large amount of energy upon oxidation. However, the cell does not release this energy all at once, as this would be an uncontrollable reaction. Instead, the electrons are removed from NADH and passed to oxygen through a series of enzymes that each release a small amount of the energy. This set of enzymes, consisting of complexes I through IV, is called the electron transport chain and is found in the inner membrane of the mitochondrion. Succinate
is also oxidized by the electron transport chain, but feeds into the pathway at a different point.
In eukaryote
s, the enzymes in this electron transport system use the energy released from the oxidation of NADH to pump proton
s across the inner membrane of the mitochondrion. This causes protons to build up in the intermembrane space
, and generates an electrochemical gradient
across the membrane. The energy stored in this potential is then used by ATP synthase to produce ATP. Oxidative phosphorylation in the eukaryotic mitochondrion is the best-understood example of this process. The mitochondrion is present in almost all eukaryotes, with the exception of anaerobic protozoa such as Trichomonas vaginalis
that instead reduce protons to hydrogen in a remnant mitochondrion called a hydrogenosome
.
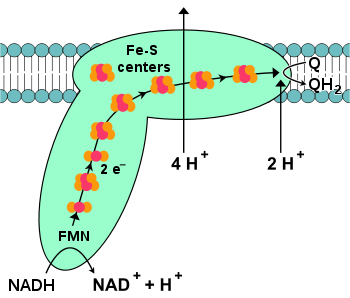 NADH-coenzyme Q oxidoreductase
NADH-coenzyme Q oxidoreductase
, also known as NADH dehydrogenase or complex I, is the first protein in the electron transport chain. Complex I is a giant enzyme
with the mammalian complex I having 46 subunits and a molecular mass of about 1,000 kilodaltons
(kDa). The structure is known in detail only from a bacterium; in most organisms the complex resembles a boot with a large “ball” poking out from the membrane into the mitochondrion. The genes that encode the individual proteins are contained in both the cell nucleus
and the mitochondrial genome, as is the case for many enzymes present in the mitochondrion.
The reaction that is catalyzed by this enzyme is the two electron reduction by NADH
of coenzyme Q10 or ubiquinone (represented as Q in the equation below), a lipid-soluble quinone
that is found in the mitochondrion membrane:

The start of the reaction, and indeed of the entire electron chain, is the binding of a NADH molecule to complex I and the donation of two electrons. The electrons enter complex I via a prosthetic group attached to the complex, flavin mononucleotide
(FMN). The addition of electrons to FMN converts it to its reduced form, FMNH2. The electrons are then transferred through a series of iron–sulfur clusters
: the second kind of prosthetic group present in the complex. There are both [2Fe–2S] and [4Fe–4S] iron–sulfur clusters in complex I.
As the electrons pass through this complex, four protons are pumped from the matrix into the intermembrane space. Exactly how this occurs is unclear, but it seems to involve conformational change
s in complex I that cause the protein to bind protons on the N-side of the membrane and release them on the P-side of the membrane. Finally, the electrons are transferred from the chain of iron–sulfur clusters to a ubiquinone molecule in the membrane. Reduction of ubiquinone also contributes to the generation of a proton gradient, as two protons are taken up from the matrix as it is reduced to ubiquinol
(QH2).
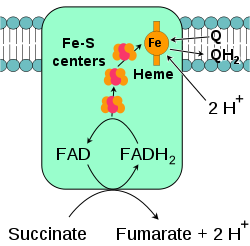 Succinate-Q oxidoreductase
Succinate-Q oxidoreductase
, also known as complex II or succinate dehydrogenase, is a second entry point to the electron transport chain. It is unusual because it is the only enzyme that is part of both the citric acid cycle and the electron transport chain. Complex II consists of four protein subunits and contains a bound flavin adenine dinucleotide
(FAD) cofactor, iron–sulfur clusters, and a heme
group that does not participate in electron transfer to coenzyme Q, but is believed to be important in decreasing production of reactive oxygen species. It oxidizes succinate
to fumarate
and reduces ubiquinone. As this reaction releases less energy than the oxidation of NADH, complex II does not transport protons across the membrane and does not contribute to the proton gradient.

In some eukaryotes, such as the parasitic worm
Ascaris suum
, an enzyme similar to complex II, fumarate reductase (menaquinol:fumarate
oxidoreductase, or QFR), operates in reverse to oxidize ubiquinol and reduce fumarate. This allows the worm to survive in the anaerobic environment of the large intestine
, carrying out anaerobic oxidative phosphorylation with fumarate as the electron acceptor. Another unconventional function of complex II is seen in the malaria
parasite Plasmodium falciparum
. Here, the reversed action of complex II as an oxidase is important in regenerating ubiquinol, which the parasite uses in an unusual form of pyrimidine
biosynthesis.
(ETF-Q oxidoreductase), also known as electron transferring-flavoprotein dehydrogenase, is a third entry point to the electron transport chain. It is an enzyme that accepts electrons from electron-transferring flavoprotein
in the mitochondrial matrix, and uses these electrons to reduce ubiquinone. This enzyme contains a flavin and a [4Fe–4S] cluster, but, unlike the other respiratory complexes, it attaches to the surface of the membrane and does not cross the lipid bilayer.

In mammals, this metabolic pathway is important in beta oxidation
of fatty acid
s and catabolism of amino acid
s and choline
, as it accepts electrons from multiple acetyl-CoA
dehydrogenases. In plants, ETF-Q oxidoreductase is also important in the metabolic responses that allow survival in extended periods of darkness.
is also known as cytochrome c reductase, cytochrome bc1 complex, or simply complex III. In mammals, this enzyme is a dimer
, with each subunit complex containing 11 protein subunits, an [2Fe-2S] iron–sulfur cluster and three cytochrome
s: one cytochrome
c1 and two b cytochromes. A cytochrome is a kind of electron-transferring protein that contains at least one heme
group. The iron atoms inside complex III’s heme groups alternate between a reduced ferrous (+2) and oxidized ferric (+3) state as the electrons are transferred through the protein.
The reaction catalyzed by complex III is the oxidation of one molecule of ubiquinol
and the reduction of two molecules of cytochrome c
, a heme protein loosely associated with the mitochondrion. Unlike coenzyme Q, which carries two electrons, cytochrome c carries only one electron.

As only one of the electrons can be transferred from the QH2 donor to a cytochrome c acceptor at a time, the reaction mechanism of complex III is more elaborate than those of the other respiratory complexes, and occurs in two steps called the Q cycle. In the first step, the enzyme binds three substrates, first, QH2, which is then oxidized, with one electron being passed to the second substrate, cytochrome c. The two protons released from QH2 pass into the intermembrane space. The third substrate is Q, which accepts the second electron from the QH2 and is reduced to Q.-, which is the ubisemiquinone
free radical. The first two substrates are released, but this ubisemiquinone intermediate remains bound. In the second step, a second molecule of QH2 is bound and again passes its first electron to a cytochrome c acceptor. The second electron is passed to the bound ubisemiquinone, reducing it to QH2 as it gains two protons from the mitochondrial matrix. This QH2 is then released from the enzyme.
As coenzyme Q is reduced to ubiquinol on the inner side of the membrane and oxidized to ubiquinone on the other, a net transfer of protons across the membrane occurs, adding to the proton gradient. The rather complex two-step mechanism by which this occurs is important, as it increases the efficiency of proton transfer. If, instead of the Q cycle, one molecule of QH2 were used to directly reduce two molecules of cytochrome c, the efficiency would be halved, with only one proton transferred per cytochrome c reduced.
 Cytochrome c oxidase
Cytochrome c oxidase
, also known as complex IV, is the final protein complex in the electron transport chain. The mammalian enzyme has an extremely complicated structure and contains 13 subunits, two heme groups, as well as multiple metal ion cofactors – in all three atoms of copper
, one of magnesium
and one of zinc
.
This enzyme mediates the final reaction in the electron transport chain and transfers electrons to oxygen, while pumping protons across the membrane. The final electron acceptor
oxygen, which is also called the terminal electron acceptor, is reduced to water in this step. Both the direct pumping of protons and the consumption of matrix protons in the reduction of oxygen contribute to the proton gradient. The reaction catalyzed is the oxidation of cytochrome c and the reduction of oxygen:

s have alternative NADH oxidases, which oxidize NADH in the cytosol rather than in the mitochondrial matrix, and pass these electrons to the ubiquinone pool. These enzymes do not transport protons, and, therefore, reduce ubiquinone without altering the electrochemical gradient across the inner membrane.
Another example of a divergent electron transport chain is the alternative oxidase
, which is found in plant
s, as well as some fungi
, protist
s, and possibly some animals. This enzyme transfers electrons directly from ubiquinol to oxygen.
The electron transport pathways produced by these alternative NADH and ubiquinone oxidases have lower ATP
yields than the full pathway. The advantages produced by a shortened pathway are not entirely clear. However, the alternative oxidase is produced in response to stresses such as cold, reactive oxygen species
, and infection by pathogens, as well as other factors that inhibit the full electron transport chain. Alternative pathways might, therefore, enhance an organisms' resistance to injury, by reducing oxidative stress
.
and archaea
possess a large variety of electron-transfer enzymes. These use an equally wide set of chemicals as substrates. In common with eukaryotes, prokaryotic electron transport uses the energy released from the oxidation of a substrate to pump ions across a membrane and generate an electrochemical gradient. In the bacteria, oxidative phosphorylation in Escherichia coli
is understood in most detail, while archaeal systems are at present poorly understood.
The main difference between eukaryotic and prokaryotic oxidative phosphorylation is that bacteria and archaea use many different substances to donate or accept electrons. This allows prokaryotes to grow under a wide variety of environmental conditions. In E. coli, for example, oxidative phosphorylation can be driven by a large number of pairs of reducing agents and oxidizing agents, which are listed below. The midpoint potential of a chemical measures how much energy is released when it is oxidized or reduced, with reducing agents having negative potentials and oxidizing agents positive potentials.
As shown above, E. coli can grow with reducing agents such as formate, hydrogen, or lactate as electron donors, and nitrate, DMSO, or oxygen as acceptors. The larger the difference in midpoint potential between an oxidizing and reducing agent, the more energy is released when they react. Out of these compounds, the succinate/fumarate pair is unusual, as its midpoint potential is close to zero. Succinate can therefore be oxidized to fumarate if a strong oxidizing agent such as oxygen is available, or fumarate can be reduced to succinate using a strong reducing agent such as formate. These alternative reactions are catalyzed by succinate dehydrogenase
and fumarate reductase
, respectively.
Some prokaryotes use redox pairs that have only a small difference in midpoint potential. For example, nitrifying
bacteria such as Nitrobacter
oxidize nitrite to nitrate, donating the electrons to oxygen. The small amount of energy released in this reaction is enough to pump protons and generate ATP, but not enough to produce NADH or NADPH directly for use in anabolism
. This problem is solved by using a nitrite oxidoreductase
to produce enough proton-motive force to run part of the electron transport chain in reverse, causing complex I to generate NADH.
Prokaryotes control their use of these electron donors and acceptors by varying which enzymes are produced, in response to environmental conditions. This flexibility is possible because different oxidases and reductases use the same ubiquinone pool. This allows many combinations of enzymes to function together, linked by the common ubiquinol intermediate. These respiratory chains therefore have a modular design
, with easily interchangeable sets of enzyme systems.
In addition to this metabolic diversity, prokaryotes also possess a range of isozyme
s – different enzymes that catalyze the same reaction. For example, in E. coli, there are two different types of ubiquinol oxidase using oxygen as an electron acceptor. Under highly aerobic conditions, the cell uses an oxidase with a low affinity for oxygen that can transport two protons per electron. However, if levels of oxygen fall, they switch to an oxidase that transfers only one proton per electron, but has a high affinity for oxygen.
(Pi). Estimates of the number of protons required to synthesize one ATP have ranged from three to four, with some suggesting cells can vary this ratio, to suit different conditions.

This phosphorylation
reaction is an equilibrium
, which can be shifted by altering the proton-motive force. In the absence of a proton-motive force, the ATP synthase reaction will run from right to left, hydrolyzing ATP and pumping protons out of the matrix across the membrane. However, when the proton-motive force is high, the reaction is forced to run in the opposite direction; it proceeds from left to right, allowing protons to flow down their concentration gradient and turning ADP into ATP. Indeed, in the closely related vacuolar type H+-ATPases
, the same reaction is used to acidify cellular compartments, by pumping protons and hydrolysing ATP.
ATP synthase is a massive protein complex with a mushroom-like shape. The mammalian enzyme complex contains 16 subunits and has a mass of approximately 600 kilodaltons. The portion embedded within the membrane is called FO and contains a ring of c subunits and the proton channel. The stalk and the ball-shaped headpiece is called F1 and is the site of ATP synthesis. The ball-shaped complex at the end of the F1 portion contains six proteins of two different kinds (three α subunits and three β subunits), whereas the "stalk" consists of one protein: the γ subunit, with the tip of the stalk extending into the ball of α and β subunits. Both the α and β subunits bind nucleotides, but only the β subunits catalyze the ATP synthesis reaction. Reaching along the side of the F1 portion and back into the membrane is a long rod-like subunit that anchors the α and β subunits into the base of the enzyme.
As protons cross the membrane through the channel in the base of ATP synthase, the FO proton-driven motor rotates. Rotation might be caused by changes in the ionization
of amino acids in the ring of c subunits causing electrostatic interactions that propel the ring of c subunits past the proton channel. This rotating ring in turn drives the rotation of the central axle
(the γ subunit stalk) within the α and β subunits. The α and β subunits are prevented from rotating themselves by the side-arm, which acts as a stator
. This movement of the tip of the γ subunit within the ball of α and β subunits provides the energy for the active sites in the β subunits to undergo a cycle of movements that produces and then releases ATP.
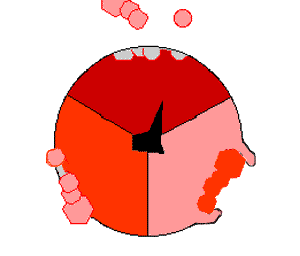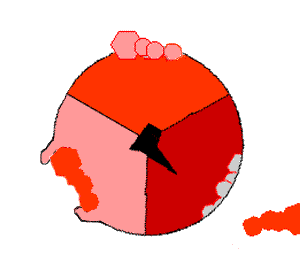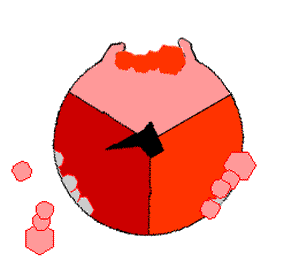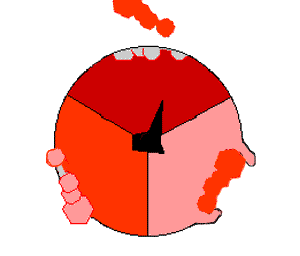 This ATP synthesis reaction is called the binding change mechanism and involves the active site of a β subunit cycling between three states. In the "open" state, ADP and phosphate enter the active site (shown in brown in the diagram). The protein then closes up around the molecules and binds them loosely – the "loose" state (shown in red). The enzyme then changes shape again and forces these molecules together, with the active site in the resulting "tight" state (shown in pink) binding the newly produced ATP molecule with very high affinity
This ATP synthesis reaction is called the binding change mechanism and involves the active site of a β subunit cycling between three states. In the "open" state, ADP and phosphate enter the active site (shown in brown in the diagram). The protein then closes up around the molecules and binds them loosely – the "loose" state (shown in red). The enzyme then changes shape again and forces these molecules together, with the active site in the resulting "tight" state (shown in pink) binding the newly produced ATP molecule with very high affinity
. Finally, the active site cycles back to the open state, releasing ATP and binding more ADP and phosphate, ready for the next cycle.
In some bacteria and archaea, ATP synthesis is driven by the movement of sodium ions through the cell membrane, rather than the movement of protons. Archaea such as Methanococcus
also contain the A1Ao synthase, a form of the enzyme that contains additional proteins with little similarity in sequence to other bacterial and eukaryotic ATP synthase subunits. It is possible that, in some species, the A1Ao form of the enzyme is a specialized sodium-driven ATP synthase, but this might not be true in all cases.
because it is a strong oxidizing agent. The reduction of oxygen does involve potentially harmful intermediates. Although the transfer of four electrons and four protons reduces oxygen to water, which is harmless, transfer of one or two electrons produces superoxide
or peroxide
anions, which are dangerously reactive.

These reactive oxygen species
and their reaction products, such as the hydroxyl
radical, are very harmful to cells, as they oxidize proteins and cause mutation
s in DNA
. This cellular damage might contribute to disease
and is proposed as one cause of aging.
The cytochrome c oxidase complex is highly efficient at reducing oxygen to water, and it releases very few partly reduced intermediates; however small amounts of superoxide anion and peroxide are produced by the electron transport chain. Particularly important is the reduction of coenzyme Q
in complex III, as a highly reactive ubisemiquinone free radical is formed as an intermediate in the Q cycle. This unstable species can lead to electron "leakage" when electrons transfer directly to oxygen, forming superoxide. As the production of reactive oxygen species by these proton-pumping complexes is greatest at high membrane potentials, it has been proposed that mitochondria regulate their activity to maintain the membrane potential within a narrow range that balances ATP production against oxidant generation. For instance, oxidants can activate uncoupling protein
s that reduce membrane potential.
To counteract these reactive oxygen species, cells contain numerous antioxidant
systems, including antioxidant vitamin
s such as vitamin C
and vitamin E
, and antioxidant enzymes such as superoxide dismutase
, catalase
, and peroxidases, which detoxify the reactive species, limiting damage to the cell.
s and toxin
s that inhibit oxidative phosphorylation. Although any one of these toxins inhibits only one enzyme in the electron transport chain, inhibition of any step in this process will halt the rest of the process. For example, if oligomycin
inhibits ATP synthase, protons cannot pass back into the mitochondrion. As a result, the proton pumps are unable to operate, as the gradient becomes too strong for them to overcome. NADH is then no longer oxidized and the citric acid cycle ceases to operate because the concentration of NAD+ falls below the concentration that these enzymes can use.
Not all inhibitors of oxidative phosphorylation are toxins. In brown adipose tissue
, regulated proton channels called uncoupling protein
s can uncouple respiration from ATP synthesis. This rapid respiration produces heat, and is particularly important as a way of maintaining body temperature for hibernating
animals, although these proteins may also have a more general function in cells' responses to stress.
of a vital role for phosphate in cellular fermentation
, but initially only sugar phosphates
were known to be involved. However, in the early 1940s, the link between the oxidation of sugars and the generation of ATP was firmly established by Herman Kalckar
, confirming the central role of ATP in energy transfer that had been proposed by Fritz Albert Lipmann
in 1941. Later, in 1949, Morris Friedkin and Albert L. Lehninger
proved that the coenzyme NADH linked metabolic pathways such as the citric acid cycle and the synthesis of ATP.
For another twenty years, the mechanism by which ATP is generated remained mysterious, with scientists searching for an elusive "high-energy intermediate" that would link oxidation and phosphorylation reactions. This puzzle was solved by Peter D. Mitchell
with the publication of the chemiosmotic theory in 1961. At first, this proposal was highly controversial, but it was slowly accepted and Mitchell was awarded a Nobel prize
in 1978. Subsequent research concentrated on purifying and characterizing the enzymes involved, with major contributions being made by David E. Green
on the complexes of the electron-transport chain, as well as Efraim Racker
on the ATP synthase. A critical step towards solving the mechanism of the ATP synthase was provided by Paul D. Boyer
, by his development in 1973 of the "binding change" mechanism, followed by his radical proposal of rotational catalysis in 1982. More recent work has included structural studies
on the enzymes involved in oxidative phosphorylation by John E. Walker
, with Walker and Boyer being awarded a Nobel Prize in 1997.
Structural resources
Metabolic pathway
In biochemistry, metabolic pathways are series of chemical reactions occurring within a cell. In each pathway, a principal chemical is modified by a series of chemical reactions. Enzymes catalyze these reactions, and often require dietary minerals, vitamins, and other cofactors in order to function...
that uses energy released by the oxidation
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
of nutrient
Nutrient
A nutrient is a chemical that an organism needs to live and grow or a substance used in an organism's metabolism which must be taken in from its environment. They are used to build and repair tissues, regulate body processes and are converted to and used as energy...
s to produce adenosine triphosphate
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
(ATP). Although the many forms of life on earth use a range of different nutrients, almost all aerobic organism
Aerobic organism
An aerobic organism or aerobe is an organism that can survive and grow in an oxygenated environment.Faculitative anaerobes grow and survive in an oxygenated environment and so do aerotolerant anaerobes.-Glucose:...
s carry out oxidative phosphorylation to produce ATP, the molecule that supplies energy to metabolism
Metabolism
Metabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
. This pathway is probably so pervasive because it is a highly efficient way of releasing energy, compared to alternative fermentation
Fermentation (biochemistry)
Fermentation is the process of extracting energy from the oxidation of organic compounds, such as carbohydrates, using an endogenous electron acceptor, which is usually an organic compound. In contrast, respiration is where electrons are donated to an exogenous electron acceptor, such as oxygen,...
processes such as anaerobic glycolysis
Glycolysis
Glycolysis is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+...
.
During oxidative phosphorylation, electrons are transferred from electron donors
Reducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
to electron acceptors
Oxidizing agent
An oxidizing agent can be defined as a substance that removes electrons from another reactant in a redox chemical reaction...
such as oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, in redox reactions. These redox reactions release energy, which is used to form ATP. In eukaryote
Eukaryote
A eukaryote is an organism whose cells contain complex structures enclosed within membranes. Eukaryotes may more formally be referred to as the taxon Eukarya or Eukaryota. The defining membrane-bound structure that sets eukaryotic cells apart from prokaryotic cells is the nucleus, or nuclear...
s, these redox reactions are carried out by a series of protein complex
Protein complex
A multiprotein complex is a group of two or more associated polypeptide chains. If the different polypeptide chains contain different protein domain, the resulting multiprotein complex can have multiple catalytic functions...
es within mitochondria
Mitochondrion
In cell biology, a mitochondrion is a membrane-enclosed organelle found in most eukaryotic cells. These organelles range from 0.5 to 1.0 micrometers in diameter...
, whereas, in prokaryote
Prokaryote
The prokaryotes are a group of organisms that lack a cell nucleus , or any other membrane-bound organelles. The organisms that have a cell nucleus are called eukaryotes. Most prokaryotes are unicellular, but a few such as myxobacteria have multicellular stages in their life cycles...
s, these proteins are located in the cells' inner membranes. These linked sets of proteins are called electron transport chain
Electron transport chain
An electron transport chain couples electron transfer between an electron donor and an electron acceptor with the transfer of H+ ions across a membrane. The resulting electrochemical proton gradient is used to generate chemical energy in the form of adenosine triphosphate...
s. In eukaryotes, five main protein complexes are involved, whereas in prokaryotes many different enzymes are present, using a variety of electron donors and acceptors.
The energy released by electrons flowing through this electron transport chain is used to transport protons across the inner mitochondrial membrane
Inner mitochondrial membrane
The mitochondrial inner membrane forms internal compartments known as cristae, which allow greater space for the proteins such as cytochromes to function properly and efficiently. The electron transport chain is located on the inner membrane of the mitochondria...
, in a process called chemiosmosis
Chemiosmosis
Chemiosmosis is the movement of ions across a selectively permeable membrane, down their electrochemical gradient. More specifically, it relates to the generation of ATP by the movement of hydrogen ions across a membrane during cellular respiration....
. This generates potential energy
Potential energy
In physics, potential energy is the energy stored in a body or in a system due to its position in a force field or due to its configuration. The SI unit of measure for energy and work is the Joule...
in the form of a pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
gradient and an electrical potential
Membrane potential
Membrane potential is the difference in electrical potential between the interior and exterior of a biological cell. All animal cells are surrounded by a plasma membrane composed of a lipid bilayer with a variety of types of proteins embedded in it...
across this membrane. This store of energy is tapped by allowing protons to flow back across the membrane and down this gradient, through a large enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
called ATP synthase
ATP synthase
right|thumb|300px|Molecular model of ATP synthase by X-ray diffraction methodATP synthase is an important enzyme that provides energy for the cell to use through the synthesis of adenosine triphosphate . ATP is the most commonly used "energy currency" of cells from most organisms...
. This enzyme uses this energy to generate ATP from adenosine diphosphate
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
(ADP), in a phosphorylation
Phosphorylation
Phosphorylation is the addition of a phosphate group to a protein or other organic molecule. Phosphorylation activates or deactivates many protein enzymes....
reaction. This reaction is driven by the proton flow, which forces the rotation
Rotation
A rotation is a circular movement of an object around a center of rotation. A three-dimensional object rotates always around an imaginary line called a rotation axis. If the axis is within the body, and passes through its center of mass the body is said to rotate upon itself, or spin. A rotation...
of a part of the enzyme; the ATP synthase is a rotary mechanical motor.
Although oxidative phosphorylation is a vital part of metabolism
Metabolism
Metabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
, it produces reactive oxygen species
Reactive oxygen species
Reactive oxygen species are chemically reactive molecules containing oxygen. Examples include oxygen ions and peroxides. Reactive oxygen species are highly reactive due to the presence of unpaired valence shell electrons....
such as superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
and hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
, which lead to propagation of free radicals
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
, damaging cells and contributing to disease
Disease
A disease is an abnormal condition affecting the body of an organism. It is often construed to be a medical condition associated with specific symptoms and signs. It may be caused by external factors, such as infectious disease, or it may be caused by internal dysfunctions, such as autoimmune...
and, possibly, aging (senescence
Senescence
Senescence or biological aging is the change in the biology of an organism as it ages after its maturity. Such changes range from those affecting its cells and their function to those affecting the whole organism...
). The enzymes carrying out this metabolic pathway are also the target of many drugs and poisons that inhibit
Enzyme inhibitor
An enzyme inhibitor is a molecule that binds to enzymes and decreases their activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used as herbicides and pesticides...
their activities.
Overview of energy transfer by chemiosmosis
Oxidative phosphorylation works by using energyEnergy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
-releasing chemical reactions to drive energy-requiring reactions: The two sets of reactions are said to be coupled. This means one cannot occur without the other. The flow of electrons through the electron transport chain, from electron donors such as NADH to electron acceptor
Electron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. It is an oxidizing agent that, by virtue of its accepting electrons, is itself reduced in the process....
s such as oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, is an exergonic
Exergonic
Exergonic means "releasing energy in the form of work". By thermodynamic standards, work, a form of energy, is defined as moving from the system to the surroundings...
process – it releases energy, whereas the synthesis of ATP is an endergonic
Endergonic
Endergonic means "absorbing energy in the form of work." Endergonic reactions are not spontaneous...
process, which requires an input of energy. Both the electron transport chain and the ATP synthase are embedded in a membrane, and energy is transferred from electron transport chain to the ATP synthase by movements of protons across this membrane, in a process called chemiosmosis
Chemiosmosis
Chemiosmosis is the movement of ions across a selectively permeable membrane, down their electrochemical gradient. More specifically, it relates to the generation of ATP by the movement of hydrogen ions across a membrane during cellular respiration....
. In practice, this is like a simple electric circuit
Electrical network
An electrical network is an interconnection of electrical elements such as resistors, inductors, capacitors, transmission lines, voltage sources, current sources and switches. An electrical circuit is a special type of network, one that has a closed loop giving a return path for the current...
, with a current of protons being driven from the negative N-side of the membrane to the positive P-side by the proton-pumping enzymes of the electron transport chain. These enzymes are like a battery
Battery (electricity)
An electrical battery is one or more electrochemical cells that convert stored chemical energy into electrical energy. Since the invention of the first battery in 1800 by Alessandro Volta and especially since the technically improved Daniell cell in 1836, batteries have become a common power...
, as they perform work
Work (thermodynamics)
In thermodynamics, work performed by a system is the energy transferred to another system that is measured by the external generalized mechanical constraints on the system. As such, thermodynamic work is a generalization of the concept of mechanical work in mechanics. Thermodynamic work encompasses...
to drive current through the circuit. The movement of protons creates an electrochemical gradient
Electrochemical gradient
An electrochemical gradient is a spatial variation of both electrical potential and chemical concentration across a membrane; that is, a combination of the membrane potential and the pH gradient...
across the membrane, which is often called the proton-motive force. This gradient has two components: a difference in proton concentration (a H+ gradient) and a difference in electric potential
Electric potential
In classical electromagnetism, the electric potential at a point within a defined space is equal to the electric potential energy at that location divided by the charge there...
, with the N-side having a negative charge. The energy is stored largely as the difference of electric potential
Electric potential
In classical electromagnetism, the electric potential at a point within a defined space is equal to the electric potential energy at that location divided by the charge there...
s in mitochondria
Mitochondrion
In cell biology, a mitochondrion is a membrane-enclosed organelle found in most eukaryotic cells. These organelles range from 0.5 to 1.0 micrometers in diameter...
, but also as a pH gradient in chloroplast
Chloroplast
Chloroplasts are organelles found in plant cells and other eukaryotic organisms that conduct photosynthesis. Chloroplasts capture light energy to conserve free energy in the form of ATP and reduce NADP to NADPH through a complex set of processes called photosynthesis.Chloroplasts are green...
s.
ATP synthase releases this stored energy by completing the circuit and allowing protons to flow down the electrochemical gradient, back to the N-side of the membrane. This enzyme is like an electric motor
Electric motor
An electric motor converts electrical energy into mechanical energy.Most electric motors operate through the interaction of magnetic fields and current-carrying conductors to generate force...
as it uses the proton-motive force to drive the rotation of part of its structure and couples this motion to the synthesis of ATP.
The amount of energy released by oxidative phosphorylation is high, compared with the amount produced by anaerobic fermentation. Glycolysis
Glycolysis
Glycolysis is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+...
produces only 2 ATP molecules, but somewhere between 30 and 36 ATPs are produced by the oxidative phosphorylation of the 10 NADH and 2 succinate molecules made by converting one molecule of glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
to carbon dioxide and water, while each cycle of beta oxidation
Beta oxidation
Beta oxidation is the process by which fatty acids, in the form of Acyl-CoA molecules, are broken down in mitochondria and/or in peroxisomes to generate Acetyl-CoA, the entry molecule for the Citric Acid cycle....
of a fatty acid
Fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
yields about 14 ATPs. These ATP yields are theoretical maximum values; in practice, some protons leak across the membrane, lowering the yield of ATP.
Electron and proton transfer molecules
The electron transport chain carries both protons and electrons, passing electrons from donors to acceptors, and transporting protons across a membrane. These processes use both soluble and protein-bound transfer molecules. In mitochondria, electrons are transferred within the intermembrane space by the water-soluble electron transfer protein cytochrome cCytochrome c
The Cytochrome complex, or cyt c is a small heme protein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins. Cytochrome c is a highly soluble protein, unlike other cytochromes, with a solubility of about 100 g/L and is an...
. This carries only electrons, and these are transferred by the reduction and oxidation of an iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
atom that the protein holds within a heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
group in its structure. Cytochrome c is also found in some bacteria, where it is located within the periplasmic space
Periplasmic space
The periplasmic space or periplasm is a space between the peptidoglycan cell wall and inner membrane of Gram-negative bacteria or the equivalent space outside the inner membrane of Gram-positive bacteria. It may constitute up to 40% of the total cell volume in Gram-negative species, and is...
.
Within the inner mitochondrial membrane, the lipid
Lipid
Lipids constitute a broad group of naturally occurring molecules that include fats, waxes, sterols, fat-soluble vitamins , monoglycerides, diglycerides, triglycerides, phospholipids, and others...
-soluble electron carrier coenzyme Q10 (Q) carries both electrons and protons by a redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
cycle. This small benzoquinone
1,4-Benzoquinone
1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic. Impure samples are often dark-colored due to the presence...
molecule is very hydrophobic
Hydrophobe
In chemistry, hydrophobicity is the physical property of a molecule that is repelled from a mass of water....
, so it diffuses freely within the membrane. When Q accepts two electrons and two protons, it becomes reduced to the ubiquinol
Hydroquinone
Hydroquinone, also benzene-1,4-diol or quinol, is an aromatic organic compound that is a type of phenol, having the chemical formula C6H42. Its chemical structure, shown in the table at right, has two hydroxyl groups bonded to a benzene ring in a para position. It is a white granular solid...
form (QH2); when QH2 releases two electrons and two protons, it becomes oxidized back to the ubiquinone (Q) form. As a result, if two enzymes are arranged so that Q is reduced on one side of the membrane and QH2 oxidized on the other, ubiquinone will couple these reactions and shuttle protons across the membrane. Some bacterial electron transport chains use different quinones, such as menaquinone
Vitamin K
Vitamin K is a group of structurally similar, fat soluble vitamins that are needed for the posttranslational modification of certain proteins required for blood coagulation and in metabolic pathways in bone and other tissue. They are 2-methyl-1,4-naphthoquinone derivatives...
, in addition to ubiquinone.
Within proteins, electrons are transferred between flavin cofactors, iron–sulfur clusters, and cytochromes. There are several types of iron–sulfur cluster. The simplest kind found in the electron transfer chain consists of two iron atoms joined by two atoms of inorganic sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
; these are called [2Fe–2S] clusters. The second kind, called [4Fe–4S], contains a cube of four iron atoms and four sulfur atoms. Each iron atom in these clusters is coordinated by an additional amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
, usually by the sulfur atom of cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
. Metal ion cofactors undergo redox reactions without binding or releasing protons, so in the electron transport chain they serve solely to transport electrons through proteins. Electrons move quite long distances through proteins by hopping along chains of these cofactors. This occurs by quantum tunnelling
Quantum tunnelling
Quantum tunnelling refers to the quantum mechanical phenomenon where a particle tunnels through a barrier that it classically could not surmount. This plays an essential role in several physical phenomena, such as the nuclear fusion that occurs in main sequence stars like the sun, and has important...
, which is rapid over distances of less than 1.4 m.
Eukaryotic electron transport chains
Many catabolic biochemical processes, such as glycolysisGlycolysis
Glycolysis is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+...
, the citric acid cycle
Citric acid cycle
The citric acid cycle — also known as the tricarboxylic acid cycle , the Krebs cycle, or the Szent-Györgyi-Krebs cycle — is a series of chemical reactions which is used by all aerobic living organisms to generate energy through the oxidization of acetate derived from carbohydrates, fats and...
, and beta oxidation
Beta oxidation
Beta oxidation is the process by which fatty acids, in the form of Acyl-CoA molecules, are broken down in mitochondria and/or in peroxisomes to generate Acetyl-CoA, the entry molecule for the Citric Acid cycle....
, produce the reduced coenzyme NADH. This coenzyme contains electrons that have a high transfer potential
Standard electrode potential
In electrochemistry, the standard electrode potential, abbreviated E° or E , is the measure of individual potential of a reversible electrode at standard state, which is with solutes at an effective concentration of 1 mol dm−3, and gases at a pressure of 1 atm...
; in other words, they will release a large amount of energy upon oxidation. However, the cell does not release this energy all at once, as this would be an uncontrollable reaction. Instead, the electrons are removed from NADH and passed to oxygen through a series of enzymes that each release a small amount of the energy. This set of enzymes, consisting of complexes I through IV, is called the electron transport chain and is found in the inner membrane of the mitochondrion. Succinate
Succinic acid
Succinic acid is a dicarboxylic acid. Succinate plays a biochemical role in the citric acid cycle. The name derives from Latin succinum, meaning amber, from which the acid may be obtained....
is also oxidized by the electron transport chain, but feeds into the pathway at a different point.
In eukaryote
Eukaryote
A eukaryote is an organism whose cells contain complex structures enclosed within membranes. Eukaryotes may more formally be referred to as the taxon Eukarya or Eukaryota. The defining membrane-bound structure that sets eukaryotic cells apart from prokaryotic cells is the nucleus, or nuclear...
s, the enzymes in this electron transport system use the energy released from the oxidation of NADH to pump proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s across the inner membrane of the mitochondrion. This causes protons to build up in the intermembrane space
Intermembrane space
The intermembrane space also known as IMS is the region between the inner membrane and the outer membrane of a mitochondrion or a chloroplast. The main function of the intermembrane space is oxidative phosphorylation....
, and generates an electrochemical gradient
Electrochemical gradient
An electrochemical gradient is a spatial variation of both electrical potential and chemical concentration across a membrane; that is, a combination of the membrane potential and the pH gradient...
across the membrane. The energy stored in this potential is then used by ATP synthase to produce ATP. Oxidative phosphorylation in the eukaryotic mitochondrion is the best-understood example of this process. The mitochondrion is present in almost all eukaryotes, with the exception of anaerobic protozoa such as Trichomonas vaginalis
Trichomonas vaginalis
Trichomonas vaginalis is an anaerobic, flagellated protozoan, a form of microorganism. The parasitic microorganism is the causative agent of trichomoniasis, and is the most common pathogenic protozoan infection of humans in industrialized countries. Infection rates between men and women are the...
that instead reduce protons to hydrogen in a remnant mitochondrion called a hydrogenosome
Hydrogenosome
A hydrogenosome is a membrane-enclosed organelle of some anaerobic ciliates, trichomonads and fungi. The hydrogenosomes of trichomonads produce molecular hydrogen, acetate, carbon dioxide and ATP by the combined actions of pyruvate:ferredoxin oxido-reductase, hydrogenase, acetate:succinate CoA...
.
| Respiratory enzyme | Redox pair Redox Redox reactions describe all chemical reactions in which atoms have their oxidation state changed.... |
Midpoint potential (Volts) |
|---|---|---|
| NADH dehydrogenase NADH dehydrogenase NADH dehydrogenase is an enzyme located in the inner mitochondrial membrane that catalyzes the transfer of electrons from NADH to coenzyme Q... |
NAD+ Nicotinamide adenine dinucleotide Nicotinamide adenine dinucleotide, abbreviated NAD, is a coenzyme found in all living cells. The compound is a dinucleotide, since it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide.In metabolism, NAD is involved... / NADH Nicotinamide adenine dinucleotide Nicotinamide adenine dinucleotide, abbreviated NAD, is a coenzyme found in all living cells. The compound is a dinucleotide, since it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide.In metabolism, NAD is involved... |
−0.32 |
| Succinate dehydrogenase | FMN Flavin mononucleotide Flavin mononucleotide , or riboflavin-5′-phosphate, is a biomolecule produced from riboflavin by the enzyme riboflavin kinase and functions as prosthetic group of various oxidoreductases including NADH dehydrogenase as well as cofactor in biological blue-light photo receptors... or FAD FAD In biochemistry, flavin adenine dinucleotide is a redox cofactor involved in several important reactions in metabolism. FAD can exist in two different redox states, which it converts between by accepting or donating electrons. The molecule consists of a riboflavin moiety bound to the phosphate... / FMNH2 or FADH2 |
−0.20 |
| Cytochrome bc1 complex Coenzyme Q - cytochrome c reductase In enzymology, a ubiquinol—cytochrome-c reductase is an enzyme that catalyzes the chemical reactionThus, the two substrates of this enzyme are dihydroquinone and ferri- cytochrome c, whereas its 3 products are quinone , ferro- cytochrome c, and H+.This enzyme belongs to the family of... |
Coenzyme Q10ox / Coenzyme Q10red | +0.06 |
| Cytochrome bc1 complex | Cytochrome b Cytochrome b Cytochrome b/b6 is the main subunit of transmembrane cytochrome bc1 and b6f complexes. In addition, it commonly refers to a region of mtDNA used for population genetics and phylogenetics.- Function :... ox / Cytochrome bred |
+0.12 |
| Complex IV Cytochrome c oxidase The enzyme cytochrome c oxidase or Complex IV is a large transmembrane protein complex found in bacteria and the mitochondrion.It is the last enzyme in the respiratory electron transport chain of mitochondria located in the mitochondrial membrane... |
Cytochrome c Cytochrome c The Cytochrome complex, or cyt c is a small heme protein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins. Cytochrome c is a highly soluble protein, unlike other cytochromes, with a solubility of about 100 g/L and is an... ox / Cytochrome cred |
+0.22 |
| Complex IV | Cytochrome aox / Cytochrome ared | +0.29 |
| Complex IV | O2 / HO− | +0.82 |
| Conditions: pH = 7 | ||
NADH-coenzyme Q oxidoreductase (complex I)

NADH dehydrogenase
NADH dehydrogenase is an enzyme located in the inner mitochondrial membrane that catalyzes the transfer of electrons from NADH to coenzyme Q...
, also known as NADH dehydrogenase or complex I, is the first protein in the electron transport chain. Complex I is a giant enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
with the mammalian complex I having 46 subunits and a molecular mass of about 1,000 kilodaltons
Atomic mass unit
The unified atomic mass unit or dalton is a unit that is used for indicating mass on an atomic or molecular scale. It is defined as one twelfth of the rest mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state, and has a value of...
(kDa). The structure is known in detail only from a bacterium; in most organisms the complex resembles a boot with a large “ball” poking out from the membrane into the mitochondrion. The genes that encode the individual proteins are contained in both the cell nucleus
Cell nucleus
In cell biology, the nucleus is a membrane-enclosed organelle found in eukaryotic cells. It contains most of the cell's genetic material, organized as multiple long linear DNA molecules in complex with a large variety of proteins, such as histones, to form chromosomes. The genes within these...
and the mitochondrial genome, as is the case for many enzymes present in the mitochondrion.
The reaction that is catalyzed by this enzyme is the two electron reduction by NADH
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide, abbreviated NAD, is a coenzyme found in all living cells. The compound is a dinucleotide, since it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide.In metabolism, NAD is involved...
of coenzyme Q10 or ubiquinone (represented as Q in the equation below), a lipid-soluble quinone
Quinone
A quinone is a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C– groups with any necessary rearrangement of double bonds," resulting in "a fully conjugated cyclic dione structure."...
that is found in the mitochondrion membrane:

The start of the reaction, and indeed of the entire electron chain, is the binding of a NADH molecule to complex I and the donation of two electrons. The electrons enter complex I via a prosthetic group attached to the complex, flavin mononucleotide
Flavin mononucleotide
Flavin mononucleotide , or riboflavin-5′-phosphate, is a biomolecule produced from riboflavin by the enzyme riboflavin kinase and functions as prosthetic group of various oxidoreductases including NADH dehydrogenase as well as cofactor in biological blue-light photo receptors...
(FMN). The addition of electrons to FMN converts it to its reduced form, FMNH2. The electrons are then transferred through a series of iron–sulfur clusters
Iron-sulfur cluster
For biological Fe-S clusters, see iron-sulfur proteins.Iron-sulfur clusters are ensembles of iron and sulfide centres. Fe-S clusters are most often discussed in the context of the biological role for iron-sulfur proteins. Many Fe-S clusters are known in the area of organometallic chemistry and as...
: the second kind of prosthetic group present in the complex. There are both [2Fe–2S] and [4Fe–4S] iron–sulfur clusters in complex I.
As the electrons pass through this complex, four protons are pumped from the matrix into the intermembrane space. Exactly how this occurs is unclear, but it seems to involve conformational change
Conformational change
A macromolecule is usually flexible and dynamic. It can change its shape in response to changes in its environment or other factors; each possible shape is called a conformation, and a transition between them is called a conformational change...
s in complex I that cause the protein to bind protons on the N-side of the membrane and release them on the P-side of the membrane. Finally, the electrons are transferred from the chain of iron–sulfur clusters to a ubiquinone molecule in the membrane. Reduction of ubiquinone also contributes to the generation of a proton gradient, as two protons are taken up from the matrix as it is reduced to ubiquinol
Ubiquinol
Ubiquinol is an electron-rich form of coenzyme Q10.The natural ubiquinol form of coenzyme Q10 is 2,3-dimethoxy-5-methyl-6-poly prenyl-1,4-benzoquinol, where the polyprenylated side chain is 9-10 units long in mammals...
(QH2).
Succinate-Q oxidoreductase (complex II)

Succinate - coenzyme Q reductase
Succinate dehydrogenase or succinate-coenzyme Q reductase or Complex II is an enzyme complex, bound to the inner mitochondrial membrane of mammalian mitochondria and many bacterial cells...
, also known as complex II or succinate dehydrogenase, is a second entry point to the electron transport chain. It is unusual because it is the only enzyme that is part of both the citric acid cycle and the electron transport chain. Complex II consists of four protein subunits and contains a bound flavin adenine dinucleotide
FAD
In biochemistry, flavin adenine dinucleotide is a redox cofactor involved in several important reactions in metabolism. FAD can exist in two different redox states, which it converts between by accepting or donating electrons. The molecule consists of a riboflavin moiety bound to the phosphate...
(FAD) cofactor, iron–sulfur clusters, and a heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
group that does not participate in electron transfer to coenzyme Q, but is believed to be important in decreasing production of reactive oxygen species. It oxidizes succinate
Succinic acid
Succinic acid is a dicarboxylic acid. Succinate plays a biochemical role in the citric acid cycle. The name derives from Latin succinum, meaning amber, from which the acid may be obtained....
to fumarate
Fumaric acid
Fumaric acid or trans-butenedioic acid is the chemical compound with the formula HO2CCH=CHCO2H. This white crystalline compound is one of two isomeric unsaturated dicarboxylic acids, the other being maleic acid. In fumaric acid the carboxylic acid groups are trans and in maleic acid they are cis...
and reduces ubiquinone. As this reaction releases less energy than the oxidation of NADH, complex II does not transport protons across the membrane and does not contribute to the proton gradient.

In some eukaryotes, such as the parasitic worm
Parasitic worm
Parasitic worms or helminths are a division of eukaryoticparasites that, unlike external parasites such as lice and fleas, live inside their host. They are worm-like organisms that live and feed off living hosts, receiving nourishment and protection while disrupting their hosts' nutrient...
Ascaris suum
Large roundworm of pigs
-Introduction:Ascaris suum, also known as large roundworm of pigs, is a parasitic nematode that causes ascariasis in pigs. Until recently it was believed that Ascaris suum could also infect humans; however, it has now been established that humans are affected by a related parasite, Ascaris...
, an enzyme similar to complex II, fumarate reductase (menaquinol:fumarate
oxidoreductase, or QFR), operates in reverse to oxidize ubiquinol and reduce fumarate. This allows the worm to survive in the anaerobic environment of the large intestine
Large intestine
The large intestine is the third-to-last part of the digestive system — — in vertebrate animals. Its function is to absorb water from the remaining indigestible food matter, and then to pass useless waste material from the body...
, carrying out anaerobic oxidative phosphorylation with fumarate as the electron acceptor. Another unconventional function of complex II is seen in the malaria
Malaria
Malaria is a mosquito-borne infectious disease of humans and other animals caused by eukaryotic protists of the genus Plasmodium. The disease results from the multiplication of Plasmodium parasites within red blood cells, causing symptoms that typically include fever and headache, in severe cases...
parasite Plasmodium falciparum
Plasmodium falciparum
Plasmodium falciparum is a protozoan parasite, one of the species of Plasmodium that cause malaria in humans. It is transmitted by the female Anopheles mosquito. Malaria caused by this species is the most dangerous form of malaria, with the highest rates of complications and mortality...
. Here, the reversed action of complex II as an oxidase is important in regenerating ubiquinol, which the parasite uses in an unusual form of pyrimidine
Pyrimidine
Pyrimidine is a heterocyclic aromatic organic compound similar to benzene and pyridine, containing two nitrogen atoms at positions 1 and 3 of the six-member ring...
biosynthesis.
Electron transfer flavoprotein-Q oxidoreductase
Electron transfer flavoprotein-ubiquinone oxidoreductaseElectron-transferring-flavoprotein dehydrogenase
Electron-transferring-flavoprotein dehydrogenase is an enzyme that transfers electrons from electron-transferring flavoprotein in the mitochondrial matrix, to the ubiquinone pool in the inner mitochondrial membrane. It is part of the electron transport chain...
(ETF-Q oxidoreductase), also known as electron transferring-flavoprotein dehydrogenase, is a third entry point to the electron transport chain. It is an enzyme that accepts electrons from electron-transferring flavoprotein
Electron-transferring flavoprotein
An electron transfer flavoprotein is a flavoprotein and functions as a specific electron acceptors for primary dehydrogenases, transferring the electrons to terminal respiratory systems such as electron-transferring-flavoprotein dehydrogenase...
in the mitochondrial matrix, and uses these electrons to reduce ubiquinone. This enzyme contains a flavin and a [4Fe–4S] cluster, but, unlike the other respiratory complexes, it attaches to the surface of the membrane and does not cross the lipid bilayer.

In mammals, this metabolic pathway is important in beta oxidation
Beta oxidation
Beta oxidation is the process by which fatty acids, in the form of Acyl-CoA molecules, are broken down in mitochondria and/or in peroxisomes to generate Acetyl-CoA, the entry molecule for the Citric Acid cycle....
of fatty acid
Fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
s and catabolism of amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s and choline
Choline
Choline is a water-soluble essential nutrient. It is usually grouped within the B-complex vitamins. Choline generally refers to the various quaternary ammonium salts containing the N,N,N-trimethylethanolammonium cation....
, as it accepts electrons from multiple acetyl-CoA
Acetyl-CoA
Acetyl coenzyme A or acetyl-CoA is an important molecule in metabolism, used in many biochemical reactions. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. In chemical structure, acetyl-CoA is the thioester...
dehydrogenases. In plants, ETF-Q oxidoreductase is also important in the metabolic responses that allow survival in extended periods of darkness.
Q-cytochrome c oxidoreductase (complex III)
Q-cytochrome c oxidoreductaseCoenzyme Q - cytochrome c reductase
In enzymology, a ubiquinol—cytochrome-c reductase is an enzyme that catalyzes the chemical reactionThus, the two substrates of this enzyme are dihydroquinone and ferri- cytochrome c, whereas its 3 products are quinone , ferro- cytochrome c, and H+.This enzyme belongs to the family of...
is also known as cytochrome c reductase, cytochrome bc1 complex, or simply complex III. In mammals, this enzyme is a dimer
Protein dimer
In biochemistry, a dimer is a macromolecular complex formed by two, usually non-covalently bound, macromolecules like proteins or nucleic acids...
, with each subunit complex containing 11 protein subunits, an [2Fe-2S] iron–sulfur cluster and three cytochrome
Cytochrome
Cytochromes are, in general, membrane-bound hemoproteins that contain heme groups and carry out electron transport.They are found either as monomeric proteins or as subunits of bigger enzymatic complexes that catalyze redox reactions....
s: one cytochrome
Cytochrome
Cytochromes are, in general, membrane-bound hemoproteins that contain heme groups and carry out electron transport.They are found either as monomeric proteins or as subunits of bigger enzymatic complexes that catalyze redox reactions....
c1 and two b cytochromes. A cytochrome is a kind of electron-transferring protein that contains at least one heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
group. The iron atoms inside complex III’s heme groups alternate between a reduced ferrous (+2) and oxidized ferric (+3) state as the electrons are transferred through the protein.
The reaction catalyzed by complex III is the oxidation of one molecule of ubiquinol
Ubiquinol
Ubiquinol is an electron-rich form of coenzyme Q10.The natural ubiquinol form of coenzyme Q10 is 2,3-dimethoxy-5-methyl-6-poly prenyl-1,4-benzoquinol, where the polyprenylated side chain is 9-10 units long in mammals...
and the reduction of two molecules of cytochrome c
Cytochrome c
The Cytochrome complex, or cyt c is a small heme protein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins. Cytochrome c is a highly soluble protein, unlike other cytochromes, with a solubility of about 100 g/L and is an...
, a heme protein loosely associated with the mitochondrion. Unlike coenzyme Q, which carries two electrons, cytochrome c carries only one electron.

As only one of the electrons can be transferred from the QH2 donor to a cytochrome c acceptor at a time, the reaction mechanism of complex III is more elaborate than those of the other respiratory complexes, and occurs in two steps called the Q cycle. In the first step, the enzyme binds three substrates, first, QH2, which is then oxidized, with one electron being passed to the second substrate, cytochrome c. The two protons released from QH2 pass into the intermembrane space. The third substrate is Q, which accepts the second electron from the QH2 and is reduced to Q.-, which is the ubisemiquinone
Semiquinone
Semiquinone is a free radical resulting from the removal of one hydrogen atom with its electron during the process of dehydrogenation of a hydroquinone to quinone or alternatively the addition of a single H atom to a quinone....
free radical. The first two substrates are released, but this ubisemiquinone intermediate remains bound. In the second step, a second molecule of QH2 is bound and again passes its first electron to a cytochrome c acceptor. The second electron is passed to the bound ubisemiquinone, reducing it to QH2 as it gains two protons from the mitochondrial matrix. This QH2 is then released from the enzyme.
As coenzyme Q is reduced to ubiquinol on the inner side of the membrane and oxidized to ubiquinone on the other, a net transfer of protons across the membrane occurs, adding to the proton gradient. The rather complex two-step mechanism by which this occurs is important, as it increases the efficiency of proton transfer. If, instead of the Q cycle, one molecule of QH2 were used to directly reduce two molecules of cytochrome c, the efficiency would be halved, with only one proton transferred per cytochrome c reduced.
Cytochrome c oxidase (complex IV)

Cytochrome c oxidase
The enzyme cytochrome c oxidase or Complex IV is a large transmembrane protein complex found in bacteria and the mitochondrion.It is the last enzyme in the respiratory electron transport chain of mitochondria located in the mitochondrial membrane...
, also known as complex IV, is the final protein complex in the electron transport chain. The mammalian enzyme has an extremely complicated structure and contains 13 subunits, two heme groups, as well as multiple metal ion cofactors – in all three atoms of copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
, one of magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
and one of zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
.
This enzyme mediates the final reaction in the electron transport chain and transfers electrons to oxygen, while pumping protons across the membrane. The final electron acceptor
Electron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. It is an oxidizing agent that, by virtue of its accepting electrons, is itself reduced in the process....
oxygen, which is also called the terminal electron acceptor, is reduced to water in this step. Both the direct pumping of protons and the consumption of matrix protons in the reduction of oxygen contribute to the proton gradient. The reaction catalyzed is the oxidation of cytochrome c and the reduction of oxygen:

Alternative reductases and oxidases
Many eukaryotic organisms have electron transport chains that differ from the much-studied mammalian enzymes described above. For example, plantPlant
Plants are living organisms belonging to the kingdom Plantae. Precise definitions of the kingdom vary, but as the term is used here, plants include familiar organisms such as trees, flowers, herbs, bushes, grasses, vines, ferns, mosses, and green algae. The group is also called green plants or...
s have alternative NADH oxidases, which oxidize NADH in the cytosol rather than in the mitochondrial matrix, and pass these electrons to the ubiquinone pool. These enzymes do not transport protons, and, therefore, reduce ubiquinone without altering the electrochemical gradient across the inner membrane.
Another example of a divergent electron transport chain is the alternative oxidase
Alternative oxidase
The alternative oxidase is an enzyme that forms part of the electron transport chain in plants, as well as some fungi, protists and possibly some animals. Sequences similar to the plant oxidase have also been identified in bacterial genomes....
, which is found in plant
Plant
Plants are living organisms belonging to the kingdom Plantae. Precise definitions of the kingdom vary, but as the term is used here, plants include familiar organisms such as trees, flowers, herbs, bushes, grasses, vines, ferns, mosses, and green algae. The group is also called green plants or...
s, as well as some fungi
Fungus
A fungus is a member of a large group of eukaryotic organisms that includes microorganisms such as yeasts and molds , as well as the more familiar mushrooms. These organisms are classified as a kingdom, Fungi, which is separate from plants, animals, and bacteria...
, protist
Protist
Protists are a diverse group of eukaryotic microorganisms. Historically, protists were treated as the kingdom Protista, which includes mostly unicellular organisms that do not fit into the other kingdoms, but this group is contested in modern taxonomy...
s, and possibly some animals. This enzyme transfers electrons directly from ubiquinol to oxygen.
The electron transport pathways produced by these alternative NADH and ubiquinone oxidases have lower ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
yields than the full pathway. The advantages produced by a shortened pathway are not entirely clear. However, the alternative oxidase is produced in response to stresses such as cold, reactive oxygen species
Reactive oxygen species
Reactive oxygen species are chemically reactive molecules containing oxygen. Examples include oxygen ions and peroxides. Reactive oxygen species are highly reactive due to the presence of unpaired valence shell electrons....
, and infection by pathogens, as well as other factors that inhibit the full electron transport chain. Alternative pathways might, therefore, enhance an organisms' resistance to injury, by reducing oxidative stress
Oxidative stress
Oxidative stress represents an imbalance between the production and manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage...
.
Organization of complexes
The original model for how the respiratory chain complexes are organized was that they diffuse freely and independently in the mitochondrial membrane. However, recent data suggest that the complexes might form higher-order structures called supercomplexes or "respirasomes." In this model, the various complexes exist as organized sets of interacting enzymes. These associations might allow channeling of substrates between the various enzyme complexes, increasing the rate and efficiency of electron transfer. Within such mammalian supercomplexes, some components would be present in higher amounts than others, with some data suggesting a ratio between complexes I/II/III/IV and the ATP synthase of approximately 1:1:3:7:4. However, the debate over this supercomplex hypothesis is not completely resolved, as some data do not appear to fit with this model.Prokaryotic electron transport chains
In contrast to the general similarity in structure and function of the electron transport chains in eukaryotes, bacteriaBacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
and archaea
Archaea
The Archaea are a group of single-celled microorganisms. A single individual or species from this domain is called an archaeon...
possess a large variety of electron-transfer enzymes. These use an equally wide set of chemicals as substrates. In common with eukaryotes, prokaryotic electron transport uses the energy released from the oxidation of a substrate to pump ions across a membrane and generate an electrochemical gradient. In the bacteria, oxidative phosphorylation in Escherichia coli
Escherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
is understood in most detail, while archaeal systems are at present poorly understood.
The main difference between eukaryotic and prokaryotic oxidative phosphorylation is that bacteria and archaea use many different substances to donate or accept electrons. This allows prokaryotes to grow under a wide variety of environmental conditions. In E. coli, for example, oxidative phosphorylation can be driven by a large number of pairs of reducing agents and oxidizing agents, which are listed below. The midpoint potential of a chemical measures how much energy is released when it is oxidized or reduced, with reducing agents having negative potentials and oxidizing agents positive potentials.
| Respiratory enzyme | Redox pair Redox Redox reactions describe all chemical reactions in which atoms have their oxidation state changed.... |
Midpoint potential (Volts) |
|---|---|---|
| Formate dehydrogenase Formate dehydrogenase Formate dehydrogenases are a set of enzymes that catalyse the oxidation of formate to bicarbonate, donating the electrons to a second substrate, such as NAD+ in formate:NAD+ oxidoreductase or to a cytochrome in formate:ferricytochrome-b1 oxidoreductase .-Biological function:NAD-dependent formate... |
Bicarbonate Bicarbonate In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid... / Formate Formate Formate or methanoate is the ion CHOO− or HCOO− . It is the simplest carboxylate anion. It is produced in large amounts in the hepatic mitochondria of embryonic cells and in cancer cells by the folate cycle Formate or methanoate is the ion CHOO− or HCOO− (formic acid minus one hydrogen ion). It... |
|
| Hydrogenase Hydrogenase A hydrogenase is an enzyme that catalyses the reversible oxidation of molecular hydrogen . Hydrogenases play a vital role in anaerobic metabolism.... |
Proton Proton The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number.... / Hydrogen Hydrogen Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly... |
|
| NADH dehydrogenase NADH dehydrogenase NADH dehydrogenase is an enzyme located in the inner mitochondrial membrane that catalyzes the transfer of electrons from NADH to coenzyme Q... |
NAD+ Nicotinamide adenine dinucleotide Nicotinamide adenine dinucleotide, abbreviated NAD, is a coenzyme found in all living cells. The compound is a dinucleotide, since it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide.In metabolism, NAD is involved... / NADH Nicotinamide adenine dinucleotide Nicotinamide adenine dinucleotide, abbreviated NAD, is a coenzyme found in all living cells. The compound is a dinucleotide, since it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide.In metabolism, NAD is involved... |
|
| Glycerol-3-phosphate dehydrogenase Glycerol-3-phosphate dehydrogenase Glycerol-3-phosphate dehydrogenase is an enzyme that catalyzes the reversible redox conversion of dihydroxyacetone phosphate to sn-glycerol 3-phosphate.... |
DHAP / Gly-3-P Glycerol 3-phosphate Glycerol 3-phosphate is an organophosphate derived from the reaction catalysed by glycerol kinase where ATP + glycerol ADP + sn-glycerol 3-phosphate. It is a component of glycerophospholipids. It should not be confused with the similarly named glycerate 3-phosphate or glyceraldehyde 3-phosphate... |
|
| Pyruvate oxidase | Acetate Acetic acid Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell... + Carbon dioxide Carbon dioxide Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom... / Pyruvate Pyruvic acid Pyruvic acid is an organic acid, a ketone, as well as the simplest of the alpha-keto acids. The carboxylate ion of pyruvic acid, CH3COCOO−, is known as pyruvate, and is a key intersection in several metabolic pathways.... |
|
| Lactate dehydrogenase Lactate dehydrogenase Lactate dehydrogenase is an enzyme present in a wide variety of organisms, including plants and animals.Lactate dehydrogenases exist in four distinct enzyme classes. Two of them are cytochrome c-dependent enzymes, each acting on either D-lactate or L-lactate... |
Pyruvate Pyruvic acid Pyruvic acid is an organic acid, a ketone, as well as the simplest of the alpha-keto acids. The carboxylate ion of pyruvic acid, CH3COCOO−, is known as pyruvate, and is a key intersection in several metabolic pathways.... / Lactate Lactic acid Lactic acid, also known as milk acid, is a chemical compound that plays a role in various biochemical processes and was first isolated in 1780 by the Swedish chemist Carl Wilhelm Scheele. Lactic acid is a carboxylic acid with the chemical formula C3H6O3... |
|
| D-amino acid dehydrogenase D-amino acid dehydrogenase D-amino-acid dehydrogenase is a bacterial enzyme that catalyses the oxidation of D-amino acids into their corresponding oxoacids. It contains both flavin and nonheme iron as cofactors... |
2-oxoacid Oxoacid An oxoacid is an acid that contains oxygen. To be more specific, it is an acid that:#contains oxygen#contains at least one other element#has at least one hydrogen atom bound to oxygen#forms an ion by the loss of one or more protons.... + ammonia Ammonia Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or... / D-amino acid Amino acid Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen... |
|
| Glucose dehydrogenase Quinoprotein glucose dehydrogenase In enzymology, a quinoprotein glucose dehydrogenase is an enzyme that catalyzes the chemical reactionThus, the two substrates of this enzyme are D-glucose and ubiquinone, whereas its two products are D-glucono-1,5-lactone and ubiquinol.... |
Gluconate Gluconic acid Gluconic acid is an organic compound with molecular formula C6H12O7 and condensed structural formula HOCH24COOH. It is one of the 16 stereoisomers of 2,3,4,5,6-pentahydroxyhexanoic acid.... / Glucose Glucose Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate... |
|
| Succinate dehydrogenase Succinate - coenzyme Q reductase Succinate dehydrogenase or succinate-coenzyme Q reductase or Complex II is an enzyme complex, bound to the inner mitochondrial membrane of mammalian mitochondria and many bacterial cells... |
Fumarate Fumaric acid Fumaric acid or trans-butenedioic acid is the chemical compound with the formula HO2CCH=CHCO2H. This white crystalline compound is one of two isomeric unsaturated dicarboxylic acids, the other being maleic acid. In fumaric acid the carboxylic acid groups are trans and in maleic acid they are cis... / Succinate Succinic acid Succinic acid is a dicarboxylic acid. Succinate plays a biochemical role in the citric acid cycle. The name derives from Latin succinum, meaning amber, from which the acid may be obtained.... |
|
| Ubiquinol oxidase Ubiquinol oxidase Ubiquinol oxidases are enzymes in the bacterial electron transport chain that oxidise ubiquinol into ubiquinone and reduce oxygen to water. These enzymes are one set of the many alternative terminal oxidases in the branched prokaryotic electron transport chain. The overall structure of the E... |
Oxygen Oxygen Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition... / Water Water Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a... |
|
| Nitrate reductase Nitrate reductase Nitrate reductases are molybdoenzymes that reduce nitrate to nitrite .* Eukaryotic nitrate reductases are part of the sulfite oxidase family of molybdoenzymes.... |
Nitrate Nitrate The nitrate ion is a polyatomic ion with the molecular formula NO and a molecular mass of 62.0049 g/mol. It is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically-bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a... / Nitrite Nitrite The nitrite ion has the chemical formula NO2−. The anion is symmetric with equal N-O bond lengths and a O-N-O bond angle of ca. 120°. On protonation the unstable weak acid nitrous acid is produced. Nitrite can be oxidised or reduced, with product somewhat dependent on the oxidizing/reducing agent... |
|
| Nitrite reductase Nitrite reductase Nitrite reductase refers to any of several classes of enzymes that catalyze the reduction of nitrite. There are two classes of NIR's. A multi haem enzyme reduces NO2 to a variety of products. Copper containing enzymes carry out a single electron transfer to produce nitric oxide.- Iron based... |
Nitrite Nitrite The nitrite ion has the chemical formula NO2−. The anion is symmetric with equal N-O bond lengths and a O-N-O bond angle of ca. 120°. On protonation the unstable weak acid nitrous acid is produced. Nitrite can be oxidised or reduced, with product somewhat dependent on the oxidizing/reducing agent... / Ammonia Ammonia Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or... |
|
| Dimethyl sulfoxide reductase DMSO reductase DMSO reductase is a molybdenum-containing enzyme capable of reducing dimethyl sulfoxide to dimethyl sulfide . This enzyme serves as the terminal reductase under anaerobic conditions in some bacteria, with DMSO being the terminal electron acceptor... |
DMSO Dimethyl sulfoxide Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water... / DMS Dimethyl sulfide Dimethyl sulfide or methylthiomethane is an organosulfur compound with the formula 2S. Dimethyl sulfide is a water-insoluble flammable liquid that boils at and has a characteristic disagreeable odor. It is a component of the smell produced from cooking of certain vegetables, notably maize,... |
|
| Trimethylamine N-oxide reductase Trimethylamine N-oxide reductase Trimethylamine N-oxide reductase is a microbial enzyme that can reduce trimethylamine N-oxide into trimethylamine , as part of the electron transport chain. The enzyme has been purified from E. coli and the photosynthetic bacteria Roseobacter denitrificans. Both the R. denitrificans and E... |
TMAO Trimethylamine N-oxide Trimethylamine N-oxide, also known by several other names and acronyms, is the organic compound with the formula 3NO. This colorless solid is usually encountered as the dihydrate. It is an oxidation product of trimethylamine and a common metabolite in animals. It is an osmolyte found in saltwater... / TMA Trimethylamine Trimethylamine is an organic compound with the formula N3. This colorless, hygroscopic, and flammable tertiary amine has a strong "fishy" odor in low concentrations and an ammonia-like odor at higher concentrations... |
|
| Fumarate reductase Fumarate reductase Fumarate reductase is the enzyme that converts fumarate to succinate, and is important in microbial metabolism as a part of anaerobic respiration.Succinate + acceptor fumarate + reduced acceptor... |
Fumarate Fumaric acid Fumaric acid or trans-butenedioic acid is the chemical compound with the formula HO2CCH=CHCO2H. This white crystalline compound is one of two isomeric unsaturated dicarboxylic acids, the other being maleic acid. In fumaric acid the carboxylic acid groups are trans and in maleic acid they are cis... / Succinate Succinic acid Succinic acid is a dicarboxylic acid. Succinate plays a biochemical role in the citric acid cycle. The name derives from Latin succinum, meaning amber, from which the acid may be obtained.... |
|
As shown above, E. coli can grow with reducing agents such as formate, hydrogen, or lactate as electron donors, and nitrate, DMSO, or oxygen as acceptors. The larger the difference in midpoint potential between an oxidizing and reducing agent, the more energy is released when they react. Out of these compounds, the succinate/fumarate pair is unusual, as its midpoint potential is close to zero. Succinate can therefore be oxidized to fumarate if a strong oxidizing agent such as oxygen is available, or fumarate can be reduced to succinate using a strong reducing agent such as formate. These alternative reactions are catalyzed by succinate dehydrogenase
Succinate - coenzyme Q reductase
Succinate dehydrogenase or succinate-coenzyme Q reductase or Complex II is an enzyme complex, bound to the inner mitochondrial membrane of mammalian mitochondria and many bacterial cells...
and fumarate reductase
Fumarate reductase
Fumarate reductase is the enzyme that converts fumarate to succinate, and is important in microbial metabolism as a part of anaerobic respiration.Succinate + acceptor fumarate + reduced acceptor...
, respectively.
Some prokaryotes use redox pairs that have only a small difference in midpoint potential. For example, nitrifying
Nitrification
Nitrification is the biological oxidation of ammonia with oxygen into nitrite followed by the oxidation of these nitrites into nitrates. Degradation of ammonia to nitrite is usually the rate limiting step of nitrification. Nitrification is an important step in the nitrogen cycle in soil...
bacteria such as Nitrobacter
Nitrobacter
Nitrobacter is genus of mostly rod-shaped, gram-negative, and chemoautotrophic bacteria.Nitrobacter plays an important role in the nitrogen cycle by oxidizing nitrite into nitrate in soil...
oxidize nitrite to nitrate, donating the electrons to oxygen. The small amount of energy released in this reaction is enough to pump protons and generate ATP, but not enough to produce NADH or NADPH directly for use in anabolism
Anabolism
Anabolism is the set of metabolic pathways that construct molecules from smaller units. These reactions require energy. One way of categorizing metabolic processes, whether at the cellular, organ or organism level is as 'anabolic' or as 'catabolic', which is the opposite...
. This problem is solved by using a nitrite oxidoreductase
Nitrite oxidoreductase
Nitrite oxidoreductase is an enzyme involved in nitrification. It is the last step in the process of aerobic ammonia oxidation, which is carried out by two groups of nitrifying bacteria: ammonia oxidizers such as Nitrosospira, Nitrosomonas and Nitrosococcus convert ammonia to nitrite, while...
to produce enough proton-motive force to run part of the electron transport chain in reverse, causing complex I to generate NADH.
Prokaryotes control their use of these electron donors and acceptors by varying which enzymes are produced, in response to environmental conditions. This flexibility is possible because different oxidases and reductases use the same ubiquinone pool. This allows many combinations of enzymes to function together, linked by the common ubiquinol intermediate. These respiratory chains therefore have a modular design
Modular design
Modular design, or "modularity in design" is an approach that subdivides a system into smaller parts that can be independently created and then used in different systems to drive multiple functionalities...
, with easily interchangeable sets of enzyme systems.
In addition to this metabolic diversity, prokaryotes also possess a range of isozyme
Isozyme
Isozymes are enzymes that differ in amino acid sequence but catalyze the same chemical reaction. These enzymes usually display different kinetic parameters Isozymes (also known as isoenzymes) are enzymes that differ in amino acid sequence but catalyze the same chemical reaction. These enzymes...
s – different enzymes that catalyze the same reaction. For example, in E. coli, there are two different types of ubiquinol oxidase using oxygen as an electron acceptor. Under highly aerobic conditions, the cell uses an oxidase with a low affinity for oxygen that can transport two protons per electron. However, if levels of oxygen fall, they switch to an oxidase that transfers only one proton per electron, but has a high affinity for oxygen.
ATP synthase (complex V)
ATP synthase, also called complex V, is the final enzyme in the oxidative phosphorylation pathway. This enzyme is found in all forms of life and functions in the same way in both prokaryotes and eukaryotes. The enzyme uses the energy stored in a proton gradient across a membrane to drive the synthesis of ATP from ADP and phosphatePhosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
(Pi). Estimates of the number of protons required to synthesize one ATP have ranged from three to four, with some suggesting cells can vary this ratio, to suit different conditions.

This phosphorylation
Phosphorylation
Phosphorylation is the addition of a phosphate group to a protein or other organic molecule. Phosphorylation activates or deactivates many protein enzymes....
reaction is an equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
, which can be shifted by altering the proton-motive force. In the absence of a proton-motive force, the ATP synthase reaction will run from right to left, hydrolyzing ATP and pumping protons out of the matrix across the membrane. However, when the proton-motive force is high, the reaction is forced to run in the opposite direction; it proceeds from left to right, allowing protons to flow down their concentration gradient and turning ADP into ATP. Indeed, in the closely related vacuolar type H+-ATPases
V-ATPase
Vacuolar-type H+-ATPase is a highly conserved evolutionarily ancient enzyme with remarkably diverse functions in eukaryotic organisms. V-ATPases acidify a wide array of intracellular organelles and pump protons across the plasma membranes of numerous cell types...
, the same reaction is used to acidify cellular compartments, by pumping protons and hydrolysing ATP.
ATP synthase is a massive protein complex with a mushroom-like shape. The mammalian enzyme complex contains 16 subunits and has a mass of approximately 600 kilodaltons. The portion embedded within the membrane is called FO and contains a ring of c subunits and the proton channel. The stalk and the ball-shaped headpiece is called F1 and is the site of ATP synthesis. The ball-shaped complex at the end of the F1 portion contains six proteins of two different kinds (three α subunits and three β subunits), whereas the "stalk" consists of one protein: the γ subunit, with the tip of the stalk extending into the ball of α and β subunits. Both the α and β subunits bind nucleotides, but only the β subunits catalyze the ATP synthesis reaction. Reaching along the side of the F1 portion and back into the membrane is a long rod-like subunit that anchors the α and β subunits into the base of the enzyme.
As protons cross the membrane through the channel in the base of ATP synthase, the FO proton-driven motor rotates. Rotation might be caused by changes in the ionization
Ionization
Ionization is the process of converting an atom or molecule into an ion by adding or removing charged particles such as electrons or other ions. This is often confused with dissociation. A substance may dissociate without necessarily producing ions. As an example, the molecules of table sugar...
of amino acids in the ring of c subunits causing electrostatic interactions that propel the ring of c subunits past the proton channel. This rotating ring in turn drives the rotation of the central axle
Axle
An axle is a central shaft for a rotating wheel or gear. On wheeled vehicles, the axle may be fixed to the wheels, rotating with them, or fixed to its surroundings, with the wheels rotating around the axle. In the former case, bearings or bushings are provided at the mounting points where the axle...
(the γ subunit stalk) within the α and β subunits. The α and β subunits are prevented from rotating themselves by the side-arm, which acts as a stator
Stator
The stator is the stationary part of a rotor system, found in an electric generator, electric motor and biological rotors.Depending on the configuration of a spinning electromotive device the stator may act as the field magnet, interacting with the armature to create motion, or it may act as the...
. This movement of the tip of the γ subunit within the ball of α and β subunits provides the energy for the active sites in the β subunits to undergo a cycle of movements that produces and then releases ATP.

Dissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant is a specific type of equilibrium constant that measures the propensity of a larger object to separate reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into...
. Finally, the active site cycles back to the open state, releasing ATP and binding more ADP and phosphate, ready for the next cycle.
In some bacteria and archaea, ATP synthesis is driven by the movement of sodium ions through the cell membrane, rather than the movement of protons. Archaea such as Methanococcus
Methanococcus
In taxonomy, Methanococcus is a genus of the Methanococcaceae.Methanococcus is a genus of coccoid methanogens. They are all mesophiles, except the thermophilic M. thermolithotrophicus and the hyperthermophilic M. jannaschii...
also contain the A1Ao synthase, a form of the enzyme that contains additional proteins with little similarity in sequence to other bacterial and eukaryotic ATP synthase subunits. It is possible that, in some species, the A1Ao form of the enzyme is a specialized sodium-driven ATP synthase, but this might not be true in all cases.
Reactive oxygen species
Molecular oxygen is an ideal terminal electron acceptorElectron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. It is an oxidizing agent that, by virtue of its accepting electrons, is itself reduced in the process....
because it is a strong oxidizing agent. The reduction of oxygen does involve potentially harmful intermediates. Although the transfer of four electrons and four protons reduces oxygen to water, which is harmless, transfer of one or two electrons produces superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
or peroxide
Peroxide
A peroxide is a compound containing an oxygen–oxygen single bond or the peroxide anion .The O−O group is called the peroxide group or peroxo group. In contrast to oxide ions, the oxygen atoms in the peroxide ion have an oxidation state of −1.The simplest stable peroxide is hydrogen peroxide...
anions, which are dangerously reactive.

These reactive oxygen species
Reactive oxygen species
Reactive oxygen species are chemically reactive molecules containing oxygen. Examples include oxygen ions and peroxides. Reactive oxygen species are highly reactive due to the presence of unpaired valence shell electrons....
and their reaction products, such as the hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
radical, are very harmful to cells, as they oxidize proteins and cause mutation
Mutation
In molecular biology and genetics, mutations are changes in a genomic sequence: the DNA sequence of a cell's genome or the DNA or RNA sequence of a virus. They can be defined as sudden and spontaneous changes in the cell. Mutations are caused by radiation, viruses, transposons and mutagenic...
s in DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
. This cellular damage might contribute to disease
Disease
A disease is an abnormal condition affecting the body of an organism. It is often construed to be a medical condition associated with specific symptoms and signs. It may be caused by external factors, such as infectious disease, or it may be caused by internal dysfunctions, such as autoimmune...
and is proposed as one cause of aging.
The cytochrome c oxidase complex is highly efficient at reducing oxygen to water, and it releases very few partly reduced intermediates; however small amounts of superoxide anion and peroxide are produced by the electron transport chain. Particularly important is the reduction of coenzyme Q
Coenzyme Q
Coenzyme Q10, also known as ubiquinone, ubidecarenone, coenzyme Q, and abbreviated at times to CoQ10 , CoQ, Q10, or Q, is a 1,4-benzoquinone, where Q refers to the quinone chemical group, and 10 refers to the number of isoprenyl chemical subunits in its tail.This oil-soluble, vitamin-like substance...
in complex III, as a highly reactive ubisemiquinone free radical is formed as an intermediate in the Q cycle. This unstable species can lead to electron "leakage" when electrons transfer directly to oxygen, forming superoxide. As the production of reactive oxygen species by these proton-pumping complexes is greatest at high membrane potentials, it has been proposed that mitochondria regulate their activity to maintain the membrane potential within a narrow range that balances ATP production against oxidant generation. For instance, oxidants can activate uncoupling protein
Uncoupling protein
An uncoupling protein is a mitochondrial inner membrane protein that can dissipate the proton gradient before it can be used to provide the energy for oxidative phosphorylation.There are five types known in mammals:* UCP1, also known as thermogenin* UCP2...
s that reduce membrane potential.
To counteract these reactive oxygen species, cells contain numerous antioxidant
Antioxidant
An antioxidant is a molecule capable of inhibiting the oxidation of other molecules. Oxidation is a chemical reaction that transfers electrons or hydrogen from a substance to an oxidizing agent. Oxidation reactions can produce free radicals. In turn, these radicals can start chain reactions. When...
systems, including antioxidant vitamin
Vitamin
A vitamin is an organic compound required as a nutrient in tiny amounts by an organism. In other words, an organic chemical compound is called a vitamin when it cannot be synthesized in sufficient quantities by an organism, and must be obtained from the diet. Thus, the term is conditional both on...
s such as vitamin C
Vitamin C
Vitamin C or L-ascorbic acid or L-ascorbate is an essential nutrient for humans and certain other animal species. In living organisms ascorbate acts as an antioxidant by protecting the body against oxidative stress...
and vitamin E
Vitamin E
Vitamin E is used to refer to a group of fat-soluble compounds that include both tocopherols and tocotrienols. There are many different forms of vitamin E, of which γ-tocopherol is the most common in the North American diet. γ-Tocopherol can be found in corn oil, soybean oil, margarine and dressings...
, and antioxidant enzymes such as superoxide dismutase
Superoxide dismutase
Superoxide dismutases are a class of enzymes that catalyze the dismutation of superoxide into oxygen and hydrogen peroxide. As such, they are an important antioxidant defense in nearly all cells exposed to oxygen...
, catalase
Catalase
Catalase is a common enzyme found in nearly all living organisms that are exposed to oxygen, where it catalyzes the decomposition of hydrogen peroxide to water and oxygen...
, and peroxidases, which detoxify the reactive species, limiting damage to the cell.
Inhibitors
There are several well-known drugDrug
A drug, broadly speaking, is any substance that, when absorbed into the body of a living organism, alters normal bodily function. There is no single, precise definition, as there are different meanings in drug control law, government regulations, medicine, and colloquial usage.In pharmacology, a...
s and toxin
Toxin
A toxin is a poisonous substance produced within living cells or organisms; man-made substances created by artificial processes are thus excluded...
s that inhibit oxidative phosphorylation. Although any one of these toxins inhibits only one enzyme in the electron transport chain, inhibition of any step in this process will halt the rest of the process. For example, if oligomycin
Oligomycin
Oligomycins are macrolides created by Streptomyces that can be poisonous to other organisms.-Function:They have use as antibiotics.In addition, oligomycin inhibits ATP synthase by blocking its proton channel , which is necessary for oxidative phosphorylation of ADP to ATP . The inhibition of ATP...
inhibits ATP synthase, protons cannot pass back into the mitochondrion. As a result, the proton pumps are unable to operate, as the gradient becomes too strong for them to overcome. NADH is then no longer oxidized and the citric acid cycle ceases to operate because the concentration of NAD+ falls below the concentration that these enzymes can use.
| Compounds | Use | Effect on oxidative phosphorylation |
|---|---|---|
| Cyanide Cyanide A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic.... Carbon monoxide Carbon monoxide Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal... Azide Azide Azide is the anion with the formula N3−. It is the conjugate base of hydrazoic acid. N3− is a linear anion that is isoelectronic with CO2 and N2O. Per valence bond theory, azide can be described by several resonance structures, an important one being N−=N+=N−... |
Poisons | Inhibit the electron transport chain by binding more strongly than oxygen to the Fe Iron Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust... –Cu Copper Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish... center in cytochrome c oxidase, preventing the reduction of oxygen. |
| Oligomycin Oligomycin Oligomycins are macrolides created by Streptomyces that can be poisonous to other organisms.-Function:They have use as antibiotics.In addition, oligomycin inhibits ATP synthase by blocking its proton channel , which is necessary for oxidative phosphorylation of ADP to ATP . The inhibition of ATP... |
Antibiotic Antibiotic An antibacterial is a compound or substance that kills or slows down the growth of bacteria.The term is often used synonymously with the term antibiotic; today, however, with increased knowledge of the causative agents of various infectious diseases, antibiotic has come to denote a broader range of... |
Inhibits ATP synthase by blocking the flow of protons through the Fo subunit. |
| CCCP Carbonyl cyanide m-chlorophenyl hydrazone Carbonyl cyanide m-chlorophenyl hydrazone , is a chemical inhibitor of oxidative phosphorylation. It is a nitrile, hydrazone and ionophore. In general, CCCP causes the gradual destruction of living cells and death of the organism. The CCCP affects the protein synthesis reactions in seedling... 2,4-Dinitrophenol 2,4-Dinitrophenol 2,4-Dinitrophenol , C6H4N2O5, is a cellular metabolic poison. It uncouples oxidative phosphorylation by carrying protons across the mitochondrial membrane, leading to a rapid consumption of energy without generation of ATP.... |
Poisons | Ionophore Ionophore An ionophore is a lipid-soluble molecule usually synthesized by microorganisms to transport ions across the lipid bilayer of the cell membrane... s that disrupt the proton gradient by carrying protons across a membrane. This ionophore uncouples proton pumping from ATP synthesis because it carries protons across the inner mitochondrial membrane. |
| Rotenone Rotenone Rotenone is an odorless chemical that is used as a broad-spectrum insecticide, piscicide, and pesticide. It occurs naturally in the roots and stems of several plants such as the jicama vine plant... |
Pesticide Pesticide Pesticides are substances or mixture of substances intended for preventing, destroying, repelling or mitigating any pest.A pesticide may be a chemical unicycle, biological agent , antimicrobial, disinfectant or device used against any pest... |
Prevents the transfer of electrons from complex I to ubiquinone by blocking the ubiquinone-binding site. |
| Malonate Malonate The malonate or propanedioate ion is CH222− . Malonate compounds include salts and esters of malonic acid, such as*diethyl malonate, 2,*dimethyl malonate, 2,... and oxaloacetate |
Competitive inhibitors of succinate dehydrogenase (complex II). |
Not all inhibitors of oxidative phosphorylation are toxins. In brown adipose tissue
Brown adipose tissue
Brown adipose tissue or brown fat is one of two types of fat or adipose tissue found in mammals....
, regulated proton channels called uncoupling protein
Uncoupling protein
An uncoupling protein is a mitochondrial inner membrane protein that can dissipate the proton gradient before it can be used to provide the energy for oxidative phosphorylation.There are five types known in mammals:* UCP1, also known as thermogenin* UCP2...
s can uncouple respiration from ATP synthesis. This rapid respiration produces heat, and is particularly important as a way of maintaining body temperature for hibernating
Hibernation
Hibernation is a state of inactivity and metabolic depression in animals, characterized by lower body temperature, slower breathing, and lower metabolic rate. Hibernating animals conserve food, especially during winter when food supplies are limited, tapping energy reserves, body fat, at a slow rate...
animals, although these proteins may also have a more general function in cells' responses to stress.
History
The field of oxidative phosphorylation began with the report in 1906 by Arthur HardenArthur Harden
Sir Arthur Harden FRS was an English biochemist. He shared the Nobel Prize in Chemistry in 1929 with Hans Karl August Simon von Euler-Chelpin for their investigations into the fermentation of sugar and fermentative enzymes....
of a vital role for phosphate in cellular fermentation
Fermentation (biochemistry)
Fermentation is the process of extracting energy from the oxidation of organic compounds, such as carbohydrates, using an endogenous electron acceptor, which is usually an organic compound. In contrast, respiration is where electrons are donated to an exogenous electron acceptor, such as oxygen,...
, but initially only sugar phosphates
Sugar phosphates
Sugar phosphates are often used in biological systems to store or transfer energy. They also form the backbone for DNA and RNA ....
were known to be involved. However, in the early 1940s, the link between the oxidation of sugars and the generation of ATP was firmly established by Herman Kalckar
Herman Kalckar
Herman Moritz Kalckar was a Danish biochemist who pioneered the study of cellular respiration. Trained as a medical doctor at the University of Copenhagen, Kalckar then conducted research for his Ph. D. in Ejnar Lundsgaard's physiology laboratory, work which helped establish a fundamental...
, confirming the central role of ATP in energy transfer that had been proposed by Fritz Albert Lipmann
Fritz Albert Lipmann
Fritz Albert Lipmann FRS was a German-American biochemist and a co-discoverer in 1945 of coenzyme A. For this, together with other research on coenzyme A, he was awarded half the Nobel Prize in Physiology or Medicine in 1953 .Lipmann was born in Königsberg, Germany to a Jewish family.Lipmann...
in 1941. Later, in 1949, Morris Friedkin and Albert L. Lehninger
Albert L. Lehninger
Albert Lester Lehninger was an American biochemist in the field of bioenergetics. He made fundamental contributions to the current understanding of metabolism at a molecular level. In 1948, he discovered, with Eugene P...
proved that the coenzyme NADH linked metabolic pathways such as the citric acid cycle and the synthesis of ATP.
For another twenty years, the mechanism by which ATP is generated remained mysterious, with scientists searching for an elusive "high-energy intermediate" that would link oxidation and phosphorylation reactions. This puzzle was solved by Peter D. Mitchell
Peter D. Mitchell
Peter Dennis Mitchell, FRS was a British biochemist who was awarded the 1978 Nobel Prize for Chemistry for his discovery of the chemiosmotic mechanism of ATP synthesis.Mitchell was born in Mitcham, Surrey, England....
with the publication of the chemiosmotic theory in 1961. At first, this proposal was highly controversial, but it was slowly accepted and Mitchell was awarded a Nobel prize
Nobel Prize
The Nobel Prizes are annual international awards bestowed by Scandinavian committees in recognition of cultural and scientific advances. The will of the Swedish chemist Alfred Nobel, the inventor of dynamite, established the prizes in 1895...
in 1978. Subsequent research concentrated on purifying and characterizing the enzymes involved, with major contributions being made by David E. Green
David E. Green
David Ezra Green was an America biochemist who made significant contributions to the study of enzymes, particularly the electron transport chain and oxidative phosphorylation. He was born in New York and was awarded a degree in biology from New York University...
on the complexes of the electron-transport chain, as well as Efraim Racker
Efraim Racker
Efraim Racker was an Austrian biochemist who was responsible for identifying and purifying Factor 1 , the first part of the ATP synthase enzyme to be characterised. F1 is only a part of a larger ATP synthase complex known as Complex V...
on the ATP synthase. A critical step towards solving the mechanism of the ATP synthase was provided by Paul D. Boyer
Paul D. Boyer
- External links :* , from the Office of Scientific and Technical Information, United States Department of Energy* * *...
, by his development in 1973 of the "binding change" mechanism, followed by his radical proposal of rotational catalysis in 1982. More recent work has included structural studies
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
on the enzymes involved in oxidative phosphorylation by John E. Walker
John E. Walker
Professor Sir John Ernest Walker is an English chemist who won the Nobel Prize in Chemistry in 1997. He is currently the director of the MRC Mitochondrial Biology Unit in Cambridge, and a Fellow of Sidney Sussex College.He was born in Halifax, Yorkshire, the son of Thomas Ernest Walker, a...
, with Walker and Boyer being awarded a Nobel Prize in 1997.
External links
General resources- Animated diagrams illustrating oxidative phosphorylation Wiley and CoJohn Wiley & SonsJohn Wiley & Sons, Inc., also referred to as Wiley, is a global publishing company that specializes in academic publishing and markets its products to professionals and consumers, students and instructors in higher education, and researchers and practitioners in scientific, technical, medical, and...
Concepts in Biochemistry - ATP synthase - the rotary engine in the cell Brief introduction, including videos of microscope images of the enzyme rotating, at Tokyo Institute of TechnologyTokyo Institute of TechnologyThe Tokyo Institute of Technology is a public research university located in Greater Tokyo Area, Japan. Tokyo Tech is the largest institution for higher education in Japan dedicated to science and technology. Tokyo Tech enrolled 4,850 undergaraduates and 5006 graduate students for 2009-2010...
- On-line biophysics lectures Antony Crofts, University of Illinois at Urbana-ChampaignUniversity of Illinois at Urbana-ChampaignThe University of Illinois at Urbana–Champaign is a large public research-intensive university in the state of Illinois, United States. It is the flagship campus of the University of Illinois system...
Structural resources
- Animations of the ATP synthase Hongyun Wang and George Oster, University of California, BerkeleyUniversity of California, BerkeleyThe University of California, Berkeley , is a teaching and research university established in 1868 and located in Berkeley, California, USA...
- PDBProtein Data BankThe Protein Data Bank is a repository for the 3-D structural data of large biological molecules, such as proteins and nucleic acids....
molecule of the month: - Interactive molecular models at Universidade Fernando Pessoa:

