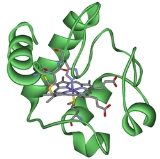
Cytochrome c
Encyclopedia
The Cytochrome complex, or cyt c is a small heme
protein
found loosely associated with the inner membrane of the mitochondrion
. It belongs to the cytochrome c family
of proteins. Cytochrome c is a highly soluble protein, unlike other cytochrome
s, with a solubility of about 100 g/L and is an essential component of the electron transport chain
, where it carries one electron. It is capable of undergoing oxidation and reduction
, but does not bind oxygen
. It transfers electrons between Complexes III
(Coenzyme Q - Cyt C reductase) and IV
(Cyt C oxidase). In humans, cytochrome c is encoded by the CYCS gene
.
in mitochondria. The heme
group of cytochrome c accepts electrons from the b-c1 complex and transfers electrons to the cytochrome oxidase complex. Cytochrome c is also involved in initiation of apoptosis
. Upon release of cytochrome c to the cytoplasm, the protein binds apoptotic protease activating factor.
Cytochrome c can catalyze several reactions such as hydroxylation
and aromatic oxidation, and shows peroxidase
activity by oxidation of various electron donors such as 2,2-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS
), 2-keto-4-thiomethyl butyric acid and 4-aminoantipyrine.
. Its primary structure consists of a chain of about 100 amino acid
s. Many higher order organisms possess a chain of 104 amino acids.
The cytochrome c molecule has been studied for the glimpse it gives into evolutionary biology. Both chicken
s and turkey
s have identical sequence homology (amino acid for amino acid), whereas duck
s possess molecules differing by one amino acid. Similarly, both human
s and chimpanzee
s have the identical molecule, while rhesus monkeys share all but one of the amino acids: the 66th amino acid is isoleucine
in the former and threonine
in the latter. Pig
s, cows and sheep also share identical cytochrome c molecules.
with COX4I2
.
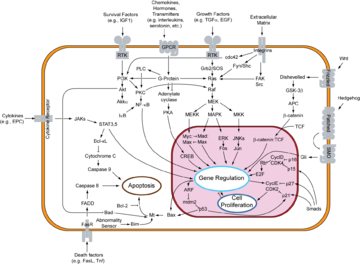 Cytochrome c is also an intermediate in apoptosis
Cytochrome c is also an intermediate in apoptosis
, a controlled form of cell death used to kill cells in the process of development or in response to infection or DNA damage.
Cytochrome c is released by the mitochondria in response to pro-apoptotic stimuli. The sustained elevation in calcium
levels precedes cyt c release from the mitochondria. The release of small amounts of cyt c leads to an interaction with the IP3 receptor
(IP3R) on the endoplasmic reticulum
(ER), causing ER calcium release. The overall increase in calcium triggers a massive release of cyt c, which then acts in the positive feedback loop to maintain ER calcium release through the IP3Rs. This explains how the ER calcium release can reach cytotoxic levels. This release of cytochrome c in turn activates caspase 9, a cysteine protease
. Caspase 9 can then go on to activate caspase 3
and caspase 7
, which are responsible for destroying the cell from within.
Cytochrome c binds to cardiolipin in the inner mitochondrial membrane, thus anchoring its presence and keeping it from releasing out of the mitochondria and initiating apoptosis. While the initial attraction between cardiolipin and cytochrome c is electrostatic due to the extreme positive charge on cytochrome c, the final interaction is hydrophobic, where a hydrophobic tail from cardiolipin inserts itself into the hydrophobic portion of cytochrome c.
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
found loosely associated with the inner membrane of the mitochondrion
Mitochondrion
In cell biology, a mitochondrion is a membrane-enclosed organelle found in most eukaryotic cells. These organelles range from 0.5 to 1.0 micrometers in diameter...
. It belongs to the cytochrome c family
Cytochrome c family
Cytochromes c are electron-transfer proteins having one or several heme c groups, bound to the protein by one or, more generally, two thioether bonds involving sulphydryl groups of cysteine residues. The fifth haem iron ligand is always provided by a histidine residue...
of proteins. Cytochrome c is a highly soluble protein, unlike other cytochrome
Cytochrome
Cytochromes are, in general, membrane-bound hemoproteins that contain heme groups and carry out electron transport.They are found either as monomeric proteins or as subunits of bigger enzymatic complexes that catalyze redox reactions....
s, with a solubility of about 100 g/L and is an essential component of the electron transport chain
Electron transport chain
An electron transport chain couples electron transfer between an electron donor and an electron acceptor with the transfer of H+ ions across a membrane. The resulting electrochemical proton gradient is used to generate chemical energy in the form of adenosine triphosphate...
, where it carries one electron. It is capable of undergoing oxidation and reduction
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
, but does not bind oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
. It transfers electrons between Complexes III
Coenzyme Q - cytochrome c reductase
In enzymology, a ubiquinol—cytochrome-c reductase is an enzyme that catalyzes the chemical reactionThus, the two substrates of this enzyme are dihydroquinone and ferri- cytochrome c, whereas its 3 products are quinone , ferro- cytochrome c, and H+.This enzyme belongs to the family of...
(Coenzyme Q - Cyt C reductase) and IV
Cytochrome c oxidase
The enzyme cytochrome c oxidase or Complex IV is a large transmembrane protein complex found in bacteria and the mitochondrion.It is the last enzyme in the respiratory electron transport chain of mitochondria located in the mitochondrial membrane...
(Cyt C oxidase). In humans, cytochrome c is encoded by the CYCS gene
Gene
A gene is a molecular unit of heredity of a living organism. It is a name given to some stretches of DNA and RNA that code for a type of protein or for an RNA chain that has a function in the organism. Living beings depend on genes, as they specify all proteins and functional RNA chains...
.
Function
Cytochrome c is a component of the electron transport chainElectron transport chain
An electron transport chain couples electron transfer between an electron donor and an electron acceptor with the transfer of H+ ions across a membrane. The resulting electrochemical proton gradient is used to generate chemical energy in the form of adenosine triphosphate...
in mitochondria. The heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
group of cytochrome c accepts electrons from the b-c1 complex and transfers electrons to the cytochrome oxidase complex. Cytochrome c is also involved in initiation of apoptosis
Apoptosis
Apoptosis is the process of programmed cell death that may occur in multicellular organisms. Biochemical events lead to characteristic cell changes and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation...
. Upon release of cytochrome c to the cytoplasm, the protein binds apoptotic protease activating factor.
Cytochrome c can catalyze several reactions such as hydroxylation
Hydroxylation
Hydroxylation is a chemical process that introduces a hydroxyl group into an organic compound. In biochemistry, hydroxylation reactions are often facilitated by enzymes called hydroxylases. Hydroxylation is the first step in the oxidative degradation of organic compounds in air...
and aromatic oxidation, and shows peroxidase
Peroxidase
Peroxidases are a large family of enzymes that typically catalyze a reaction of the form:For many of these enzymes the optimal substrate is hydrogen peroxide, but others are more active with organic hydroperoxides such as lipid peroxides...
activity by oxidation of various electron donors such as 2,2-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS
ABTS
In biochemistry, 2,2'-azino-bis or ABTS is chemical compound used to observe the reaction kinetics of specific enzymes...
), 2-keto-4-thiomethyl butyric acid and 4-aminoantipyrine.
Species distribution
Cytochrome c wael is a highly conserved protein across the spectrum of species, found in plants, animals, and many unicellular organisms. This, along with its small size (molecular weight about 12,000 daltons), makes it useful in studies of cladisticsCladistics
Cladistics is a method of classifying species of organisms into groups called clades, which consist of an ancestor organism and all its descendants . For example, birds, dinosaurs, crocodiles, and all descendants of their most recent common ancestor form a clade...
. Its primary structure consists of a chain of about 100 amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s. Many higher order organisms possess a chain of 104 amino acids.
The cytochrome c molecule has been studied for the glimpse it gives into evolutionary biology. Both chicken
Chicken
The chicken is a domesticated fowl, a subspecies of the Red Junglefowl. As one of the most common and widespread domestic animals, and with a population of more than 24 billion in 2003, there are more chickens in the world than any other species of bird...
s and turkey
Turkey
Turkey , known officially as the Republic of Turkey , is a Eurasian country located in Western Asia and in East Thrace in Southeastern Europe...
s have identical sequence homology (amino acid for amino acid), whereas duck
Duck
Duck is the common name for a large number of species in the Anatidae family of birds, which also includes swans and geese. The ducks are divided among several subfamilies in the Anatidae family; they do not represent a monophyletic group but a form taxon, since swans and geese are not considered...
s possess molecules differing by one amino acid. Similarly, both human
Human
Humans are the only living species in the Homo genus...
s and chimpanzee
Chimpanzee
Chimpanzee, sometimes colloquially chimp, is the common name for the two extant species of ape in the genus Pan. The Congo River forms the boundary between the native habitat of the two species:...
s have the identical molecule, while rhesus monkeys share all but one of the amino acids: the 66th amino acid is isoleucine
Isoleucine
Isoleucine is an α-amino acid with the chemical formula HO2CCHCHCH2CH3. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested. Its codons are AUU, AUC and AUA....
in the former and threonine
Threonine
Threonine is an α-amino acid with the chemical formula HO2CCHCHCH3. Its codons are ACU, ACA, ACC, and ACG. This essential amino acid is classified as polar...
in the latter. Pig
Pig
A pig is any of the animals in the genus Sus, within the Suidae family of even-toed ungulates. Pigs include the domestic pig, its ancestor the wild boar, and several other wild relatives...
s, cows and sheep also share identical cytochrome c molecules.
Classes
In 1991 R. P. Ambler recognized four classes of cytochrome c:- Class I includes the lowspin soluble cytochrome c of mitochondria and bacteria. It has the heme-attachment site towards the N terminus of histidine and the sixth ligand provided by a methionine residue towards the C terminus.
- Class II includes the highspin cytochrome c'. It has the heme-attachment site closed to the N terminus of histidine.
- Class III comprises the low redox potential multiple heme cytochromes. The heme c groups are structurally and functionally nonequivalent and present different redox potentials in the range 0 to -400 mV.
- Class IV was originally created to hold the complex proteins that have other prosthetic groups as well as heme c.
Applications
Cytochrome c is suspected to be the functional complex in so called LLLT: Low-level laser therapy. In LLLT, red light and some near infra-red wavelengths penetrate tissue in order to increase cellular regeneration. Light of this wavelength appears capable of increasing activity of cytochrome c, thus increasing metabolic activity and freeing up more energy for the cells to repair the tissue.Interactions
Cytochrome c has been shown to interactProtein-protein interaction
Protein–protein interactions occur when two or more proteins bind together, often to carry out their biological function. Many of the most important molecular processes in the cell such as DNA replication are carried out by large molecular machines that are built from a large number of protein...
with COX4I2
COX4I2
Cytochrome c oxidase subunit 4 isoform 2, mitochondrial is an enzyme that in humans is encoded by the COX4I2 gene.-Interactions:COX4I2 has been shown to interact with Cytochrome c.-Further reading:...
.
Role in apoptosis

Apoptosis
Apoptosis is the process of programmed cell death that may occur in multicellular organisms. Biochemical events lead to characteristic cell changes and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation...
, a controlled form of cell death used to kill cells in the process of development or in response to infection or DNA damage.
Cytochrome c is released by the mitochondria in response to pro-apoptotic stimuli. The sustained elevation in calcium
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
levels precedes cyt c release from the mitochondria. The release of small amounts of cyt c leads to an interaction with the IP3 receptor
Inositol triphosphate receptor
Inositol trisphosphate receptor is a membrane glycoprotein complex acting as Ca2+ channel activated by inositol trisphosphate . InsP3R is very diverse among organisms, and is necessary for the control of cellular and physiological processes including cell division, cell proliferation, apoptosis,...
(IP3R) on the endoplasmic reticulum
Endoplasmic reticulum
The endoplasmic reticulum is an organelle of cells in eukaryotic organisms that forms an interconnected network of tubules, vesicles, and cisternae...
(ER), causing ER calcium release. The overall increase in calcium triggers a massive release of cyt c, which then acts in the positive feedback loop to maintain ER calcium release through the IP3Rs. This explains how the ER calcium release can reach cytotoxic levels. This release of cytochrome c in turn activates caspase 9, a cysteine protease
Protease
A protease is any enzyme that conducts proteolysis, that is, begins protein catabolism by hydrolysis of the peptide bonds that link amino acids together in the polypeptide chain forming the protein....
. Caspase 9 can then go on to activate caspase 3
Caspase 3
Caspase 3 is a caspase protein that interacts with caspase 8 and caspase 9. It is encoded by the CASP3 gene. CASP3 orthologs have been identified in numerous mammals for which complete genome data are available...
and caspase 7
Caspase 7
Caspase-7, apoptosis-related cysteine peptidase, also known as CASP7, is a human protein encoded by the CASP7 gene.CASP7 orthologs have been identified in nearly all mammals for which complete genome data are available...
, which are responsible for destroying the cell from within.
Cytochrome c binds to cardiolipin in the inner mitochondrial membrane, thus anchoring its presence and keeping it from releasing out of the mitochondria and initiating apoptosis. While the initial attraction between cardiolipin and cytochrome c is electrostatic due to the extreme positive charge on cytochrome c, the final interaction is hydrophobic, where a hydrophobic tail from cardiolipin inserts itself into the hydrophobic portion of cytochrome c.
External links
- The Cytochrome c Protein
- Apoptosis & Caspase 3 - PMAP The Proteolysis MapThe Proteolysis MapThe Proteolysis MAP is an integrated web resource focused on proteases.-Rationale:PMAP is to aid the protease researchers in reasoning about proteolytic networks and metabolic pathways.-History and funding:...
-animation - Calculated orientations of cytochromes c in the lipid bilayer

