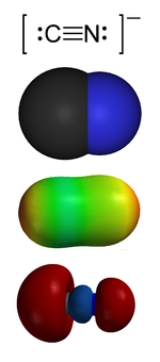
Cyanide
Overview
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
that contains the cyano group, -C≡N, which consists of a carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
triple-bonded to a nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic.
In organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
compounds containing a -C≡N group are known as nitrile
Nitrile
A nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called...
s and compounds that contain the -N≡C group are known as isocyanide
Isocyanide
An isocyanide is an organic compound with the functional group -N≡C. It is the isomer of the related cyanide , hence the prefix iso....
s. Organic nitriles and isocyanides are far less toxic because they do not release cyanide ions easily.
The dye Prussian blue
Prussian blue
Prussian blue is a dark blue pigment with the idealized formula Fe718. Another name for the color Prussian blue is Berlin blue or, in painting, Parisian blue. Turnbull's blue is the same substance but is made from different reagents....
had been first accidentally made, it is presumed around 1706, from substances containing iron and carbon and nitrogen, and the (then unknown) cyanide was formed during the manufacture of the dye.

