Fatty acid
Encyclopedia
In chemistry
, especially biochemistry
, a fatty acid is a carboxylic acid
with a long unbranched aliphatic tail (chain
), which is either saturated
or unsaturated
. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from triglyceride
s or phospholipid
s. When they are not attached to other molecules, they are known as "free" fatty acids. Fatty acids are important sources of fuel because, metabolized, they yield large quantities of ATP
. Many cell types can use either glucose
or fatty acids for this purpose. In particular, heart and skeletal muscle prefer fatty acids. The brain cannot use fatty acids as a source of fuel; it relies on glucose or ketone bodies
.
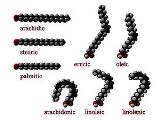
 Fatty acids that have double bond
Fatty acids that have double bond
s are known as unsaturated. Fatty acids without double bonds are known as saturated. They differ in length as well.
 Unsaturated fatty acids have one or more double bond
Unsaturated fatty acids have one or more double bond
s between carbon atoms. (Pairs of carbon atoms connected by double bonds can be saturated by adding hydrogen atoms to them, converting the double bonds to single bonds. Therefore, the double bonds are called unsaturated.)
The two carbon atoms in the chain that are bound next to either side of the double bond can occur in a cis or trans configuration.
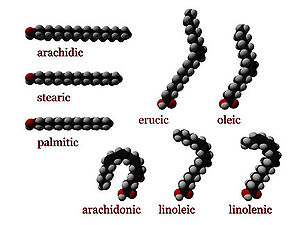
cis : A cis configuration means that adjacent hydrogen atoms are on the same side of the double bond. The rigidity of the double bond freezes its conformation and, in the case of the cis isomer, causes the chain to bend and restricts the conformational freedom of the fatty acid. The more double bonds the chain has in the cis configuration, the less flexibility it has. When a chain has many cis bonds, it becomes quite curved in its most accessible conformations. For example, oleic acid
, with one double bond, has a "kink" in it, whereas linoleic acid
, with two double bonds, has a more pronounced bend. Alpha-linolenic acid
, with three double bonds, favors a hooked shape. The effect of this is that, in restricted environments, such as when fatty acids are part of a phospholipid in a lipid bilayer, or triglycerides in lipid droplets, cis bonds limit the ability of fatty acids to be closely packed, and therefore could affect the melting temperature of the membrane or of the fat.
trans : A trans configuration, by contrast, means that the next two hydrogen atoms are bound to opposite sides of the double bond. As a result, they do not cause the chain to bend much, and their shape is similar to straight saturated fatty acids.
In most naturally occurring unsaturated fatty acids, each double bond has three n carbon atoms after it, for some n, and all are cis bonds. Most fatty acids in the trans configuration (trans fat
s) are not found in nature and are the result of human processing (e.g., hydrogenation
).
The differences in geometry between the various types of unsaturated fatty acids, as well as between saturated and unsaturated fatty acids, play an important role in biological processes, and in the construction of biological structures (such as cell membranes).
removed from the methyl end; the other has a double bond six carbon atoms
removed from the methyl end. Humans lack the ability to introduce double bonds in fatty acids beyond carbons 9 and 10, as counted from the carboxylic acid side. Two essential fatty acids are linoleic acid
(LA) and alpha-linolenic acid
(ALA). They are widely distributed in plant oils. The human body has a limited ability to convert ALA into the longer-chain n-3 fatty acids eicosapentaenoic acid
(EPA) and docosahexaenoic acid
(DHA), which can also be obtained from fish.
of triglyceride
s, with the removal of glycerol
(see oleochemical
s). Phospholipid
s represent another source. Some fatty acids are produced synthetically by hydrocarboxylation
of alkenes.
of fatty acids involves the condensation of acetyl-CoA
. Since this coenzyme carries a two-carbon-atom group, almost all natural fatty acids have even numbers of carbon atoms.
The "uncombined fatty acids" or "free fatty acids" found in organisms come from the breakdown of a triglyceride. Because they are insoluble in water, these fatty acids are transported (solubilized, circulated) while bound to plasma protein albumin. The levels of "free fatty acid" in the blood are limited by the availability of albumin binding sites.
, for example, has a pKa of 4.96, being only slightly weaker than acetic acid (4.76). As the chain length increases the solubility of the fatty acids in water decreases very rapidly, so that the longer-chain fatty acids have minimal effect on the pH
of an aqueous solution. Even those fatty acids that are insoluble in water will dissolve in warm ethanol
, and can be titrated
with sodium hydroxide solution using phenolphthalein
as an indicator to a pale-pink endpoint. This analysis is used to determine the free fatty acid content of fats; i.e., the proportion of the triglycerides that have been hydrolyze
d.
of unsaturated fatty acids is widely practiced to give saturated fatty acids, which are less prone toward rancidification
. Since the saturated fatty acids are higher melting that the unsaturated relatives, the process is called hardening. This technology is used to convert vegetable oils into margarine. During partial hydrogenation, unsaturated fatty acids can be isomerized from cis to trans configuration.
More forcing hydrogenation, i.e. using higher pressures of H2 and higher temperatures, converts fatty acids fatty alcohol
s. Fatty alcohols are, however, more easily produced from fatty acid esters.
In the Varrentrapp reaction
certain unsaturated fatty acids are cleaved in molten alkali, a reaction at one time of relevance to structure elucidation.
. Fats and oils often are treated with chelating agents
such as citric acid
to remove the metal catalysts.
((CH2)7(CO2H)2) from oleic acid
.
and protein (protein coat) into a compound called a chylomicron
.
Within the villi, the chylomicron enters a lymphatic capillary called a lacteal
, which merges into larger lymphatic vessels. It is transported via the lymphatic system and the thoracic duct
up to a location near the heart (where the arteries and veins are larger). The thoracic duct empties the chylomicrons into the bloodstream via the left subclavian vein
. At this point the chylomicrons can transport the triglycerides to tissues where they are stored or metabolized for energy.
through beta oxidation
.
s (VLDL) and low density lipoprotein
s (LDL) after processing in the liver. In addition, when released from adipocytes, fatty acids exist in the blood as free fatty acids.
It is proposed that the blend of fatty acids exuded by mammalian skin, together with lactic acid
and pyruvic acid
, is distinctive and enables animals with a keen sense of smell to differentiate individuals.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, especially biochemistry
Biochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
, a fatty acid is a carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
with a long unbranched aliphatic tail (chain
Chain
A chain is a sequence of connected links.Chain may also refer to:Chain may refer to:* Necklace - a jewelry which is worn around the neck* Mail , a type of armor made of interlocking chain links...
), which is either saturated
Saturation (chemistry)
In chemistry, saturation has six different meanings, all based on reaching a maximum capacity...
or unsaturated
Unsaturated compound
In organic chemistry, a saturated compound is a chemical compound that has of a chain of carbon atoms linked together by single bonds and has hydrogen atoms filling all of the other bonding orbitals of the carbon atoms. Alkanes are an example of saturated compounds...
. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from triglyceride
Triglyceride
A triglyceride is an ester derived from glycerol and three fatty acids. There are many triglycerides, depending on the oil source, some are highly unsaturated, some less so....
s or phospholipid
Phospholipid
Phospholipids are a class of lipids that are a major component of all cell membranes as they can form lipid bilayers. Most phospholipids contain a diglyceride, a phosphate group, and a simple organic molecule such as choline; one exception to this rule is sphingomyelin, which is derived from...
s. When they are not attached to other molecules, they are known as "free" fatty acids. Fatty acids are important sources of fuel because, metabolized, they yield large quantities of ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
. Many cell types can use either glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
or fatty acids for this purpose. In particular, heart and skeletal muscle prefer fatty acids. The brain cannot use fatty acids as a source of fuel; it relies on glucose or ketone bodies
Ketone bodies
Ketone bodies are three water-soluble compounds that are produced as by-products when fatty acids are broken down for energy in the liver and kidney. They are used as a source of energy in the heart and brain. In the brain, they are a vital source of energy during fasting...
.
Types of fatty acids

Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
s are known as unsaturated. Fatty acids without double bonds are known as saturated. They differ in length as well.
Length of free fatty acid chains
Fatty acid chains differ by length, often categorized as short, medium, or long.- Short-chain fatty acids (SCFA) are fatty acids with aliphatic tails of fewer than six carbons (i.e. butyric acidButyric acidButyric acid , also known under the systematic name butanoic acid, is a carboxylic acid with the structural formula CH3CH2CH2-COOH. Salts and esters of butyric acid are known as butyrates or butanoates...
). - Medium-chain fatty acid (MCFA) are fatty acids with aliphatic tails of 6–12 carbons, which can form medium-chain triglycerides.
- Long-chain fatty acid (LCFA) are fatty acids with aliphatic tails longer than 12 carbons.
- Very long chain fatty acidVery long chain fatty acidA very long chain fatty acid is a fatty acid with aliphatic tails longer than 22 carbons.Unlike most fatty acids, VLCFAs are too long to be metabolized in the mitochondria, and must be metabolized in peroxisomes....
(VLCFA) are fatty acids with aliphatic tails longer than 22 carbons
Unsaturated fatty acids

Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
s between carbon atoms. (Pairs of carbon atoms connected by double bonds can be saturated by adding hydrogen atoms to them, converting the double bonds to single bonds. Therefore, the double bonds are called unsaturated.)
The two carbon atoms in the chain that are bound next to either side of the double bond can occur in a cis or trans configuration.
cis : A cis configuration means that adjacent hydrogen atoms are on the same side of the double bond. The rigidity of the double bond freezes its conformation and, in the case of the cis isomer, causes the chain to bend and restricts the conformational freedom of the fatty acid. The more double bonds the chain has in the cis configuration, the less flexibility it has. When a chain has many cis bonds, it becomes quite curved in its most accessible conformations. For example, oleic acid
Oleic acid
Oleic acid is a monounsaturated omega-9 fatty acid found in various animal and vegetable fats. It has the formula CH37CH=CH7COOH. It is an odorless, colourless oil, although commercial samples may be yellowish. The trans isomer of oleic acid is called elaidic acid...
, with one double bond, has a "kink" in it, whereas linoleic acid
Linoleic acid
Linoleic acid is an unsaturated n-6 fatty acid. It is a colorless liquid at room temperature. In physiological literature, it has a lipid number of 18:2...
, with two double bonds, has a more pronounced bend. Alpha-linolenic acid
Alpha-linolenic acid
α-Linolenic acid is an organic compound found in many common vegetable oils. In terms of its structure, it is named all-cis-9,12,15-octadecatrienoic acid. In physiological literature, it is given the name 18:3 ....
, with three double bonds, favors a hooked shape. The effect of this is that, in restricted environments, such as when fatty acids are part of a phospholipid in a lipid bilayer, or triglycerides in lipid droplets, cis bonds limit the ability of fatty acids to be closely packed, and therefore could affect the melting temperature of the membrane or of the fat.
trans : A trans configuration, by contrast, means that the next two hydrogen atoms are bound to opposite sides of the double bond. As a result, they do not cause the chain to bend much, and their shape is similar to straight saturated fatty acids.
In most naturally occurring unsaturated fatty acids, each double bond has three n carbon atoms after it, for some n, and all are cis bonds. Most fatty acids in the trans configuration (trans fat
Trans fat
Trans fat is the common name for unsaturated fat with trans-isomer fatty acid. Because the term refers to the configuration of a double carbon-carbon bond, trans fats are sometimes monounsaturated or polyunsaturated, but never saturated....
s) are not found in nature and are the result of human processing (e.g., hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
).
The differences in geometry between the various types of unsaturated fatty acids, as well as between saturated and unsaturated fatty acids, play an important role in biological processes, and in the construction of biological structures (such as cell membranes).
| Common name | Chemical structure | Δx | C:D | n−x |
|---|---|---|---|---|
| Myristoleic acid Myristoleic acid Myristoleic acid, or 9-tetradecenoic acid, is an omega-5 fatty acid. It is biosynthesized from myristic acid by the enzyme delta-9 desaturase, but it is uncommon in nature... |
CH3(CH2)3CH=CH(CH2)7COOH | cis-Δ9 | 14:1 | n−5 |
| Palmitoleic acid Palmitoleic acid Palmitoleic acid, or -9-hexadecenoic acid, is an omega-7 monounsaturated fatty acid with the formula CH35CH=CH7COOH that is a common constituent of the glycerides of human adipose tissue. It is present in all tissues, but generally found in higher concentrations in the liver... |
CH3(CH2)5CH=CH(CH2)7COOH | cis-Δ9 | 16:1 | n−7 |
| Sapienic acid Sapienic acid Sapienic acid is a fatty acid that is a major component of human sebum. Among hair-bearing animals, sapienic acid is unique to humans . The equivalent fatty acid in mouse sebum is palmitoleic acid... |
CH3(CH2)8CH=CH(CH2)4COOH | cis-Δ6 | 16:1 | n−10 |
| Oleic acid Oleic acid Oleic acid is a monounsaturated omega-9 fatty acid found in various animal and vegetable fats. It has the formula CH37CH=CH7COOH. It is an odorless, colourless oil, although commercial samples may be yellowish. The trans isomer of oleic acid is called elaidic acid... |
CH3(CH2)7CH=CH(CH2)7COOH | cis-Δ9 | 18:1 | n−9 Omega-9 fatty acid n−9 fatty acids are a family of unsaturated fatty acids which have in common a final carbon–carbon double bond in the n−9 position; that is, the ninth bond from the end of the fatty acid.-Background:Some n−9s are common components of animal fat and vegetable oil... |
| Elaidic acid Elaidic acid Elaidic acid is the major trans fat found in hydrogenated vegetable oils and occurs in small amounts in caprine and bovine milk . It is the trans isomer of oleic acid. The name of the elaidinization reaction comes from elaidic acid.Elaidic acid increases CETP activity, which in turn raises VLDL... |
CH3(CH2)7CH=CH(CH2)7COOH | trans-Δ9 | 18:1 | n−9 Omega-9 fatty acid n−9 fatty acids are a family of unsaturated fatty acids which have in common a final carbon–carbon double bond in the n−9 position; that is, the ninth bond from the end of the fatty acid.-Background:Some n−9s are common components of animal fat and vegetable oil... |
| Vaccenic acid Vaccenic acid Vaccenic acid is an omega-7 fatty acid. It is a naturally occurring trans-fatty acid found in the fat of ruminants and in dairy products such as milk, butter, and yogurt. It is also the predominant fatty acid comprising trans fat in human milk.... |
CH3(CH2)5CH=CH(CH2)9COOH | trans-Δ11 | 18:1 | n−7 |
| Linoleic acid Linoleic acid Linoleic acid is an unsaturated n-6 fatty acid. It is a colorless liquid at room temperature. In physiological literature, it has a lipid number of 18:2... |
CH3(CH2)4CH=CHCH2CH=CH(CH2)7COOH | cis,cis-Δ9,Δ12 | 18:2 | n−6 Omega-6 fatty acid n−6 fatty acids are a family of unsaturated fatty acids that have in common a final carbon–carbon double bond in the n−6 position, that is, the sixth bond, counting from the methyl end.The biological effects of the n−6 fatty acids are largely mediated by their conversion to n-6 eicosanoids... |
| Linoelaidic acid Linoelaidic acid Linoelaidic acid is an omega-6 trans fatty acid and is a geometric isomer of linoleic acid. It is found in partially hydrogenated vegetable oils.... |
CH3(CH2)4CH=CHCH2CH=CH(CH2)7COOH | trans,trans-Δ9,Δ12 | 18:2 | n−6 Omega-6 fatty acid n−6 fatty acids are a family of unsaturated fatty acids that have in common a final carbon–carbon double bond in the n−6 position, that is, the sixth bond, counting from the methyl end.The biological effects of the n−6 fatty acids are largely mediated by their conversion to n-6 eicosanoids... |
| α-Linolenic acid Alpha-linolenic acid α-Linolenic acid is an organic compound found in many common vegetable oils. In terms of its structure, it is named all-cis-9,12,15-octadecatrienoic acid. In physiological literature, it is given the name 18:3 .... |
CH3CH2CH=CHCH2CH=CHCH2CH=CH(CH2)7COOH | cis,cis,cis-Δ9,Δ12,Δ15 | 18:3 | n−3 Omega-3 fatty acid N−3 fatty acids are essential unsaturated fatty acids with a double bond starting after the third carbon atom from the end of the carbon chain.... |
| Arachidonic acid Arachidonic acid Arachidonic acid is a polyunsaturated omega-6 fatty acid 20:4.It is the counterpart to the saturated arachidic acid found in peanut oil, Arachidonic acid (AA, sometimes ARA) is a polyunsaturated omega-6 fatty acid 20:4(ω-6).It is the counterpart to the saturated arachidic acid found in peanut oil,... |
CH3(CH2)4CH=CHCH2CH=CHCH2CH=CHCH2CH=CH(CH2)3COOHNIST | cis,cis,cis,cis-Δ5Δ8,Δ11,Δ14 | 20:4 | n−6 Omega-6 fatty acid n−6 fatty acids are a family of unsaturated fatty acids that have in common a final carbon–carbon double bond in the n−6 position, that is, the sixth bond, counting from the methyl end.The biological effects of the n−6 fatty acids are largely mediated by their conversion to n-6 eicosanoids... |
| Eicosapentaenoic acid Eicosapentaenoic acid Eicosapentaenoic acid is an omega-3 fatty acid. In physiological literature, it is given the name 20:5. It also has the trivial name timnodonic acid... |
CH3CH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CH(CH2)3COOH | cis,cis,cis,cis,cis-Δ5,Δ8,Δ11,Δ14,Δ17 | 20:5 | n−3 Omega-3 fatty acid N−3 fatty acids are essential unsaturated fatty acids with a double bond starting after the third carbon atom from the end of the carbon chain.... |
| Erucic acid Erucic acid Erucic acid is a monounsaturated omega-9 fatty acid, denoted 22:1 ω-9. It has the formula CH37CH=CH11COOH. It is prevalent in rapeseed, wallflower seed, and mustard seed, making up 40-50% of their oils... |
CH3(CH2)7CH=CH(CH2)11COOH | cis-Δ13 | 22:1 | n−9 Omega-9 fatty acid n−9 fatty acids are a family of unsaturated fatty acids which have in common a final carbon–carbon double bond in the n−9 position; that is, the ninth bond from the end of the fatty acid.-Background:Some n−9s are common components of animal fat and vegetable oil... |
| Docosahexaenoic acid Docosahexaenoic acid Docosahexaenoic acid is an omega-3 fatty acid that is a primary structural component of the human brain and retina. In chemical structure, DHA is a carboxylic acid with a 22-carbon chain and six cis double bonds; the first double bond is located at the third carbon from the omega end... |
CH3CH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CH(CH2)2COOH | cis,cis,cis,cis,cis,cis-Δ4,Δ7,Δ10,Δ13,Δ16,Δ19 | 22:6 | n−3 Omega-3 fatty acid N−3 fatty acids are essential unsaturated fatty acids with a double bond starting after the third carbon atom from the end of the carbon chain.... |
Essential fatty acids
Fatty acids that are required by the human body but cannot be made in sufficient quantity from other substrates, and therefore must be obtained from food, are called essential fatty acids. There are two series of essential fatty acids: one has a double bond three carbon atomsOmega-3 fatty acid
N−3 fatty acids are essential unsaturated fatty acids with a double bond starting after the third carbon atom from the end of the carbon chain....
removed from the methyl end; the other has a double bond six carbon atoms
Omega-6 fatty acid
n−6 fatty acids are a family of unsaturated fatty acids that have in common a final carbon–carbon double bond in the n−6 position, that is, the sixth bond, counting from the methyl end.The biological effects of the n−6 fatty acids are largely mediated by their conversion to n-6 eicosanoids...
removed from the methyl end. Humans lack the ability to introduce double bonds in fatty acids beyond carbons 9 and 10, as counted from the carboxylic acid side. Two essential fatty acids are linoleic acid
Linoleic acid
Linoleic acid is an unsaturated n-6 fatty acid. It is a colorless liquid at room temperature. In physiological literature, it has a lipid number of 18:2...
(LA) and alpha-linolenic acid
Alpha-linolenic acid
α-Linolenic acid is an organic compound found in many common vegetable oils. In terms of its structure, it is named all-cis-9,12,15-octadecatrienoic acid. In physiological literature, it is given the name 18:3 ....
(ALA). They are widely distributed in plant oils. The human body has a limited ability to convert ALA into the longer-chain n-3 fatty acids eicosapentaenoic acid
Eicosapentaenoic acid
Eicosapentaenoic acid is an omega-3 fatty acid. In physiological literature, it is given the name 20:5. It also has the trivial name timnodonic acid...
(EPA) and docosahexaenoic acid
Docosahexaenoic acid
Docosahexaenoic acid is an omega-3 fatty acid that is a primary structural component of the human brain and retina. In chemical structure, DHA is a carboxylic acid with a 22-carbon chain and six cis double bonds; the first double bond is located at the third carbon from the omega end...
(DHA), which can also be obtained from fish.
Saturated fatty acids
Saturated fatty acids are long-chain carboxylic acids that usually have between 12 and 24 carbon atoms and have no double bonds. Thus, saturated fatty acids are saturated with hydrogen (since double bonds reduce the number of hydrogens on each carbon). Because saturated fatty acids have only single bonds, each carbon atom within the chain has 2 hydrogen atoms (except for the omega carbon at the end that has 3 hydrogens).| Common name | Chemical structure | C:D |
|---|---|---|
| Caprylic acid Caprylic acid Caprylic acid is the common name for the eight-carbon saturated fatty acid known by the systematic name octanoic acid. It is found naturally in the milk of various mammals, and it is a minor constituent of coconut oil and palm kernel oil... |
CH3(CH2)6COOH | 8:0 |
| Capric acid | CH3(CH2)8COOH | 10:0 |
| Lauric acid Lauric acid Lauric acid , the saturated fatty acid with a 12-carbon atom chain, is a white, powdery solid with a faint odor of bay oil or soap.-Occurrence:... |
CH3(CH2)10COOH | 12:0 |
| Myristic acid Myristic acid Myristic acid, also called tetradecanoic acid, is a common saturated fatty acid with the molecular formula CH312COOH. A myristate is a salt or ester of myristic acid.... |
CH3(CH2)12COOH | 14:0 |
| Palmitic acid Palmitic acid Palmitic acid, or hexadecanoic acid in IUPAC nomenclature, is one of the most common saturated fatty acids found in animals and plants. Its molecular formula is CH314CO2H. As its name indicates, it is a major component of the oil from palm trees . Palmitate is a term for the salts and esters of... |
CH3(CH2)14COOH | 16:0 |
| Stearic acid Stearic acid Stearic acid is the saturated fatty acid with an 18 carbon chain and has the IUPAC name octadecanoic acid. It is a waxy solid, and its chemical formula is CH316CO2H. Its name comes from the Greek word στέαρ "stéatos", which means tallow. The salts and esters of stearic acid are called stearates... |
CH3(CH2)16COOH | 18:0 |
| Arachidic acid Arachidic acid Arachidic acid, also called eicosanoic acid, is the saturated fatty acid with a 20 carbon chain. It is as a minor constituent of peanut oil and corn oil . Its name derives from the Latin arachis — peanut... |
CH3(CH2)18COOH | 20:0 |
| Behenic acid Behenic acid Behenic acid is a normal carboxylic acid, the saturated fatty acid with formula C21H43COOH. In appearance, it consists of white to cream color crystals or powder with a melting point of 80°C and boiling point of 306°C.-Sources:... |
CH3(CH2)20COOH | 22:0 |
| Lignoceric acid Lignoceric acid Lignoceric acid, or tetracosanoic acid, is the saturated fatty acid with formula C23H47COOH. It is found in wood tar, various cerebrosides, and in small amounts in most natural fats. The fatty acids of peanut oil contain small amounts of lignoceric acid . This fatty acid is also a byproduct of... |
CH3(CH2)22COOH | 24:0 |
| Cerotic acid Cerotic acid Cerotic acid, or hexacosanoic acid, is a 26-carbon long-chain saturated fatty acid with the chemical formula CH324COOH. It is most commonly found in beeswax and carnauba wax, and is a white crystalline solid.... |
CH3(CH2)24COOH | 26:0 |
Nomenclature
Several different systems of nomenclature are used for fatty acids. The following table describes the most common systems.| System | Example | Explanation |
|---|---|---|
| Trivial nomenclature | Palmitoleic acid Palmitoleic acid Palmitoleic acid, or -9-hexadecenoic acid, is an omega-7 monounsaturated fatty acid with the formula CH35CH=CH7COOH that is a common constituent of the glycerides of human adipose tissue. It is present in all tissues, but generally found in higher concentrations in the liver... |
Trivial name Trivial name In chemistry, a trivial name is a common name or vernacular name; it is a non-systematic name or non-scientific name. That is, the name is not recognised according to the rules of any formal system of nomenclature... s (or common names) are non-systematic historical names, which are the most frequent naming system used in literature. Most common fatty acids have trivial names in addition to their systematic names (see below). These names frequently do not follow any pattern, but they are concise and often unambiguous. |
| Systematic nomenclature | (9Z)-octadecenoic acid Oleic acid Oleic acid is a monounsaturated omega-9 fatty acid found in various animal and vegetable fats. It has the formula CH37CH=CH7COOH. It is an odorless, colourless oil, although commercial samples may be yellowish. The trans isomer of oleic acid is called elaidic acid... |
Systematic name Systematic name A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection... s (or IUPAC names) derive from the standard IUPAC Rules for the Nomenclature of Organic Chemistry IUPAC nomenclature of organic chemistry The IUPAC nomenclature of organic chemistry is a systematic method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry . Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be drawn. ... , published in 1979, along with a recommendation published specifically for lipids in 1977. Counting begins from the carboxylic acid Carboxylic acid Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group... end. Double bond Double bond A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in... s are labelled with cis-/trans- notation or E E-Z notation E-Z notation, or the E-Z convention, is the IUPAC preferred method of describing the stereochemistry of double bonds in organic chemistry... -/Z E-Z notation E-Z notation, or the E-Z convention, is the IUPAC preferred method of describing the stereochemistry of double bonds in organic chemistry... - notation, where appropriate. This notation is generally more verbose than common nomenclature, but has the advantage of being more technically clear and descriptive. |
| Δx nomenclature | cis,cis-Δ9,Δ12 octadecadienoic acid Linoleic acid Linoleic acid is an unsaturated n-6 fatty acid. It is a colorless liquid at room temperature. In physiological literature, it has a lipid number of 18:2... |
In Δx (or delta-x) nomenclature, each double bond is indicated by Δx, where the double bond is located on the xth carbon–carbon bond, counting from the carboxylic acid end. Each double bond is preceded by a cis- or trans- prefix, indicating the conformation of the molecule around the bond. For example, linoleic acid Linoleic acid Linoleic acid is an unsaturated n-6 fatty acid. It is a colorless liquid at room temperature. In physiological literature, it has a lipid number of 18:2... is designated "cis-Δ9, cis-Δ12 octadecadienoic acid". This nomenclature has the advantage of being less verbose than systematic nomenclature, but is no more technically clear or descriptive. |
| n−x nomenclature | n−3 Omega-3 fatty acid N−3 fatty acids are essential unsaturated fatty acids with a double bond starting after the third carbon atom from the end of the carbon chain.... |
n−x (n minus x; also ω−x or omega-x) nomenclature both provides names for individual compounds and classifies them by their likely biosynthetic properties in animals. A double bond is located on the xth carbon–carbon bond, counting from the terminal methyl carbon (designated as n or ω) toward the carbonyl Carbonyl In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups.... carbon. For example, α-Linolenic acid Alpha-linolenic acid α-Linolenic acid is an organic compound found in many common vegetable oils. In terms of its structure, it is named all-cis-9,12,15-octadecatrienoic acid. In physiological literature, it is given the name 18:3 .... is classified as a n−3 Omega-3 fatty acid N−3 fatty acids are essential unsaturated fatty acids with a double bond starting after the third carbon atom from the end of the carbon chain.... or omega-3 fatty acid, and so it is likely to share a biosynthetic pathway with other compounds of this type. The ω−x, omega-x, or "omega" notation is common in popular nutritional literature, but IUPAC IUPAC nomenclature A chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry .... has deprecated it in favor of n−x notation in technical documents. The most commonly researched fatty acid biosynthetic pathways are n−3 Omega-3 fatty acid N−3 fatty acids are essential unsaturated fatty acids with a double bond starting after the third carbon atom from the end of the carbon chain.... and n−6 Omega-6 fatty acid n−6 fatty acids are a family of unsaturated fatty acids that have in common a final carbon–carbon double bond in the n−6 position, that is, the sixth bond, counting from the methyl end.The biological effects of the n−6 fatty acids are largely mediated by their conversion to n-6 eicosanoids... , which are hypothesized to decrease or increase, respectively, inflammation. |
| Lipid numbers | 18:3 18:3, n−6 Gamma-Linolenic acid γ-Linolenic acid is a fatty acid found primarily in vegetable oils... 18:3, cis,cis,cis-Δ9,Δ12,Δ15 Alpha-linolenic acid α-Linolenic acid is an organic compound found in many common vegetable oils. In terms of its structure, it is named all-cis-9,12,15-octadecatrienoic acid. In physiological literature, it is given the name 18:3 .... |
Lipid numbers take the form C:D, where C is the number of carbon atoms in the fatty acid and D is the number of double bonds in the fatty acid. This notation can be ambiguous, as some different fatty acids can have the same numbers. Consequently, when ambiguity exists this notation is usually paired with either a Δx or n−x term. |
Production
Fatty acids are usually produced industrially by the hydrolysisHydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of triglyceride
Triglyceride
A triglyceride is an ester derived from glycerol and three fatty acids. There are many triglycerides, depending on the oil source, some are highly unsaturated, some less so....
s, with the removal of glycerol
Glycerol
Glycerol is a simple polyol compound. It is a colorless, odorless, viscous liquid that is widely used in pharmaceutical formulations. Glycerol has three hydroxyl groups that are responsible for its solubility in water and its hygroscopic nature. The glycerol backbone is central to all lipids...
(see oleochemical
Oleochemical
Oleochemicals are chemicals derived from plant and animal fats. They are analogous to petrochemicals derived from petroleum.The formation of basic oleochemical substances like fatty acids, fatty acid methyl esters , fatty alcohols, fatty amines and glycerols are by various chemical and enzymatic...
s). Phospholipid
Phospholipid
Phospholipids are a class of lipids that are a major component of all cell membranes as they can form lipid bilayers. Most phospholipids contain a diglyceride, a phosphate group, and a simple organic molecule such as choline; one exception to this rule is sphingomyelin, which is derived from...
s represent another source. Some fatty acids are produced synthetically by hydrocarboxylation
Carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry.-Organic chemistry:...
of alkenes.
Free fatty acids
The biosynthesisBiosynthesis
Biosynthesis is an enzyme-catalyzed process in cells of living organisms by which substrates are converted to more complex products. The biosynthesis process often consists of several enzymatic steps in which the product of one step is used as substrate in the following step...
of fatty acids involves the condensation of acetyl-CoA
Acetyl-CoA
Acetyl coenzyme A or acetyl-CoA is an important molecule in metabolism, used in many biochemical reactions. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. In chemical structure, acetyl-CoA is the thioester...
. Since this coenzyme carries a two-carbon-atom group, almost all natural fatty acids have even numbers of carbon atoms.
The "uncombined fatty acids" or "free fatty acids" found in organisms come from the breakdown of a triglyceride. Because they are insoluble in water, these fatty acids are transported (solubilized, circulated) while bound to plasma protein albumin. The levels of "free fatty acid" in the blood are limited by the availability of albumin binding sites.
Fatty acids in dietary fats
The following table gives the fatty acid, vitamin E and cholesterol composition of some common dietary fats.| Saturated | Monounsaturated | Polyunsaturated | Cholesterol | Vitamin E | |
|---|---|---|---|---|---|
| g/100g | g/100g | g/100g | mg/100g | mg/100g | |
| Animal fats | |||||
| Lard Lard Lard is pig fat in both its rendered and unrendered forms. Lard was commonly used in many cuisines as a cooking fat or shortening, or as a spread similar to butter. Its use in contemporary cuisine has diminished because of health concerns posed by its saturated-fat content and its often negative... |
40.8 | 43.8 | 9.6 | 93 | 0.00 |
| Duck fat | 33.2 | 49.3 | 12.9 | 100 | 2.70 |
| Butter Butter Butter is a dairy product made by churning fresh or fermented cream or milk. It is generally used as a spread and a condiment, as well as in cooking applications, such as baking, sauce making, and pan frying... |
54.0 | 19.8 | 2.6 | 230 | 2.00 |
| Vegetable fats | |||||
| Coconut oil Coconut oil Coconut oil is an edible oil extracted from the kernel or meat of matured coconuts harvested from the coconut palm . Throughout the tropical world, it has provided the primary source of fat in the diets of millions of people for generations. It has various applications in food, medicine, and industry... |
85.2 | 6.6 | 1.7 | 0 | .66 |
| Palm oil Palm oil Palm oil, coconut oil and palm kernel oil are edible plant oils derived from the fruits of palm trees. Palm oil is extracted from the pulp of the fruit of the oil palm Elaeis guineensis; palm kernel oil is derived from the kernel of the oil palm and coconut oil is derived from the kernel of the... |
45.3 | 41.6 | 8.3 | 0 | 33.12 |
| Cottonseed oil Cottonseed oil Cottonseed oil is a cooking oil extracted from the seeds of cotton plant of various species, mainly Gossypium hirsutum and Gossypium herbaceum... |
25.5 | 21.3 | 48.1 | 0 | 42.77 |
| Wheat germ oil Wheat germ oil Wheat germ oil is extracted from the germ of the wheat kernel, which makes up only 2½% by weight of the kernel Wheat germ oil is particularly high in octacosanol - a 28 carbon long-chain saturated primary alcohol found in a number of different vegetable waxes. Octacosanol has been studied as an... |
18.8 | 15.9 | 60.7 | 0 | 136.65 |
| Soya oil | 14.5 | 23.2 | 56.5 | 0 | 16.29 |
| Olive oil Olive oil Olive oil is an oil obtained from the olive , a traditional tree crop of the Mediterranean Basin. It is commonly used in cooking, cosmetics, pharmaceuticals, and soaps and as a fuel for traditional oil lamps... |
14.0 | 69.7 | 11.2 | 0 | 5.10 |
| Corn oil Corn oil Corn oil is oil extracted from the germ of corn . Its main use is in cooking, where its high smoke point makes refined corn oil a valuable frying oil. It is also a key ingredient in some margarines. Corn oil is generally less expensive than most other types of vegetable oils. One bushel of corn... |
12.7 | 24.7 | 57.8 | 0 | 17.24 |
| Sunflower oil Sunflower oil Sunflower oil is the non-volatile oil expressed from sunflower seeds. Sunflower oil is commonly used in food as a frying oil, and in cosmetic formulations as an emollient. Sunflower oil was first industrially produced in 1835 in the Russian Empire.- Composition :Sunflower oil is mainly a... |
11.9 | 20.2 | 63.0 | 0 | 49.0 |
| Safflower oil | 10.2 | 12.6 | 72.1 | 0 | 40.68 |
| Hemp oil Hemp oil Hempseed oil is pressed from the seed of the hemp plant irrespective of the strain of cannabis. Cold pressed, unrefined hemp oil is dark to clear light green in color, with a pleasant nutty flavor. The darker the color, the grassier the flavour.... |
10 | 15 | 75 | 0 | |
| Canola/Rapeseed oil Canola Canola refers to a cultivar of either Rapeseed or Field Mustard . Its seeds are used to produce edible oil suitable for consumption by humans and livestock. The oil is also suitable for use as biodiesel.Originally, Canola was bred naturally from rapeseed in Canada by Keith Downey and Baldur R... |
5.3 | 64.3 | 24.8 | 0 | 22.21 |
Reactions of fatty acids
Fatty acids exhibit reactions like other carboxylic acid, i.e. they undergo esterification and acid-base reactions.Acidity
Fatty acids do not show a great variation in their acidities, as indicated by their pKas. Nonanoic acidNonanoic acid
Nonanoic acid, also called pelargonic acid, is an organic compound composed of a nine-carbon chain terminating in a carboxylic acid with structural formula CH37COOH. Nonanoic acid forms esters—nonanoates. It is a clear, oily liquid with an unpleasant, rancid odor...
, for example, has a pKa of 4.96, being only slightly weaker than acetic acid (4.76). As the chain length increases the solubility of the fatty acids in water decreases very rapidly, so that the longer-chain fatty acids have minimal effect on the pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
of an aqueous solution. Even those fatty acids that are insoluble in water will dissolve in warm ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
, and can be titrated
Titration
Titration, also known as titrimetry, is a common laboratory method of quantitative chemical analysis that is used to determine the unknown concentration of an identified analyte. Because volume measurements play a key role in titration, it is also known as volumetric analysis. A reagent, called the...
with sodium hydroxide solution using phenolphthalein
Phenolphthalein
Phenolphthalein is a chemical compound with the formula C20H14O4 and is often written as "HIn" or "phph" in shorthand notation. Often used in titrations, it turns colorless in acidic solutions and pink in basic solutions...
as an indicator to a pale-pink endpoint. This analysis is used to determine the free fatty acid content of fats; i.e., the proportion of the triglycerides that have been hydrolyze
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
d.
Hydrogenation and hardening
HydrogenationHydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
of unsaturated fatty acids is widely practiced to give saturated fatty acids, which are less prone toward rancidification
Rancidification
Rancidification is the chemical decomposition of fats, oils and other lipids . When these processes occur in food, undesirable odors and flavors can result. In some cases, however, the flavors can be desirable . In processed meats, these flavors are collectively known as "warmed over flavor"...
. Since the saturated fatty acids are higher melting that the unsaturated relatives, the process is called hardening. This technology is used to convert vegetable oils into margarine. During partial hydrogenation, unsaturated fatty acids can be isomerized from cis to trans configuration.
More forcing hydrogenation, i.e. using higher pressures of H2 and higher temperatures, converts fatty acids fatty alcohol
Fatty alcohol
Fatty alcohols are aliphatic alcohols consisting of a chain of 8 to 22 carbon atoms. Fatty alcohols usually have even number of carbon atoms and a single alcohol group attached to the terminal carbon. Some are unsaturated and some are branched...
s. Fatty alcohols are, however, more easily produced from fatty acid esters.
In the Varrentrapp reaction
Varrentrapp reaction
The Varrentrapp reaction is an organic reaction involving the chemical decomposition of an α,β-unsaturated acid into two other acid fragments by action of molten alkali. This reaction pioneered by F...
certain unsaturated fatty acids are cleaved in molten alkali, a reaction at one time of relevance to structure elucidation.
Auto-oxidation and rancidity
Unsaturated fatty acids undergo a chemical change known as auto-oxidation. The process requires oxygen (air) and is accelerated by the presence of trace metals. Vegetable oils resists this process because they contain antioxidants, such as tocopherolTocopherol
Tocopherols are a class of chemical compounds of which many have vitamin E activity. It is a series of organic compounds consisting of various methylated phenols...
. Fats and oils often are treated with chelating agents
Chelation
Chelation is the formation or presence of two or more separate coordinate bonds between apolydentate ligand and a single central atom....
such as citric acid
Citric acid
Citric acid is a weak organic acid. It is a natural preservative/conservative and is also used to add an acidic, or sour, taste to foods and soft drinks...
to remove the metal catalysts.
Ozonolysis
Unsaturated fatty acids are susceptible to degradation by ozone. This reaction is practiced in the production azelaic acidAzelaic acid
Azelaic acid is an organic compound with the formula 72. This saturated dicarboxylic acid exists as a white powder. It is found in wheat, rye, and barley. It is a component of a number of hair and skin conditioners.-Production:...
((CH2)7(CO2H)2) from oleic acid
Oleic acid
Oleic acid is a monounsaturated omega-9 fatty acid found in various animal and vegetable fats. It has the formula CH37CH=CH7COOH. It is an odorless, colourless oil, although commercial samples may be yellowish. The trans isomer of oleic acid is called elaidic acid...
.
Digestion and intake
Short- and medium-chain fatty acids are absorbed directly into the blood via intestine capillaries and travel through the portal vein just as other absorbed nutrients do. However, long-chain fatty acids are not directly released into the intestinal capillaries. Instead they are absorbed into the fatty walls of the intestine villi and reassembled again into triglycerides. The triglycerides are coated with cholesterolCholesterol
Cholesterol is a complex isoprenoid. Specifically, it is a waxy steroid of fat that is produced in the liver or intestines. It is used to produce hormones and cell membranes and is transported in the blood plasma of all mammals. It is an essential structural component of mammalian cell membranes...
and protein (protein coat) into a compound called a chylomicron
Chylomicron
Chylomicrons are lipoprotein particles that consist of triglycerides , phospholipids , cholesterol and proteins .They transport dietary lipids from the intestines to other locations in the body...
.
Within the villi, the chylomicron enters a lymphatic capillary called a lacteal
Lacteal
A lacteal is a lymphatic capillary that absorbs dietary fats in the villi of the small intestine.Triglycerides are emulsified by bile and hydrolyzed by the enzyme lipase, resulting in a mixture of fatty acids and monoglycerides. These then pass from the intestinal lumen into the enterocyte, where...
, which merges into larger lymphatic vessels. It is transported via the lymphatic system and the thoracic duct
Thoracic duct
In human anatomy, the thoracic duct of the lymphatic system is the largest lymphatic vessel in the body. It is also known as the left lymphatic duct, alimentary duct, chyliferous duct, and Van Hoorne's canal....
up to a location near the heart (where the arteries and veins are larger). The thoracic duct empties the chylomicrons into the bloodstream via the left subclavian vein
Subclavian vein
The subclavian veins are two large veins, one on either side of the body. Their diameter is approximately that of the smallest finger.-Path:Each subclavian vein is a continuation of the axillary vein and runs from the outer border of the first rib to the medial border of anterior scalene muscle...
. At this point the chylomicrons can transport the triglycerides to tissues where they are stored or metabolized for energy.
Metabolism
Fatty acids (provided either by ingestion or by drawing on triglycerides stored in fatty tissues) are distributed to cells to serve as a fuel for muscular contraction and general metabolism. They are consumed by mitochondria to produce ATPAdenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
through beta oxidation
Beta oxidation
Beta oxidation is the process by which fatty acids, in the form of Acyl-CoA molecules, are broken down in mitochondria and/or in peroxisomes to generate Acetyl-CoA, the entry molecule for the Citric Acid cycle....
.
Distribution
Blood fatty acids are in different forms in different stages in the blood circulation. They are taken in through the intestine in chylomicrons, but also exist in very low density lipoproteinVery low density lipoprotein
Very-low-density lipoprotein is a type of lipoprotein made by the liver. VLDL is one of the five major groups of lipoproteins that enable fats and cholesterol to move within the water-based solution of the bloodstream...
s (VLDL) and low density lipoprotein
Low density lipoprotein
Low-density lipoprotein is one of the five major groups of lipoproteins, which in order of size, largest to smallest, are chylomicrons, VLDL, IDL, LDL, and HDL, that enable transport of cholesterol within the water-based bloodstream...
s (LDL) after processing in the liver. In addition, when released from adipocytes, fatty acids exist in the blood as free fatty acids.
It is proposed that the blend of fatty acids exuded by mammalian skin, together with lactic acid
Lactic acid
Lactic acid, also known as milk acid, is a chemical compound that plays a role in various biochemical processes and was first isolated in 1780 by the Swedish chemist Carl Wilhelm Scheele. Lactic acid is a carboxylic acid with the chemical formula C3H6O3...
and pyruvic acid
Pyruvic acid
Pyruvic acid is an organic acid, a ketone, as well as the simplest of the alpha-keto acids. The carboxylate ion of pyruvic acid, CH3COCOO−, is known as pyruvate, and is a key intersection in several metabolic pathways....
, is distinctive and enables animals with a keen sense of smell to differentiate individuals.
See also
- Essential fatty acidEssential fatty acidEssential fatty acids, or EFAs, are fatty acids that humans and other animals must ingest because the body requires them for good health but cannot synthesize them...
- Fatty acid metabolismFatty acid metabolismFatty acids are an important source of energy and adenosine triphosphate for many cellular organisms. Excess fatty acids, glucose, and other nutrients can be stored efficiently as fat. Triglycerides yield more than twice as much energy for the same mass as do carbohydrates or proteins. All cell...
- Fatty acid synthaseFatty acid synthaseFatty acid synthase is an enzyme that in humans is encoded by the FASN gene.Fatty acid synthase is a multi-enzyme protein that catalyzes fatty acid synthesis...
- Fatty acid synthesisFatty acid synthesisFatty acid synthesis is the creation of fatty acids from acetyl-CoA and malonyl-CoA precursors through action of enzymes called fatty acid synthases...
- List of saturated fatty acids
- Saturated fatSaturated fatSaturated fat is fat that consists of triglycerides containing only saturated fatty acids. Saturated fatty acids have no double bonds between the individual carbon atoms of the fatty acid chain. That is, the chain of carbon atoms is fully "saturated" with hydrogen atoms...
- Unsaturated fatUnsaturated fatAn unsaturated fat is a fat or fatty acid in which there is at least one double bond within the fatty acid chain. A fat molecule is monounsaturated if it contains one double bond, and polyunsaturated if it contains more than one double bond. Where double bonds are formed, hydrogen atoms are...
- Vegetable oils

