1,4-Benzoquinone
Encyclopedia
1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound
with the formula
C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine
, bleach
, and hot plastic
. Impure samples are often dark-colored due to the presence of quinhydrone (1:1 complex of quinone with hydroquinone
). This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone
. The molecule is multifunctional: It exhibits properties of a ketone
, forming an oxime
; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound.
as the oxidiser and iodine
or an iodine salt as a catalyst for the oxidation occurring in a polar solvent, e.g., isopropyl alcohol
.
Benzoquinone compounds are a metabolite of paracetamol
and, as such, it can also be prepared by the oxidation of paracetamol with nitric acid
.
When heated to its melting point, the product sublimates at atmospheric pressure, and, when prepared from hydroquinone, the substrate boils at a significantly higher temperature than 1,4-benzoquinones melting point, allowing for an effective separation of the two. However, iodine also sublimates within this region and so must be fully removed by filtration prior to sublimation if it has been used as a catalyst during production.
Because the compound degrades to quinhydrone over time, ideally it is prepared immediately before use, but it can also be stored in a freezer and sublimated prior to use at a later date.
It was first commercially prepared in 1919.
. 1,4-Benzoquinone serves as a dehydrogenation
reagent. It is also used as a dienophile in Diels Alder reactions.
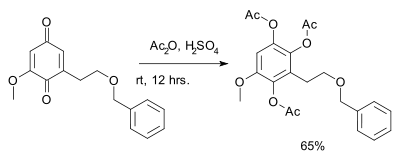
Benzoquinone reacts with acetic anhydride
and sulfuric acid
to the triacetate of hydroxyquinol
. This reaction is called the Thiele reaction after Johannes Thiele
, who first described the reaction in 1898. An application is found in total synthesis :
Benzoquinone is also used to suppress double-bond migration during Olefin Metathesis reactions.
An acidic potassium iodide
solution reduces a solution of benzoquinone to hydroquinone, which is oxidized back with a solution of silver nitrate
.
Due to its ability to function as an oxidiser, 1,4-benzoquinone can be found in methods utilising Wacker-Tsuji oxidation, wherein a palladium salt functions to catalytically oxidise an alkene to its corresponding ketone. This reaction is typically carried out using pressurised oxygen as the oxidiser, and can be done instead with methyl nitrite in solution, but benzoquinone can sometimes be preferential, as it does not require the handling of gases, making it easy to measure out by mass.
and can be used to track exposure to benzene
or mixtures containing benzene and benzene compounds, such as petrol. The compound can interfere with cellular respiration, and kidney damage has been found in animals receiving severe exposure. It is excreted in its original form and also as variations of its own metabolite, hydroquinone.
(redness, rashes on skin) and lead on to localised tissue necrosis
. It is particularly irritating to the eyes and respiratory system. Its ability to sublimate at commonly encountered temperatures allows for a greater airborne exposure risk than might be expected for a room-temperature solid. IARC
has found insufficient evidence to comment on the compound's carcinogenicity, but has noted that it can easily pass into the bloodstream and that it showed activity in depressing bone marrow production in mice and can inhibit protease
enzymes involved in cellular apoptosis
.
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
with the formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
, bleach
Bleach
Bleach refers to a number of chemicals that remove color, whiten, or disinfect, often via oxidation. Common chemical bleaches include household chlorine bleach , lye, oxygen bleach , and bleaching powder...
, and hot plastic
Plastic
A plastic material is any of a wide range of synthetic or semi-synthetic organic solids used in the manufacture of industrial products. Plastics are typically polymers of high molecular mass, and may contain other substances to improve performance and/or reduce production costs...
. Impure samples are often dark-colored due to the presence of quinhydrone (1:1 complex of quinone with hydroquinone
Hydroquinone
Hydroquinone, also benzene-1,4-diol or quinol, is an aromatic organic compound that is a type of phenol, having the chemical formula C6H42. Its chemical structure, shown in the table at right, has two hydroxyl groups bonded to a benzene ring in a para position. It is a white granular solid...
). This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone
Hydroquinone
Hydroquinone, also benzene-1,4-diol or quinol, is an aromatic organic compound that is a type of phenol, having the chemical formula C6H42. Its chemical structure, shown in the table at right, has two hydroxyl groups bonded to a benzene ring in a para position. It is a white granular solid...
. The molecule is multifunctional: It exhibits properties of a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
, forming an oxime
Oxime
An oxime is a chemical compound belonging to the imines, with the general formula R1R2C=NOH, where R1 is an organic side chain and R2 may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds...
; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound.
Preparation
1,4-Benzoquinone can be prepared from hydroquinone via a number of oxidation methods. One such method makes use of hydrogen peroxideHydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
as the oxidiser and iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
or an iodine salt as a catalyst for the oxidation occurring in a polar solvent, e.g., isopropyl alcohol
Isopropyl alcohol
Isopropyl alcohol is a common name for a chemical compound with the molecular formula C3H8O. It is a colorless, flammable chemical compound with a strong odor...
.
Benzoquinone compounds are a metabolite of paracetamol
Paracetamol
Paracetamol INN , or acetaminophen USAN , is a widely used over-the-counter analgesic and antipyretic . It is commonly used for the relief of headaches and other minor aches and pains and is a major ingredient in numerous cold and flu remedies...
and, as such, it can also be prepared by the oxidation of paracetamol with nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
.
When heated to its melting point, the product sublimates at atmospheric pressure, and, when prepared from hydroquinone, the substrate boils at a significantly higher temperature than 1,4-benzoquinones melting point, allowing for an effective separation of the two. However, iodine also sublimates within this region and so must be fully removed by filtration prior to sublimation if it has been used as a catalyst during production.
Because the compound degrades to quinhydrone over time, ideally it is prepared immediately before use, but it can also be stored in a freezer and sublimated prior to use at a later date.
It was first commercially prepared in 1919.
Applications in organic synthesis
It is used as a hydrogen acceptor and oxidant in organic synthesisOrganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
. 1,4-Benzoquinone serves as a dehydrogenation
Dehydrogenation
Dehydrogenation is a chemical reaction that involves the elimination of hydrogen . It is the reverse process of hydrogenation. Dehydrogenation reactions may be either large scale industrial processes or smaller scale laboratory procedures....
reagent. It is also used as a dienophile in Diels Alder reactions.
Benzoquinone reacts with acetic anhydride
Acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
and sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
to the triacetate of hydroxyquinol
Hydroxyquinol
Hydroxyquinol is a benzenetriol.It is a catechin biodegradation product formed by Bradyrhizobium japonicum.Hydroxyquinol 1,2-dioxygenase is an enzyme that uses hydroxyquinol and O2 to produce 3-hydroxy-cis,cis-muconate....
. This reaction is called the Thiele reaction after Johannes Thiele
Johannes Thiele (chemist)
Friedrich Karl Johannes Thiele was a German chemist and a prominent professor at several universities, including those in Munich and Strasbourg. He developed many laboratory techniques related to isolation of organic compounds...
, who first described the reaction in 1898. An application is found in total synthesis :
Benzoquinone is also used to suppress double-bond migration during Olefin Metathesis reactions.
An acidic potassium iodide
Potassium iodide
Potassium iodide is an inorganic compound with the chemical formula KI. This white salt is the most commercially significant iodide compound, with approximately 37,000 tons produced in 1985. It is less hygroscopic than sodium iodide, making it easier to work with...
solution reduces a solution of benzoquinone to hydroquinone, which is oxidized back with a solution of silver nitrate
Silver nitrate
Silver nitrate is an inorganic compound with chemical formula . This compound is a versatile precursor to many other silver compounds, such as those used in photography. It is far less sensitive to light than the halides...
.
Due to its ability to function as an oxidiser, 1,4-benzoquinone can be found in methods utilising Wacker-Tsuji oxidation, wherein a palladium salt functions to catalytically oxidise an alkene to its corresponding ketone. This reaction is typically carried out using pressurised oxygen as the oxidiser, and can be done instead with methyl nitrite in solution, but benzoquinone can sometimes be preferential, as it does not require the handling of gases, making it easy to measure out by mass.
Metabolism
1,4-Benzoquinone is a toxic metabolite found in human bloodBlood
Blood is a specialized bodily fluid in animals that delivers necessary substances such as nutrients and oxygen to the cells and transports metabolic waste products away from those same cells....
and can be used to track exposure to benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
or mixtures containing benzene and benzene compounds, such as petrol. The compound can interfere with cellular respiration, and kidney damage has been found in animals receiving severe exposure. It is excreted in its original form and also as variations of its own metabolite, hydroquinone.
Safety
1,4-Benzoquinone is able to stain skin dark brown, cause erythemaErythema
Erythema is redness of the skin, caused by hyperemia of the capillaries in the lower layers of the skin. It occurs with any skin injury, infection, or inflammation...
(redness, rashes on skin) and lead on to localised tissue necrosis
Necrosis
Necrosis is the premature death of cells in living tissue. Necrosis is caused by factors external to the cell or tissue, such as infection, toxins, or trauma. This is in contrast to apoptosis, which is a naturally occurring cause of cellular death...
. It is particularly irritating to the eyes and respiratory system. Its ability to sublimate at commonly encountered temperatures allows for a greater airborne exposure risk than might be expected for a room-temperature solid. IARC
International Agency for Research on Cancer
The International Agency for Research on Cancer is an intergovernmental agency forming part of the World Health Organisation of the United Nations....
has found insufficient evidence to comment on the compound's carcinogenicity, but has noted that it can easily pass into the bloodstream and that it showed activity in depressing bone marrow production in mice and can inhibit protease
Protease
A protease is any enzyme that conducts proteolysis, that is, begins protein catabolism by hydrolysis of the peptide bonds that link amino acids together in the polypeptide chain forming the protein....
enzymes involved in cellular apoptosis
Apoptosis
Apoptosis is the process of programmed cell death that may occur in multicellular organisms. Biochemical events lead to characteristic cell changes and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation...
.
Related 1,4-benzoquinones
A variety of derivatives and analogues are known. Illustrative examples:- 1,4-NaphthoquinoneNaphthoquinoneNaphthoquinone is a class of natural phenols based on the C6-C4 skeleton.1,4-Naphthoquinone can be viewed as derivatives of naphthalene through the replacement of two hydrogen atoms by two ketone groups....
, derived by oxidation of naphthalene with chromium trioxideChromium trioxideChromium trioxide is the inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name.This compound is a dark-red/orange brown solid, which dissolves in water concomitant with hydrolysis...
. - 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone2,3-Dichloro-5,6-dicyano-1,4-benzoquinone2,3-Dichloro-5,6-dicyano-1,4-benzoquinone is the chemical reagent with formula C8Cl2N2O2. This oxidant is useful for the dehydrogenation of alcohols, phenols and steroid ketones in organic chemistry. DDQ decomposes in water, but is stable in aqueous mineral acid.-Preparation:Synthesis of DDQ...
(DDQ), a stronger oxidant and dehydrogenation agent than 1,4-benzoquinone. - Ubiquinone-1, a naturally occurring 1,4-benzoquinone.
- Chloro-p-benzoquinone, (CAS no. [695-99-8])
- Chloranil, 1,4-C6Cl4O2, a stronger oxidant and dehydrogenation agent than 1,4-benzoquinone.
See also
- Tetrahydroxybenzoquinone
- Benzoquinonetetracarboxylic acidBenzoquinonetetracarboxylic acidIn chemistry, 1,4-benzoquinonetetracarboxylic acid is an organic compound with formula , or 4, which can be viewed as deriving from para-benzoquinone through replacement of the four hydrogen atoms by carboxyl functional groups -OH....
- 1,2-Benzoquinone1,2-Benzoquinone1,2-Benzoquinone, also called ortho-benzoquinone or cyclohexa-3,5-diene-1,2-dione, is a ketone, with formula C6H4O2. It is one of the two isomers of quinone, the other being 1,4-benzoquinone....
- Quinones
- DuroquinoneDuroquinoneDuroquinone is a derivative of 1,4-benzoquinone.It is also used in the formation of a "nano brain" ....