
Beta oxidation
Encyclopedia
Fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
s, in the form of Acyl-CoA
Acyl-CoA
Acyl-CoA is a group of coenzymes involved in the metabolism of fatty acids. It is a temporary compound formed when coenzyme A attaches to the end of a long-chain fatty acid inside living cells. The compound undergoes beta oxidation, forming one or more molecules of acetyl-CoA...
molecules, are broken down in mitochondria and/or in peroxisome
Peroxisome
Peroxisomes are organelles found in virtually all eukaryotic cells. They are involved in the catabolism of very long chain fatty acids, branched chain fatty acids, D-amino acids, polyamines, and biosynthesis of plasmalogens, etherphospholipids critical for the normal function of mammalian brains...
s to generate Acetyl-CoA
Acetyl-CoA
Acetyl coenzyme A or acetyl-CoA is an important molecule in metabolism, used in many biochemical reactions. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. In chemical structure, acetyl-CoA is the thioester...
, the entry molecule for the Citric Acid cycle
Citric acid cycle
The citric acid cycle — also known as the tricarboxylic acid cycle , the Krebs cycle, or the Szent-Györgyi-Krebs cycle — is a series of chemical reactions which is used by all aerobic living organisms to generate energy through the oxidization of acetate derived from carbohydrates, fats and...
.
The beta oxidation of fatty acids involve three stages:
- Activation of fatty acids in the cytosol
- Transport of fatty acids into mitochondria (carnitine shuttle)
- Beta oxidation proper in the mitochondrial matrix
Fatty acids are oxidized by most of the tissues in the body. However, the brain can hardly utilize fatty acids for energy requirements, while erythrocytes and adrenal medulla
Adrenal medulla
The adrenal medulla is part of the adrenal gland. It is located at the center of the gland, being surrounded by the adrenal cortex. It is the innermost part of the adrenal gland, consisting of cells that secrete epinephrine , norepinephrine , and a small amount of dopamine in response to...
cannot use them at all.
Activation of fatty acids
Free fatty acids can penetrate the plasma membrane due to their poor water solubility and high fat solubility. Once in the cytosolCytosol
The cytosol or intracellular fluid is the liquid found inside cells, that is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into compartments....
, activation of the fatty acid is catalyzed by long fatty acyl CoA synthetase
Long fatty acyl CoA synthetase
The Long chain fatty acyl-CoA synthetase enzyme is a member of the ligase family that activates the breakdown of complex fatty acids. Long chain fatty acyl-CoA synthetase plays a crucial role in intermediary metabolism by catalyzing the formation of fatty acyl-CoA by a two-step process proceeding...
. A fatty acid reacts with ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
to give a fatty acyl adenylate, plus inorganic pyrophosphate, which then reacts with free coenzyme A
Coenzyme A
Coenzyme A is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All sequenced genomes encode enzymes that use coenzyme A as a substrate, and around 4% of cellular enzymes use it as a substrate...
to give a fatty acyl-CoA ester plus AMP
Adenosine monophosphate
Adenosine monophosphate , also known as 5'-adenylic acid, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid and the nucleoside adenosine. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine...
. The fatty acyl-CoA is then reacted with carnitine
Carnitine
Carnitine is a quaternary ammonium compound biosynthesized from the amino acids lysine and methionine. In living cells, it is required for the transport of fatty acids from the cytosol into the mitochondria during the breakdown of lipids for the generation of metabolic energy. It is widely...
to form acylcarnitine, which is transported across the inner mitochondrial membrane by a monosodium glutamate
Monosodium glutamate
Monosodium glutamate, also known as sodium glutamate or MSG, is the sodium salt of glutamic acid, one of the most abundant naturally occurring non-essential amino acids....
.
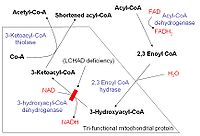
Four recurring steps
Once inside the mitochondria, each cycle of β-oxidation, liberating a two carbon unit-acetyl CoA, occurs in a sequence of four reactions:| Description | Diagram | Enzyme | End product >- | Dehydrogenation Dehydrogenation Dehydrogenation is a chemical reaction that involves the elimination of hydrogen . It is the reverse process of hydrogenation. Dehydrogenation reactions may be either large scale industrial processes or smaller scale laboratory procedures.... by FAD FAD In biochemistry, flavin adenine dinucleotide is a redox cofactor involved in several important reactions in metabolism. FAD can exist in two different redox states, which it converts between by accepting or donating electrons. The molecule consists of a riboflavin moiety bound to the phosphate... : The first step is the oxidation of the fatty acid by Acyl-CoA-Dehydrogenase. The enzyme catalyzes the formation of a double bond Double bond A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in... between the C-2 and C-3. |
 |
acyl CoA dehydrogenase Acyl CoA dehydrogenase Acyl-CoA dehydrogenases are a class of enzymes that function to catalyze the initial step in each cycle of fatty acid β -oxidation in the mitochondria of cells. Their action results in the introduction of a trans double-bond between C2 and C3 of the acyl-CoA thioester substrate... |
2-enoyl-CoA >- | Hydration: The next step is the hydration Hydration reaction In organic chemistry, a hydration reaction is a chemical reaction in which a hydroxyl group and a hydrogen cation are added to the two carbon atoms bonded together in the carbon-carbon double bond which makes up an alkene functional group. The reaction usually runs in a strong acidic, aqueous... of the bond between C-2 and C-3. The reaction is stereospecific, forming only the L isomer Isomer In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical... . |
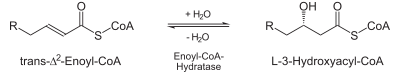 |
enoyl CoA hydratase | >- | 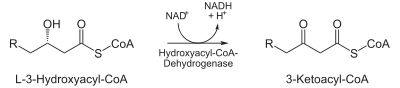 |
L-β-hydroxyacyl CoA dehydrogenase | >- | 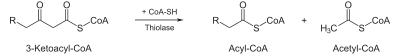 |
β-ketothiolase | An acetyl CoA molecule, and an acyl CoA molecule that is two carbons shorter |
This process continues until the entire chain is cleaved into acetyl CoA units. The final cycle produces two separate acetyl CoAs, instead of one acyl CoA and one acetyl CoA. For every cycle, the Acyl CoA unit is shortened by two carbon atoms. Concomitantly, one molecule of FADH2, NADH and acetyl CoA are formed.
β-Oxidation of unsaturated fatty acids
β-Oxidation of unsaturated fatty acids poses a problem since the location of a cis bond can prevent the formation of a trans-Δ2 bond. These situations are handled by an additional two enzymes.Whatever the conformation of the hydrocarbon chain, β-oxidation occurs normally until the acyl CoA (because of the presence of a double bond) is not an appropriate substrate for acyl CoA dehydrogenase, or enoyl CoA hydratase:
- If the acyl CoA contains a cis-Δ3 bond, then cis-Δ3-Enoyl CoA isomeraseEnoyl CoA isomeraseEnoyl CoA isomerase or dodecenoyl-coenzyme A delta-isomerase is an enzyme that catalyzes conversion of cis-double bonds of fatty acids at position 3 to trans double bonds at position 2. It has a special importance in metabolism of unsaturated fatty acids.-External links:...
will convert the bond to a trans-Δ2 bond, which is a regular substrate.
- If the acyl CoA contains a cis-Δ4 double bond, then its dehydrogenation yields a 2,4-dienoyl intermediate, which is not a substrate for enoyl CoA hydratase. However, the enzyme 2,4 Dienoyl CoA reductase reduces the intermediate, using NADPH, into trans-Δ3-enoyl CoA. As in the above case, this compound is converted into a suitable intermediate by 3,2-Enoyl CoA isomerase.
To summarize:
- Odd-numbered double bonds are handled by the isomerase.
- Even-numbered double bonds by the reductase (which creates an odd-numbered double bond)
β-Oxidation of odd-numbered chains
In general, fatty acids with an odd number of carbon are found in the lipids of plants and some marine organisms. Many ruminant animals form large amount of 3-carbon propionate during fermentation of carbohydrate in rumen.Chains with an odd-number of carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
s are oxidized in the same manner as even-numbered chains, but the final products are propionyl-CoA
Propionyl-CoA
Propionyl-CoA is a coenzyme A derivative of propionic acid.-Production:There are several different ways in which it is formed:* It is formed as a product of beta-oxidation of odd-chain fatty acids....
and acetyl-CoA
Acetyl-CoA
Acetyl coenzyme A or acetyl-CoA is an important molecule in metabolism, used in many biochemical reactions. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. In chemical structure, acetyl-CoA is the thioester...
.
Propionyl-CoA is first carboxylated using a bicarbonate
Bicarbonate
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid...
ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
into D-stereoisomer of methylmalonyl-CoA, in a reaction that involves a biotin
Biotin
Biotin, also known as Vitamin H or Coenzyme R, is a water-soluble B-complex vitamin discovered by Bateman in 1916. It is composed of a ureido ring fused with a tetrahydrothiophene ring. A valeric acid substituent is attached to one of the carbon atoms of the tetrahydrothiophene ring...
co-factor
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
, ATP, and the enzyme propionyl-CoA carboxylase
Propionyl-CoA carboxylase
Propionyl-CoA carboxylase catalyses the carboxylation reaction of propionyl CoA in the mitochondrial matrix. The enzyme is biotin dependent. The product of the reaction is -methylmalonyl CoA. Propionyl CoA is the end product of metabolism of odd-chain fatty acids, and is also a metabolite of most...
. The bicarbonate ion's carbon is added to the middle carbon of propionyl-CoA, forming a D-methylmalonyl-CoA. However, the D conformation is enzymatically converted into the L conformation by methylmalonyl-CoA epimerase, then it undergoes intramolecular rearrangement, which is catalyzed by methylmalonyl-CoA mutase
Methylmalonyl-CoA mutase
Methylmalonyl Coenzyme A mutase, also known as MCM is an enzyme that catalyzes the isomerization of methylmalonyl-CoA to succinyl-CoA and it is involved in key metabolic pathways...
(requiring B12 as a coenzyme) to form succinyl-CoA. The succinyl-CoA formed can then enter the citric acid cycle.
Because it cannot be completely metabolized in the citric acid cycle, the products of its partial reaction must be removed in a process called cataplerosis. This allows regeneration of the citric acid cycle intermediates, possibly an important process in certain metabolic diseases.
Oxidation in peroxisomes
Fatty acid oxidation also occurs in peroxisomePeroxisome
Peroxisomes are organelles found in virtually all eukaryotic cells. They are involved in the catabolism of very long chain fatty acids, branched chain fatty acids, D-amino acids, polyamines, and biosynthesis of plasmalogens, etherphospholipids critical for the normal function of mammalian brains...
s, when the fatty acid chains are too long to be handled by the mitochondria. However, the oxidation ceases at octanyl CoA. It is believed that very long chain (greater than C-22) fatty acids undergo initial oxidation in peroxisomes which is followed by mitochondrial oxidation.
One significant difference is that oxidation in peroxisomes is not coupled to ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
synthesis. Instead, the high-potential electrons are transferred to O2, which yields H2O2. The enzyme catalase
Catalase
Catalase is a common enzyme found in nearly all living organisms that are exposed to oxygen, where it catalyzes the decomposition of hydrogen peroxide to water and oxygen...
, found exclusively in peroxisomes, converts the hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
into water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
.
Peroxisomal β-oxidation also requires enzymes specific to the peroxisome and to very long fatty acids. There are three key differences between the enzymes used for mitochondrial and peroxisomal β-oxidation:
- β-oxidation in the peroxisome requires the use of a peroxisomal carnitine acyltransferase (instead of carnitine acyltransferase I and II used by the mitochondria) for transport of the activated acyl group into the peroxisome.
- The first oxidation step in the peroxisome is catalyzed by the enzyme acyl CoA oxidaseAcyl-CoA oxidaseIn enzymology, an acyl-CoA oxidase is an enzyme that catalyzes the chemical reactionThus, the two substrates of this enzyme are acyl-CoA and O2, whereas its two products are trans-2,3-dehydroacyl-CoA and H2O2....
. - The β-ketothiolase used in peroxisomal β-oxidation has an altered substrate specificity, different from the mitochondrial β-ketothiolase.
Peroxisomal oxidation is induced by high-fat diet and administration of hypolipidemic drugs like clofibrate.
Energy yield
The ATP yield for every oxidation cycle is 14 ATP (according to the P/O ratio), broken down as follows:| Source | ATP | Total >- | 1 FADH2 |
x 1.5 ATP | >- | x 2.5 ATP | >- | x 10 ATP | >- | = 14 ATP |
For an even-numbered saturated fat (C2n), n - 1 oxidations are necessary, and the final process yields an additional acetyl CoA. In addition, two equivalents of ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
are lost during the activation of the fatty acid. Therefore, the total ATP yield can be stated as:
* 14 + 10 - 2 = total ATP
For instance, the ATP yield of palmitate (C16, n = 8) is:
* 14 + 10 - 2 = 106 ATP
Represented in table form:
| Source | ATP | Total >- | 7 FADH2 |
x 1.5 ATP | >- | x 2.5 ATP | >- | x 10 ATP | >- | >- | = 106 ATP |
For sources that use the larger ATP production numbers described above, the total would be 129 ATP ={(8-1)*17+12-2} equivalents per palmitate.
Beta-oxidation of unsaturated fatty acids changes the ATP yield due to the requirement of two possible additional enzymes.

