.gif)
Radical (chemistry)
Encyclopedia

Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s, molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s, or ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s with unpaired electron
Unpaired electron
In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. As the formation of electron pairs is often energetically favourable, either in the form of a chemical bond or as a lone pair, unpaired electrons are relatively...
s on an open shell
Open shell
In the context of atomic orbitals, an open shell is a valence shell which is not completely filled with electrons or that has not given all of its valence electrons through chemical bonds with other atoms or molecules during a chemical reaction. Atoms generally reach a noble gas configuration in a...
configuration. Free radicals may have positive, negative, or zero charge. With some exceptions, the unpaired electrons cause radicals to be highly chemically reactive
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
.
Radicals, if allowed to run free in the body, are believed to be involved in degenerative diseases, senescence
Senescence
Senescence or biological aging is the change in the biology of an organism as it ages after its maturity. Such changes range from those affecting its cells and their function to those affecting the whole organism...
(the aging process), and cancers.
Free radicals play an important role in combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
, atmospheric chemistry
Atmospheric chemistry
Atmospheric chemistry is a branch of atmospheric science in which the chemistry of the Earth's atmosphere and that of other planets is studied. It is a multidisciplinary field of research and draws on environmental chemistry, physics, meteorology, computer modeling, oceanography, geology and...
, polymerization
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
, plasma
Plasma (physics)
In physics and chemistry, plasma is a state of matter similar to gas in which a certain portion of the particles are ionized. Heating a gas may ionize its molecules or atoms , thus turning it into a plasma, which contains charged particles: positive ions and negative electrons or ions...
chemistry, biochemistry
Biochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
, and many other chemical processes. In chemical biology, superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
and nitric oxide
Nitric oxide
Nitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry...
regulate many processes, such as controlling vascular tone. Such radicals can even be messengers in a phenomenon dubbed redox signaling
Redox signaling
Redox signaling is when free radicals, reactive oxygen species , and other electronically activated species such as nitric oxide act as biological messengers. Arguably, hydrogen sulfide and carbon monoxide are also redox signaling molecules...
. A radical may be trapped within a solvent cage
Cage effect (chemistry)
The cage effect in chemistry describes how properties of a molecule are affected by its surroundings.In a solvent a molecule is often more accurately described existing in a cage of solvent molecules, the so-called solvent cage. Reactions occur when a molecule occasionally "jumps out" and meets...
or be otherwise bound.
History
The first organic free radical identified was triphenylmethyl radicalTriphenylmethyl radical
The triphenylmethyl radical is a persistent radical and the first-ever radical described in organic chemistry. It can be prepared by homolysis of triphenylmethyl chloride 1 by a metal like silver or zinc in benzene or diethyl ether. The radical 2 forms a chemical equilibrium with the quinoid type...
, by Moses Gomberg
Moses Gomberg
Moses Gomberg was a chemistry professor at the University of Michigan....
in 1900 at the University of Michigan
University of Michigan
The University of Michigan is a public research university located in Ann Arbor, Michigan in the United States. It is the state's oldest university and the flagship campus of the University of Michigan...
.
Historically, the term radical has also been used for bound parts of the molecule, especially when they remain unchanged in reactions. These are now called functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s. For example, methyl alcohol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
was described as consisting of a methyl "radical" and a hydroxyl "radical". Neither are radicals in the modern chemical sense, as they are permanently bound to each other, and have no unpaired, reactive electrons. They can, however, be observed as radicals in mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
when broken apart by irradiation with energetic electrons.
Depiction in chemical reactions
In chemical equations, free radicals are frequently denoted by a dot placed immediately to the right of the atomic symbol or molecular formula as follows:
- Chlorine gas can be broken down by ultraviolet light to form atomic chlorine radicals.
Radical reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
s use single-headed arrows to depict the movement of single electrons:
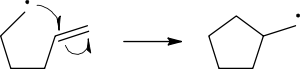
Homolysis
In general it means breakdown to equal pieces There are separate meanings for the word in chemistry and biology.-Homolysis in chemistry:...
cleavage of the breaking bond is drawn with a 'fish-hook' arrow to distinguish from the usual movement of two electrons depicted by a standard curly arrow. It should be noted that the second electron of the breaking bond also moves to pair up with the attacking radical electron; this is not explicitly indicated in this case.
Free radicals take part in radical addition and radical substitution
Radical substitution
In organic chemistry, a radical substitution reaction is a substitution reaction involving free radicals as a reactive intermediate.The reaction always involves at least two steps, and possibly a third....
as reactive intermediate
Reactive intermediate
In chemistry a reactive intermediate is a short-lived, high energy, highly reactive molecule. When generated in a chemical reaction it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation...
s. Chain reactions involving free radicals can usually be divided into three distinct processes: initiation, propagation, and termination.
- Initiation reactions are those that result in a net increase in the number of free radicals. They may involve the formation of free radicals from stable species as in Reaction 1 above or they may involve reactions of free radicals with stable species to form more free radicals.
- Propagation reactions are those reactions involving free radicals in which the total number of free radicals remains the same.
- Termination reactions are those reactions resulting in a net decrease in the number of free radicals. Typically two free radicals combine to form a more stable species, for example: 2Cl·→ Cl2
Formation
The formation of radicals may involve breaking of covalent bonds homolyticallyHomolysis
In general it means breakdown to equal pieces There are separate meanings for the word in chemistry and biology.-Homolysis in chemistry:...
, a process that requires significant amounts of energy. For example, splitting H2 into 2H· has a ΔH° of +435 kJ/mol, and Cl2 into 2Cl· has a ΔH° of +243 kJ/mol. This is known as the homolytic bond dissociation energy
Bond dissociation energy
In chemistry, bond-dissociation energy or D0, is one measure of the bond strength in a chemical bond. It is defined as the standard enthalpy change when a bond is cleaved by homolysis, with reactants and products of the homolysis reaction at 0 K...
, and is usually abbreviated as the symbol DH°. The bond energy between two covalently bonded atoms is affected by the structure of the molecule as a whole, not just the identity of the two atoms, and radicals requiring more energy to form are less stable than those requiring less energy. Homolytic bond cleavage
Cleavage
Cleavage may refer to:*Cleavage , partial exposure of the separation between a woman's breasts.**Cleavage enhancement, methods of making a person's breast cleavage look more substantial than it really is....
most often happens between two atoms of similar electronegativity. In organic chemistry this is often the O-O bond in peroxide
Organic peroxide
Organic peroxides are organic compounds containing the peroxide functional group . If the R' is hydrogen, the compound is called an organic hydroperoxide. Peresters have general structure RCOOR. The O-O bond easily breaks and forms free radicals of the form RO·...
species or O-N bonds. Sometimes radical formation is spin-forbidden, presenting an additional barrier.
However, propagation is a very exothermic reaction
Exothermic reaction
An exothermic reaction is a chemical reaction that releases energy in the form of light or heat. It is the opposite of an endothermic reaction. Expressed in a chemical equation:-Overview:...
. Note that most species are electrically neutral although radical ion
Radical ion
A radical ion is a free radical species that carries a charge. Radical ions are encountered in organic chemistry as reactive intermediates and in mass spectrometry as gas phase ions...
s do exist.
Radicals may also be formed by single electron oxidation or reduction
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
of an atom or molecule. An example is the production of superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
by the electron transport chain
Electron transport chain
An electron transport chain couples electron transfer between an electron donor and an electron acceptor with the transfer of H+ ions across a membrane. The resulting electrochemical proton gradient is used to generate chemical energy in the form of adenosine triphosphate...
. Early studies of organometallic chemistry, especially tetra-alkyl lead species by F.A. Paneth and K. Hahnfeld in the 1930s supported heterolytic fission of bonds and a radical based mechanism.
Persistence and stability
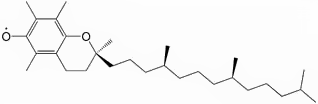
Stable radicals
The prime example of a stable radical is molecular dioxygen (O2). Another common example is nitric oxide (NO). Organic radicals can be long lived if they occur in a conjugated π system, such as the radical derived from α-tocopherol
Tocopherol
Tocopherols are a class of chemical compounds of which many have vitamin E activity. It is a series of organic compounds consisting of various methylated phenols...
(vitamin E
Vitamin E
Vitamin E is used to refer to a group of fat-soluble compounds that include both tocopherols and tocotrienols. There are many different forms of vitamin E, of which γ-tocopherol is the most common in the North American diet. γ-Tocopherol can be found in corn oil, soybean oil, margarine and dressings...
). There are also hundreds of examples of thiazyl radicals, which show remarkable kinetic and thermodynamic stability with only a very limited extent of π resonance stabilization.
Persistent radicals
Persistent radical compounds are those whose longevity is due to steric crowding
Steric effects
Steric effects arise from the fact that each atom within a molecule occupies a certain amount of space. If atoms are brought too close together, there is an associated cost in energy due to overlapping electron clouds , and this may affect the molecule's preferred shape and reactivity.-Steric...
around the radical center, which makes it physically difficult for the radical to react with another molecule. Examples of these include Gomberg's triphenylmethyl radical
Triphenylmethyl radical
The triphenylmethyl radical is a persistent radical and the first-ever radical described in organic chemistry. It can be prepared by homolysis of triphenylmethyl chloride 1 by a metal like silver or zinc in benzene or diethyl ether. The radical 2 forms a chemical equilibrium with the quinoid type...
, Fremy's salt
Fremy's salt
Frémy's salt, discovered in 1845 by Edmond Frémy , is a chemical compound and a strong oxidizing agent. The formal name is disodium nitrosodisulfonate or Na2NO2, but the expression "Frémy's salt" refers equally well to potassium nitrosodisulfonate, also known as potassium peroxylamine disulfonate...
(Potassium nitrosodisulfonate, (KSO3)2NO·), nitroxides, (general formula R2NO·) such as TEMPO
TEMPO
oxyl, or oxidanyl or TEMPO is a chemical compound with the formula 32NO . This heterocycle is a red-orange, sublimable solid. As a stable radical, it has applications throughout chemistry and biochemistry. TEMPO was discovered by Lebedev and Kazarnowskii in 1960...
, TEMPOL, verdazys, nitronyl nitroxides, and azephenylenyls and radicals derived from PTM (perchlorophenylmethyl radical) and TTM (tris(2,4,6-trichlorophenylmethyl radical). The longest-lived free radical is melanin
Melanin
Melanin is a pigment that is ubiquitous in nature, being found in most organisms . In animals melanin pigments are derivatives of the amino acid tyrosine. The most common form of biological melanin is eumelanin, a brown-black polymer of dihydroxyindole carboxylic acids, and their reduced forms...
, which may persist for millions of years. Persistent radicals are generated in great quantity during combustion, and "may be responsible for the oxidative stress resulting in cardiopulmonary disease and probably cancer that has been attributed to exposure to airborne fine particles."
Diradicals
Diradical
Diradical
A diradical in organic chemistry is a molecular species with two electrons occupying two degenerate molecular orbitals . They are known by their higher reactivities and shorter lifetimes. In a broader definition diradicals are even-electron molecules that have one bond less than the number...
s are molecules containing two radical centers. Multiple radical centers can exist in a molecule. Atmospheric oxygen naturally exists as a diradical in its ground state as triplet oxygen
Triplet oxygen
Triplet oxygen is the ground state of the oxygen molecule. The electron configuration of the molecule has two unpaired electrons occupying two degenerate molecular orbitals...
. The high reactivity of atmospheric oxygen is due to its diradical state. Non-radical states of dioxygen are actually less stable. The relative stability of the oxygen diradical is primarily due to the spin-forbidden nature of the triplet-singlet transition required for it to grab electrons. The diradical state of oxygen also results in its paramagnetic character, which is demonstrated by its attraction to an external magnet.
Reactivity
Radical alkyl intermediates are stabilized by similar criteria as carbocations: the more substituted the radical center is, the more stable it is. This will direct their reactions: formation of a tertiary radical (R3C·) is favored over secondary (R2HC·), which is favored over primary (RH2C·). Radicals next to functional groups such as carbonyl, nitrile, and ether are more stable than tertiary alkyl radicals.Radicals attack double bonds, but unlike similar ions, they are not as much directed by electrostatic interactions. For example, the reactivity of nucleophilic ions with α,β-unsaturated compounds (C=C–C=O) is directed by the electron-withdrawing effect of the oxygen, resulting in a partial positive charge on the carbonyl carbon. There are two reactions that are observed in the ionic case: the carbonyl is attacked in a direct addition to carbonyl, or the vinyl is attacked in conjugate addition, and in either case, the charge on the nucleophile is taken by the oxygen. Radicals add rapidly to the double bond, and the resulting α-radical carbonyl is relatively stable; it can couple with another molecule or be oxidized. Nonetheless, the electrophilic/neutrophilic character of radicals has been shown in a variety of instances (e.g., in the alternating tendency of the copolymerization of maleic anhydride
Maleic anhydride
Maleic anhydride is an organic compound with the formula C2H22O. It is the acid anhydride of maleic acid and in its pure state it is a colourless or white solid with an acrid odour....
(electrophilic) and styrene (slightly nucleophilic).
In intramolecular reactions, precise control can be achieved despite the extreme reactivity of radicals. Radicals will attack the closest reactive site the most readily. Therefore, when there is a choice, a preference for five-membered rings is observed: four-membered rings are too strained, and collisions with carbons six or more atoms away in the chain are infrequent.
Carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
s and nitrene
Nitrene
In chemistry, a nitrene is the nitrogen analogue of a carbene. The nitrogen atom has only 6 valence electrons and is therefore considered an electrophile...
s, which are diradicals, have distinctive chemistry.
Combustion
Probably the most familiar free-radical reaction for most people is combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
. The oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
molecule is a stable diradical
Diradical
A diradical in organic chemistry is a molecular species with two electrons occupying two degenerate molecular orbitals . They are known by their higher reactivities and shorter lifetimes. In a broader definition diradicals are even-electron molecules that have one bond less than the number...
, best represented by ·O-O·, which is stable because the spins
Spin (physics)
In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
of the electrons are parallel. The ground state
Ground state
The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
of oxygen is an unreactive spin-unpaired (triplet
Triplet oxygen
Triplet oxygen is the ground state of the oxygen molecule. The electron configuration of the molecule has two unpaired electrons occupying two degenerate molecular orbitals...
) diradical, but an extremely reactive spin-paired (singlet
Singlet oxygen
Singlet oxygen is the common name used for the diamagnetic form of molecular oxygen , which is less stable than the normal triplet oxygen. Because of its unusual properties, singlet oxygen can persist for over an hour at room temperature, depending on the environment...
) state is available. For combustion to occur, the energy barrier between these must be overcome. This barrier can be overcome by heat, requiring high temperatures. The triplet-singlet transition is also "forbidden
Forbidden mechanism
In physics, a forbidden mechanism or forbidden line is a spectral line emitted by atoms undergoing nominally "forbidden" energy transitions not normally allowed by the selection rules of quantum mechanics. In formal physics, this means that the process cannot proceed via the most efficient route...
". This presents an additional barrier to the reaction. It also means molecular oxygen is relatively unreactive at room temperature except in the presence of a catalytic heavy atom such as iron or copper.
Combustion consists of various radical chain reactions that the singlet radical can initiate. The flammability
Flammability
Flammability is defined as how easily something will burn or ignite, causing fire or combustion. The degree of difficulty required to cause the combustion of a substance is quantified through fire testing. Internationally, a variety of test protocols exist to quantify flammability...
of a given material is strongly dependent on the concentration of free radicals that must be obtained before initiation and propagation reactions dominate leading to combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
of the material. Once the combustible material has been consumed, termination reactions again dominate and the flame dies out. Propagation or termination reactions can be promoted to alter flammability. Tetraethyl lead was once commonly added to gasoline, because lead itself deactivates free radicals in the gasoline-air mixture. This prevents the combustion from initiating in an uncontrolled manner or in unburnt residues (engine knocking
Engine knocking
Knocking in spark-ignition internal combustion engines occurs when combustion of the air/fuel mixture in the cylinder starts off correctly in response to ignition by the spark plug, but one or more pockets of air/fuel mixture explode outside the envelope of the normal combustion front.The...
) or premature ignition (preignition).
When a hydrocarbon is burned, a large number of different oxygen radicals are involved. The first thing to form is a hydroperoxide radical (HOO·), which reacts further into hydroperoxide
Peroxide
A peroxide is a compound containing an oxygen–oxygen single bond or the peroxide anion .The O−O group is called the peroxide group or peroxo group. In contrast to oxide ions, the oxygen atoms in the peroxide ion have an oxidation state of −1.The simplest stable peroxide is hydrogen peroxide...
s that break up into hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
radicals.
Polymerization
In addition to combustion, many polymerizationPolymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
reactions involve free radicals. As a result many plastics, enamels, and other polymers are formed through radical polymerization
Radical polymerization
Free radical polymerization is a method of polymerization by which a polymer forms by the successive addition of free radical building blocks. Free radicals can be formed via a number of different mechanisms usually involving separate initiator molecules...
.
Recent advances in radical polymerization methods, known as living radical polymerization
Living polymerization
In polymer chemistry, living polymerization is a form of addition polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is...
, include:
- Reversible addition-fragmentation chain transfer (RAFTRAFT (chemistry)Reversible Addition-Fragmentation chain Transfer or RAFT polymerization is one kind of controlled radical polymerization. Discovered at the Commonwealth Scientific and Industrial Research Organisation in 1998, RAFT polymerization is a relatively new method for the synthesis of living radical...
) - Atom transfer radical polymerization (ATRPATRP (chemistry)Atom transfer radical polymerization is an example of a living polymerization or a controlled/living radical polymerization . Like its counter part, ATRA or atom transfer radical addition, it is a means of forming carbon-carbon bond through transition metal catalyst...
) - Nitroxide mediated polymerization (NMP)
These methods produce polymers with a much narrower distribution of molecular weights.
Atmospheric radicals
The most common radical in the lower atmosphere is molecular dioxygen. Other free radicals are produced through photodissociationPhotodissociation
Photodissociation, photolysis, or photodecomposition is a chemical reaction in which a chemical compound is broken down by photons. It is defined as the interaction of one or more photons with one target molecule....
of source molecules. In the lower atmosphere, the most important examples of free radical production are the photodissociation of nitrogen dioxide
Nitrogen dioxide
Nitrogen dioxide is the chemical compound with the formula it is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year. This reddish-brown toxic gas has a characteristic sharp, biting odor and is a prominent...
to give an oxygen atom and nitric oxide
Nitric oxide
Nitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry...
, which plays a key role in smog formation—and the photodissociation of ozone to give the excited oxygen atom O(1D). In the upper atmosphere, a particularly important source of radicals is the photodissociation of normally unreactive chlorofluorocarbon
Chlorofluorocarbon
A chlorofluorocarbon is an organic compound that contains carbon, chlorine, and fluorine, produced as a volatile derivative of methane and ethane. A common subclass are the hydrochlorofluorocarbons , which contain hydrogen, as well. They are also commonly known by the DuPont trade name Freon...
s by solar ultraviolet radiation, or by reactions with other stratospheric constituents. These free radicals, among them the hydroxyl radical
Hydroxyl radical
The hydroxyl radical, •OH, is the neutral form of the hydroxide ion . Hydroxyl radicals are highly reactive and consequently short-lived; however, they form an important part of radical chemistry. Most notably hydroxyl radicals are produced from the decomposition of hydroperoxides or, in...
OH· predominates, then react with ozone
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
in a catalytic chain reaction
Chain reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events....
, in which destroys the ozone
Ozone depletion
Ozone depletion describes two distinct but related phenomena observed since the late 1970s: a steady decline of about 4% per decade in the total volume of ozone in Earth's stratosphere , and a much larger springtime decrease in stratospheric ozone over Earth's polar regions. The latter phenomenon...
but regenerates the free radical, allowing it to participate in additional reactions. Such reactions are believed to be the primary cause of depletion of the ozone layer
Ozone layer
The ozone layer is a layer in Earth's atmosphere which contains relatively high concentrations of ozone . This layer absorbs 97–99% of the Sun's high frequency ultraviolet light, which is potentially damaging to the life forms on Earth...
and this is why the use of chlorofluorocarbons as refrigerant
Refrigerant
A refrigerant is a substance used in a heat cycle usually including, for enhanced efficiency, a reversible phase change from a liquid to a gas. Traditionally, fluorocarbons, especially chlorofluorocarbons, were used as refrigerants, but they are being phased out because of their ozone depletion...
s has been restricted.
In biology
Free radicals play an important role in a number of biological processes, some of them necessary for life, such as the intracellular killing of bacteria by phagocytic cells such as granulocyteGranulocyte
Granulocytes are a category of white blood cells characterized by the presence of granules in their cytoplasm. They are also called polymorphonuclear leukocytes because of the varying shapes of the nucleus, which is usually lobed into three segments...
s and macrophage
Macrophage
Macrophages are cells produced by the differentiation of monocytes in tissues. Human macrophages are about in diameter. Monocytes and macrophages are phagocytes. Macrophages function in both non-specific defense as well as help initiate specific defense mechanisms of vertebrate animals...
s. Researchers have also implicated free radicals in certain cell signalling
Signal transduction
Signal transduction occurs when an extracellular signaling molecule activates a cell surface receptor. In turn, this receptor alters intracellular molecules creating a response...
processes. This is dubbed redox signaling
Redox signaling
Redox signaling is when free radicals, reactive oxygen species , and other electronically activated species such as nitric oxide act as biological messengers. Arguably, hydrogen sulfide and carbon monoxide are also redox signaling molecules...
.
The two most important oxygen-centered free radicals are superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
and hydroxyl radical
Hydroxyl radical
The hydroxyl radical, •OH, is the neutral form of the hydroxide ion . Hydroxyl radicals are highly reactive and consequently short-lived; however, they form an important part of radical chemistry. Most notably hydroxyl radicals are produced from the decomposition of hydroperoxides or, in...
. They derive from molecular oxygen under reducing conditions. However, because of their reactivity, these same free radicals can participate in unwanted side reactions resulting in cell damage. Excessive amounts of these free radicals can lead to cell injury and death
Death
Death is the permanent termination of the biological functions that sustain a living organism. Phenomena which commonly bring about death include old age, predation, malnutrition, disease, and accidents or trauma resulting in terminal injury....
, which results in many diseases such as cancer
Cancer
Cancer , known medically as a malignant neoplasm, is a large group of different diseases, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invade nearby parts of the body. The cancer may also spread to more distant parts of the...
, stroke
Stroke
A stroke, previously known medically as a cerebrovascular accident , is the rapidly developing loss of brain function due to disturbance in the blood supply to the brain. This can be due to ischemia caused by blockage , or a hemorrhage...
, myocardial infarction
Myocardial infarction
Myocardial infarction or acute myocardial infarction , commonly known as a heart attack, results from the interruption of blood supply to a part of the heart, causing heart cells to die...
, diabetes and major disorders. Many forms of cancer
Cancer
Cancer , known medically as a malignant neoplasm, is a large group of different diseases, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invade nearby parts of the body. The cancer may also spread to more distant parts of the...
are thought to be the result of reactions between free radicals and DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
, resulting in mutation
Mutation
In molecular biology and genetics, mutations are changes in a genomic sequence: the DNA sequence of a cell's genome or the DNA or RNA sequence of a virus. They can be defined as sudden and spontaneous changes in the cell. Mutations are caused by radiation, viruses, transposons and mutagenic...
s that can adversely affect the cell cycle
Cell cycle
The cell cycle, or cell-division cycle, is the series of events that takes place in a cell leading to its division and duplication . In cells without a nucleus , the cell cycle occurs via a process termed binary fission...
and potentially lead to malignancy. Some of the symptoms of aging
Senescence
Senescence or biological aging is the change in the biology of an organism as it ages after its maturity. Such changes range from those affecting its cells and their function to those affecting the whole organism...
such as atherosclerosis
Atherosclerosis
Atherosclerosis is a condition in which an artery wall thickens as a result of the accumulation of fatty materials such as cholesterol...
are also attributed to free-radical induced oxidation of many of the chemicals making up the body. In addition free radicals contribute to alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
-induced liver
Liver
The liver is a vital organ present in vertebrates and some other animals. It has a wide range of functions, including detoxification, protein synthesis, and production of biochemicals necessary for digestion...
damage, perhaps more than alcohol itself. Radicals in cigarette
Cigarette
A cigarette is a small roll of finely cut tobacco leaves wrapped in a cylinder of thin paper for smoking. The cigarette is ignited at one end and allowed to smoulder; its smoke is inhaled from the other end, which is held in or to the mouth and in some cases a cigarette holder may be used as well...
smoke
Smoke
Smoke is a collection of airborne solid and liquid particulates and gases emitted when a material undergoes combustion or pyrolysis, together with the quantity of air that is entrained or otherwise mixed into the mass. It is commonly an unwanted by-product of fires , but may also be used for pest...
have been implicated in inactivation of alpha 1-antitrypsin
Alpha 1-antitrypsin
Alpha 1-Antitrypsin or α1-antitrypsin is a protease inhibitor belonging to the serpin superfamily. It is generally known as serum trypsin inhibitor. Alpha 1-antitrypsin is also referred to as alpha-1 proteinase inhibitor because it inhibits a wide variety of proteases...
in the lung
Lung
The lung is the essential respiration organ in many air-breathing animals, including most tetrapods, a few fish and a few snails. In mammals and the more complex life forms, the two lungs are located near the backbone on either side of the heart...
. This process promotes the development of emphysema
Emphysema
Emphysema is a long-term, progressive disease of the lungs that primarily causes shortness of breath. In people with emphysema, the tissues necessary to support the physical shape and function of the lungs are destroyed. It is included in a group of diseases called chronic obstructive pulmonary...
.
Free radicals may also be involved in Parkinson's disease
Parkinson's disease
Parkinson's disease is a degenerative disorder of the central nervous system...
, senile and drug-induced deafness, schizophrenia
Schizophrenia
Schizophrenia is a mental disorder characterized by a disintegration of thought processes and of emotional responsiveness. It most commonly manifests itself as auditory hallucinations, paranoid or bizarre delusions, or disorganized speech and thinking, and it is accompanied by significant social...
, and Alzheimer's. The classic free-radical syndrome, the iron-storage disease hemochromatosis, is typically associated with a constellation of free-radical-related symptoms including movement disorder, psychosis, skin pigmentary melanin
Melanin
Melanin is a pigment that is ubiquitous in nature, being found in most organisms . In animals melanin pigments are derivatives of the amino acid tyrosine. The most common form of biological melanin is eumelanin, a brown-black polymer of dihydroxyindole carboxylic acids, and their reduced forms...
abnormalities, deafness, arthritis, and diabetes mellitus. The free radical theory of aging
Free-radical theory
The free-radical theory of aging states that organisms age because cells accumulate free radical damage over time. A free radical is any atom or molecule that has a single unpaired electron in an outer shell. While a few free radicals such as melanin are not chemically reactive, most...
proposes that free radicals underlie the aging process
Senescence
Senescence or biological aging is the change in the biology of an organism as it ages after its maturity. Such changes range from those affecting its cells and their function to those affecting the whole organism...
itself, whereas the process of mitohormesis
Hormesis
Hormesis is the term for generally favorable biological responses to low exposures to toxins and other stressors. A pollutant or toxin showing hormesis thus has the opposite effect in small doses as in large doses...
suggests that repeated exposure to free radicals may extend life span.
Because free radicals are necessary for life, the body has a number of mechanisms to minimize free-radical-induced damage and to repair damage that occurs, such as the enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s superoxide dismutase
Superoxide dismutase
Superoxide dismutases are a class of enzymes that catalyze the dismutation of superoxide into oxygen and hydrogen peroxide. As such, they are an important antioxidant defense in nearly all cells exposed to oxygen...
, catalase
Catalase
Catalase is a common enzyme found in nearly all living organisms that are exposed to oxygen, where it catalyzes the decomposition of hydrogen peroxide to water and oxygen...
, glutathione peroxidase
Glutathione peroxidase
Glutathione peroxidase is the general name of an enzyme family with peroxidase activity whose main biological role is to protect the organism from oxidative damage...
and glutathione reductase
Glutathione reductase
Glutathione reductase, also known as GSR or GR, is an enzyme that reduces glutathione disulfide to the sulfhydryl form GSH, which is an important cellular antioxidant....
. In addition, antioxidant
Antioxidant
An antioxidant is a molecule capable of inhibiting the oxidation of other molecules. Oxidation is a chemical reaction that transfers electrons or hydrogen from a substance to an oxidizing agent. Oxidation reactions can produce free radicals. In turn, these radicals can start chain reactions. When...
s play a key role in these defense mechanisms. These are often the three vitamins, vitamin A
Vitamin A
Vitamin A is a vitamin that is needed by the retina of the eye in the form of a specific metabolite, the light-absorbing molecule retinal, that is necessary for both low-light and color vision...
, vitamin C
Vitamin C
Vitamin C or L-ascorbic acid or L-ascorbate is an essential nutrient for humans and certain other animal species. In living organisms ascorbate acts as an antioxidant by protecting the body against oxidative stress...
and vitamin E
Vitamin E
Vitamin E is used to refer to a group of fat-soluble compounds that include both tocopherols and tocotrienols. There are many different forms of vitamin E, of which γ-tocopherol is the most common in the North American diet. γ-Tocopherol can be found in corn oil, soybean oil, margarine and dressings...
and polyphenol antioxidant
Polyphenol antioxidant
A polyphenol antioxidant is a type of antioxidant containing a polyphenolic substructure. Numbering over 4,000 distinct species, many of these compounds have antioxidant activity in vitro but are unlikely to have antioxidant roles in vivo...
s. Further, there is good evidence bilirubin
Bilirubin
Bilirubin is the yellow breakdown product of normal heme catabolism. Heme is found in hemoglobin, a principal component of red blood cells. Bilirubin is excreted in bile and urine, and elevated levels may indicate certain diseases...
and uric acid
Uric acid
Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the formula C5H4N4O3. It forms ions and salts known as urates and acid urates such as ammonium acid urate. Uric acid is created when the body breaks down purine nucleotides. High blood concentrations of uric acid...
can act as antioxidants to help neutralize certain free radicals. Bilirubin comes from the breakdown of red blood cell
Red blood cell
Red blood cells are the most common type of blood cell and the vertebrate organism's principal means of delivering oxygen to the body tissues via the blood flow through the circulatory system...
s' contents, while uric acid is a breakdown product of purine
Purine
A purine is a heterocyclic aromatic organic compound, consisting of a pyrimidine ring fused to an imidazole ring. Purines, including substituted purines and their tautomers, are the most widely distributed kind of nitrogen-containing heterocycle in nature....
s. Too much bilirubin, though, can lead to jaundice
Jaundice
Jaundice is a yellowish pigmentation of the skin, the conjunctival membranes over the sclerae , and other mucous membranes caused by hyperbilirubinemia . This hyperbilirubinemia subsequently causes increased levels of bilirubin in the extracellular fluid...
, which could eventually damage the central nervous system, while too much uric acid causes gout
Gout
Gout is a medical condition usually characterized by recurrent attacks of acute inflammatory arthritis—a red, tender, hot, swollen joint. The metatarsal-phalangeal joint at the base of the big toe is the most commonly affected . However, it may also present as tophi, kidney stones, or urate...
.
Reactive oxygen species
Reactive oxygen speciesReactive oxygen species
Reactive oxygen species are chemically reactive molecules containing oxygen. Examples include oxygen ions and peroxides. Reactive oxygen species are highly reactive due to the presence of unpaired valence shell electrons....
or ROSs are species such as superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
, hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
, and hydroxyl radical
Hydroxyl radical
The hydroxyl radical, •OH, is the neutral form of the hydroxide ion . Hydroxyl radicals are highly reactive and consequently short-lived; however, they form an important part of radical chemistry. Most notably hydroxyl radicals are produced from the decomposition of hydroperoxides or, in...
and are associated with cell damage. ROSs form as a natural by-product of the normal metabolism of oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and have important roles in cell signaling.
Loose definition of radicals
In most fields of chemistry, the historical definition of radicals contends that the molecules have nonzero spin. However in fields including spectroscopySpectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
, chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
, and astrochemistry
Astrochemistry
Astrochemistry is the study of the abundance and reactions of chemical elements and molecules in the universe, and their interaction with radiation. The discipline is an overlap of astronomy and chemistry. The word "astrochemistry" may be applied to both the Solar System and the interstellar medium...
, the definition is slightly different. Gerhard Herzberg
Gerhard Herzberg
Gerhard Heinrich Friedrich Otto Julius Herzberg, was a pioneering physicist and physical chemist, who won the Nobel Prize for Chemistry in 1971, "for his contributions to the knowledge of electronic structure and geometry of molecules, particularly free radicals". Herzberg's main work concerned...
, who won the Nobel prize for his research into the electron structure and geometry of radicals, suggested a looser definition of free radicals: "any transient (chemically unstable) species (atom, molecule, or ion)". The main point of his suggestion is that there are many chemically unstable molecules that have zero spin, such as C2, C3, CH2 and so on. This definition is more convenient for discussions of transient chemical processes and astrochemistry; therefore researchers in these fields prefer to use this loose definition.
Diagnostics
Free radical diagnostic techniques include:- Electron spin resonance
- A widely used technique for studying free radicals, and other paramagnetic species, is electron spin resonance spectroscopy (ESR). This is alternately referred to as "electron paramagnetic resonanceElectron paramagnetic resonanceElectron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion...
" (EPR) spectroscopy. It is conceptually related to nuclear magnetic resonanceNuclear magnetic resonanceNuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation...
, though electrons resonate with higher-frequency fields at a given fixed magnetic fieldMagnetic fieldA magnetic field is a mathematical description of the magnetic influence of electric currents and magnetic materials. The magnetic field at any given point is specified by both a direction and a magnitude ; as such it is a vector field.Technically, a magnetic field is a pseudo vector;...
than do most nuclei.- Nuclear magnetic resonanceNuclear magnetic resonanceNuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation...
using a phenomenon called CIDNPCIDNPCIDNP , often pronounced like "kidnap", is a non-Boltzmann nuclear spin state distribution produced in thermal or photochemical reactions, usually from colligation and diffusion, or disproportionation of radical pairs, and detected by NMR spectroscopy as enhanced absorption or emission signals... - Chemical labelling
- Nuclear magnetic resonance
- Chemical labelling by quenching with free radicals, e.g. with nitric oxideNitric oxideNitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry...
(NO) or DPPHDPPHDPPH is a common abbreviation for an organic chemical compound 2,2-diphenyl-1-picrylhydrazyl. It is a dark-colored crystalline powder composed of stable free-radical molecules...
(2,2-diphenyl-1-picrylhydrazyl), followed by spectroscopic methods like X-ray photoelectron spectroscopyX-ray photoelectron spectroscopyX-ray photoelectron spectroscopy is a quantitative spectroscopic technique that measures the elemental composition, empirical formula, chemical state and electronic state of the elements that exist within a material...
(XPS) or absorption spectroscopyAbsorption spectroscopyAbsorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a...
, respectively.- Use of free radical markers
- Stable, specific or non-specific derivates of physiological substances can be measured e.g. lipid peroxidation products (isoprostanes, TBARSTBARSTBARS is the abbreviation for Thiobarbituric Acid Reactive Substances, substances formed as a byproduct of lipid peroxidation which can be detected by the TBARS assay using thiobarbituric acid as a reagent....
), amino acidAmino acidAmino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
oxidation products (meta-tyrosineTyrosineTyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
, ortho-tyrosineTyrosineTyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
, hydroxy-Leu, dityrosineTyrosineTyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
etc.), peptide oxidation products (oxidized glutathioneGlutathioneGlutathione is a tripeptide that contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side-chain...
- GSSG)- Indirect method
- Measurement of the decrease in the amount of antioxidants (e.g. TAS, reduced glutathioneGlutathioneGlutathione is a tripeptide that contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side-chain...
- GSH)- Trapping agentsChemical trapIn chemistry, a chemical trap is a chemical compound that is used to detect a certain molecule when* The concentration of this molecule is very small and below detection limit...
- Trapping agents
- Using a chemical species that reacts with free radicals to form a stable product that can then be readily measured (Hydroxyl radical and salicylic acid)
See also
- -yl
- Electron pairElectron pairIn chemistry, an electron pair consists of two electrons that occupy the same orbital but have opposite spins.Because electrons are fermions, the Pauli exclusion principle forbids these particles from having exactly the same quantum numbers. Therefore the only way to occupy the same orbital, i.e....
- Free-radical theoryFree-radical theoryThe free-radical theory of aging states that organisms age because cells accumulate free radical damage over time. A free radical is any atom or molecule that has a single unpaired electron in an outer shell. While a few free radicals such as melanin are not chemically reactive, most...
- Globally Harmonized System of Classification and Labelling of Chemicals
- MitohormesisHormesisHormesis is the term for generally favorable biological responses to low exposures to toxins and other stressors. A pollutant or toxin showing hormesis thus has the opposite effect in small doses as in large doses...
- Oxidative stressOxidative stressOxidative stress represents an imbalance between the production and manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage...
- Reactive oxygen speciesReactive oxygen speciesReactive oxygen species are chemically reactive molecules containing oxygen. Examples include oxygen ions and peroxides. Reactive oxygen species are highly reactive due to the presence of unpaired valence shell electrons....
- Unpaired electronUnpaired electronIn chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. As the formation of electron pairs is often energetically favourable, either in the form of a chemical bond or as a lone pair, unpaired electrons are relatively...
- Hofmann-Löffler reactionHofmann-Löffler reactionThe Hofmann-Löffler reaction is an organic reaction in which a cyclic amine 2 is generated by thermal or photochemical decomposition of N-halogenated amine 1 in the presence of a strong acid...

