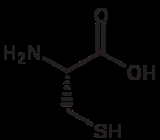
Cysteine
Overview
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
with the chemical formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
HO2CCH(NH2)CH2SH. It is a non-essential amino acid
Essential amino acid
An essential amino acid or indispensable amino acid is an amino acid that cannot be synthesized de novo by the organism , and therefore must be supplied in the diet.-Essentiality vs. conditional essentiality in humans:...
, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
, which is polar and thus cysteine is usually classified as a hydrophilic amino acid. The thiol side chain often participates in enzymatic reactions, serving as a nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
. The thiol is susceptible to oxidization to give the disulfide
Disulfide bond
In chemistry, a disulfide bond is a covalent bond, usually derived by the coupling of two thiol groups. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore R-S-S-R. The terminology is widely used in biochemistry...
derivative cystine
Cystine
Cystine is a dimeric amino acid formed by the oxidation of two cysteine residues that covalently link to make a disulfide bond. This organosulfur compound has the formula 2. It is a white solid, and melts at 247-249 °C...
, which serves an important structural role in many protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s.
Although classified as a non-essential amino acid, in rare cases, cysteine may be essential for infants, the elderly, and individuals with certain metabolic disease or who suffer from malabsorption
Malabsorption
Malabsorption is a state arising from abnormality in absorption of food nutrients across the gastrointestinal tract.Impairment can be of single or multiple nutrients depending on the abnormality...
syndrome
Syndrome
In medicine and psychology, a syndrome is the association of several clinically recognizable features, signs , symptoms , phenomena or characteristics that often occur together, so that the presence of one or more features alerts the physician to the possible presence of the others...
s.

