
Cytochrome
Encyclopedia
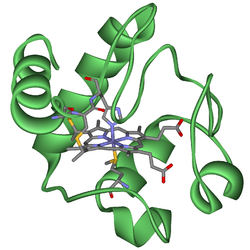
Hemoprotein
A hemeprotein , or heme protein, is a metalloprotein containing a heme prosthetic group- an organic compound that allows a protein to carry out a function that it cannot do alone....
s that contain heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
groups and carry out electron transport.
They are found either as monomeric protein
Protein subunit
In structural biology, a protein subunit or subunit protein is a single protein molecule that assembles with other protein molecules to form a protein complex: a multimeric or oligomeric protein. Many naturally occurring proteins and enzymes are multimeric...
s (e.g., cytochrome c
Cytochrome c
The Cytochrome complex, or cyt c is a small heme protein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins. Cytochrome c is a highly soluble protein, unlike other cytochromes, with a solubility of about 100 g/L and is an...
) or as subunits
Protein subunit
In structural biology, a protein subunit or subunit protein is a single protein molecule that assembles with other protein molecules to form a protein complex: a multimeric or oligomeric protein. Many naturally occurring proteins and enzymes are multimeric...
of bigger enzymatic complexes that catalyze redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
reactions.
History
Cytochromes were initially described in 1884 by MacMunn as respiratory pigments (myohematin or histohematin). In the 1920s, Keilin rediscovered these respiratory pigments and named them the cytochromes, or “cellular pigments”, and classified these heme proteins, on the basis of the position of their lowest energy absorption band in the reduced state, ascytochromes a (605 nm), b (~565 nm), and c (550 nm). The UV-visible spectroscopic signatures of hemes are still used to identify heme type from the reduced bis-pyridine-ligated state, i.e., the pyridine hemochrome method. Within each class, cytochrome a, b, or c, early cytochromes are numbered consecutively, e.g. cyt c, cyt c1, and cyt c2, with more recent examples designated by their reduced state R-band maximum, e.g. cyt c559.
Structure and function
The hemeHeme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
group is a highly-conjugated ring system (which allows its electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s to be very mobile) surrounding a metal ion, which readily interconverts between the oxidation states. For many cytochromes, the metal ion present is that of iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
, which interconverts between Fe2+ (reduced) and Fe3+ (oxidised) states (electron-transfer
Electron transfer
Electron transfer is the process by which an electron moves from an atom or a chemical species to another atom or chemical species...
processes) or between Fe2+ (reduced) and Fe3+ (formal, oxidised) states (oxidative processes). Cytochromes are, thus, capable of performing oxidation and reduction
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
. Because the cytochromes (as well as other complexes) are held within membranes in an organized way, the redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
reactions are carried out in the proper sequence for maximum efficiency.
In the process of oxidative phosphorylation
Oxidative phosphorylation
Oxidative phosphorylation is a metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate . Although the many forms of life on earth use a range of different nutrients, almost all aerobic organisms carry out oxidative phosphorylation to produce ATP,...
, which is the principal energy-generating process undertaken by organisms, which need oxygen to survive, other membrane-bound and -soluble complex
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
es and cofactor
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
s are involved in the chain of redox reactions, with the additional net effect that protons (H+) are transported across the mitochondrial inner membrane. The resulting transmembrane proton gradient ([protonmotive force]) is used to generate ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
, which is the universal chemical energy currency of life. ATP is consumed to drive cellular processes that require energy (such as synthesis of macromolecules, active transport of molecules across the membrane, and assembly of flagella
Flagellum
A flagellum is a tail-like projection that protrudes from the cell body of certain prokaryotic and eukaryotic cells, and plays the dual role of locomotion and sense organ, being sensitive to chemicals and temperatures outside the cell. There are some notable differences between prokaryotic and...
).
Types
Several kinds of cytochrome exist and can be distinguished by spectroscopySpectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
, exact structure of the heme group, inhibitor sensitivity, and reduction potential.
Three types of cytochrome are distinguished by their prosthetic groups:
| Type | prosthetic group >- | Cytochrome a |
heme a Heme a Heme A is a heme, a coordination complex consisting of a macrocyclic ligand called a porphyrin, chelating an iron atom. Heme a is a biomolecule and is produced naturally by many organisms.-Relationship to other hemes:... >- | Cytochrome b Cytochrome b Cytochrome b/b6 is the main subunit of transmembrane cytochrome bc1 and b6f complexes. In addition, it commonly refers to a region of mtDNA used for population genetics and phylogenetics.- Function :... |
heme b Heme b Heme B or haem B is the most abundant heme, both hemoglobin and myoglobin are examples of oxygen transport proteins that contain heme B. The peroxidase family of enzymes also contain heme B... >- | Cytochrome d |
tetrapyrrolic chelate of iron Iron Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust... |
The definition of cytochrome c
Cytochrome c
The Cytochrome complex, or cyt c is a small heme protein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins. Cytochrome c is a highly soluble protein, unlike other cytochromes, with a solubility of about 100 g/L and is an...
is not defined in terms of the heme group. There is no "cytochrome e," but there is a cytochrome f
Cytochrome f
Cytochrome f is the largest subunit of cytochrome b6f complex . In its structure and functions, the cytochrome b6f complex bears extensive analogy to the cytochrome bc1 complex of mitochondria and photosynthetic purple bacteria...
, which is often considered a type of cytochrome c.
In mitochondria
Mitochondrion
In cell biology, a mitochondrion is a membrane-enclosed organelle found in most eukaryotic cells. These organelles range from 0.5 to 1.0 micrometers in diameter...
and chloroplast
Chloroplast
Chloroplasts are organelles found in plant cells and other eukaryotic organisms that conduct photosynthesis. Chloroplasts capture light energy to conserve free energy in the form of ATP and reduce NADP to NADPH through a complex set of processes called photosynthesis.Chloroplasts are green...
s, these cytochromes are often combined in electron transport and related metabolic pathways:
| Cytochromes | Combination >- | a and a3 |
Cytochrome c oxidase Cytochrome c oxidase The enzyme cytochrome c oxidase or Complex IV is a large transmembrane protein complex found in bacteria and the mitochondrion.It is the last enzyme in the respiratory electron transport chain of mitochondria located in the mitochondrial membrane... ("Complex IV") with electrons delivered to complex by soluble cytochrome c Cytochrome c The Cytochrome complex, or cyt c is a small heme protein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins. Cytochrome c is a highly soluble protein, unlike other cytochromes, with a solubility of about 100 g/L and is an... (hence the name) >- | b and c1 Cytochrome C1 Cytochrome C1 is formed in the cytosol and targeted to the mitochondrial intermembrane space. It is one of the constituents of complex III, which forms the third proton pump in the mitochondrial electron transport chain.... |
Coenzyme Q - cytochrome c reductase Coenzyme Q - cytochrome c reductase In enzymology, a ubiquinol—cytochrome-c reductase is an enzyme that catalyzes the chemical reactionThus, the two substrates of this enzyme are dihydroquinone and ferri- cytochrome c, whereas its 3 products are quinone , ferro- cytochrome c, and H+.This enzyme belongs to the family of... ("Complex III") >- | b6 and f Cytochrome f Cytochrome f is the largest subunit of cytochrome b6f complex . In its structure and functions, the cytochrome b6f complex bears extensive analogy to the cytochrome bc1 complex of mitochondria and photosynthetic purple bacteria... |
Plastoquinol—plastocyanin reductase |
A completely distinct family of cytochromes is known as the cytochrome P450 oxidase
Cytochrome P450 oxidase
The cytochrome P450 superfamily is a large and diverse group of enzymes. The function of most CYP enzymes is to catalyze the oxidation of organic substances. The substrates of CYP enzymes include metabolic intermediates such as lipids and steroidal hormones, as well as xenobiotic substances...
s, so named for the characteristic Soret peak
Soret peak
A Soret Peak or Soret Band is an intense peak in the blue wavelength region of the visible spectrum. The peak is named after its discoverer Jacques-Louis Soret. The term is commonly used in absorption spectroscopy, corresponding to a wavelength of maximum absorption...
formed by absorbance of light at wavelengths near 450 nm when the heme iron is reduced (with sodium dithionite
Sodium dithionite
Sodium dithionite is a white crystalline powder with a weak sulfurous odor. It is a sodium salt of dithionous acid. Although it is stable under most conditions, it will decompose in hot water and in acid solutions...
) and complexed to carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
. These enzymes are primarily involved in steroidogenesis and detoxification.

