
Electron transport chain
Encyclopedia
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
transfer between an electron donor
Electron donor
An electron donor is a chemical entity that donates electrons to another compound. It is a reducing agent that, by virtue of its donating electrons, is itself oxidized in the process....
(such as NADH
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide, abbreviated NAD, is a coenzyme found in all living cells. The compound is a dinucleotide, since it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide.In metabolism, NAD is involved...
) and an electron acceptor
Electron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. It is an oxidizing agent that, by virtue of its accepting electrons, is itself reduced in the process....
(such as O2
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
) with the transfer of H+ ions
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
(protons) across a membrane
Cell membrane
The cell membrane or plasma membrane is a biological membrane that separates the interior of all cells from the outside environment. The cell membrane is selectively permeable to ions and organic molecules and controls the movement of substances in and out of cells. It basically protects the cell...
. The resulting electrochemical proton gradient
Electrochemical gradient
An electrochemical gradient is a spatial variation of both electrical potential and chemical concentration across a membrane; that is, a combination of the membrane potential and the pH gradient...
is used to generate chemical energy in the form of adenosine triphosphate
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
(ATP). Electron transport chains are the cellular mechanisms used for extracting energy from sunlight in photosynthesis
Photosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
and also from redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
reactions, such as the oxidation of sugars (respiration
Cellular respiration
Cellular respiration is the set of the metabolic reactions and processes that take place in the cells of organisms to convert biochemical energy from nutrients into adenosine triphosphate , and then release waste products. The reactions involved in respiration are catabolic reactions that involve...
).
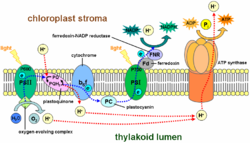
In chloroplast
Chloroplast
Chloroplasts are organelles found in plant cells and other eukaryotic organisms that conduct photosynthesis. Chloroplasts capture light energy to conserve free energy in the form of ATP and reduce NADP to NADPH through a complex set of processes called photosynthesis.Chloroplasts are green...
s, light drives the conversion of water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
to oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and NADP+
Nicotinamide adenine dinucleotide phosphate
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or TPN in older notation , is a coenzyme used in anabolic reactions, such as lipid and nucleic acid synthesis, which require NADPH as a reducing agent....
to NADPH with transfer of H+ ions across chloroplast membranes. In mitochondria, it is the conversion of oxygen to water, NADH to NAD+ and succinate to fumarate that generates a proton. Electron transport chains are major sites of premature electron leakage to oxygen, generating superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
and potentially resulting in increased oxidative stress
Oxidative stress
Oxidative stress represents an imbalance between the production and manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage...
.
Background
The electron transport chain consists of a spatially separated series of redox reactions in which electrons are transferred from a donor molecule to an acceptor molecule. The underlying force driving these reactions is the Gibbs free energyGibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
of the reactants and products. The Gibbs free energy is the energy available ("free") to do work. Any reaction that decreases the overall Gibbs free energy of a system is thermodynamically spontaneous.
The function of the electron transport chain is to produce a trans membrane proton electrochemical gradient
Electrochemical gradient
An electrochemical gradient is a spatial variation of both electrical potential and chemical concentration across a membrane; that is, a combination of the membrane potential and the pH gradient...
as a result of the redox reactions. If protons flow back through the membrane, they enable mechanical work, such as rotating bacterial flagella. ATP synthase
ATP synthase
right|thumb|300px|Molecular model of ATP synthase by X-ray diffraction methodATP synthase is an important enzyme that provides energy for the cell to use through the synthesis of adenosine triphosphate . ATP is the most commonly used "energy currency" of cells from most organisms...
, an enzyme highly conserved
Conserved sequence
In biology, conserved sequences are similar or identical sequences that occur within nucleic acid sequences , protein sequences, protein structures or polymeric carbohydrates across species or within different molecules produced by the same organism...
among all domains of life, converts this mechanical into chemical energy by producing ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
, which powers most cellular reactions.
A small amount of ATP is available from substrate-level phosphorylation
Substrate-level phosphorylation
Substrate-level phosphorylation is a type of metabolism that results in the formation and creation of adenosine triphosphate or guanosine triphosphate by the direct transfer and donation of a phosphoryl group to adenosine diphosphate or guanosine diphosphate from a phosphorylated reactive...
, for example, in glycolysis
Glycolysis
Glycolysis is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+...
. In most organisms the majority of ATP is generated in electron transport chains, while only some obtain ATP by fermentation
Fermentation (biochemistry)
Fermentation is the process of extracting energy from the oxidation of organic compounds, such as carbohydrates, using an endogenous electron acceptor, which is usually an organic compound. In contrast, respiration is where electrons are donated to an exogenous electron acceptor, such as oxygen,...
.
Electron transport chains in mitochondria
Most eukaryotic cells have mitochondria, which produce ATP from products of the Krebs cycle, fatty acid oxidationFatty acid metabolism
Fatty acids are an important source of energy and adenosine triphosphate for many cellular organisms. Excess fatty acids, glucose, and other nutrients can be stored efficiently as fat. Triglycerides yield more than twice as much energy for the same mass as do carbohydrates or proteins. All cell...
, and amino acid oxidation. At the mitochondrial inner membrane, electrons from NADH and succinate pass through the electron transport chain to oxygen, which is reduced to water. The electron transport chain comprises an enzymatic series of electron donors and acceptors. Each electron donor passes electrons to a more electronegative acceptor, which in turn donates these electrons to another acceptor, a process that continues down the series until electrons are passed to oxygen, the most electronegative and terminal electron acceptor in the chain. Passage of electrons between donor and acceptor releases energy, which is used to generate a proton gradient across the mitochondrial membrane by actively “pumping” protons
Proton pump
A proton pump is an integral membrane protein that is capable of moving protons across a cell membrane, mitochondrion, or other organelle. Mechanisms are based on conformational changes of the protein structure or on the Q cycle.-Function:...
into the intermembrane space, producing a thermodynamic state that has the potential to do work. The entire process is called oxidative phosphorylation
Oxidative phosphorylation
Oxidative phosphorylation is a metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate . Although the many forms of life on earth use a range of different nutrients, almost all aerobic organisms carry out oxidative phosphorylation to produce ATP,...
, since ADP is phosphorylated to ATP using the energy of hydrogen oxidation in many steps.
A small percentage of electrons do not complete the whole series and instead directly leak to oxygen, resulting in the formation of the free-radical
Reactive oxygen species
Reactive oxygen species are chemically reactive molecules containing oxygen. Examples include oxygen ions and peroxides. Reactive oxygen species are highly reactive due to the presence of unpaired valence shell electrons....
superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
, a highly reactive molecule that contributes to oxidative stress
Oxidative stress
Oxidative stress represents an imbalance between the production and manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage...
and has been implicated in a number of diseases and aging
Senescence
Senescence or biological aging is the change in the biology of an organism as it ages after its maturity. Such changes range from those affecting its cells and their function to those affecting the whole organism...
.
Mitochondrial redox carriers
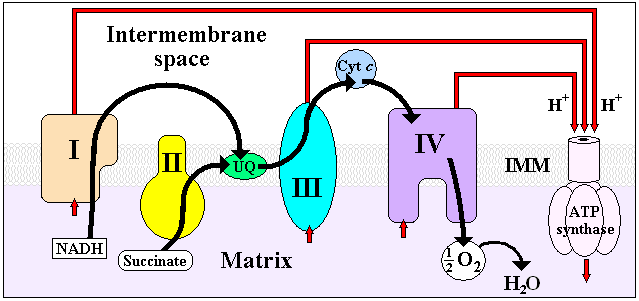
Mitochondrial matrix
In the mitochondrion, the matrix contains soluble enzymes that catalyze the oxidation of pyruvate and other small organic molecules.The mitochondrial matrix also contains the mitochondria's DNA and ribosomes. The word "matrix" stems from the fact that this space is viscous, compared to the...
into the intermembrane space, creating an electrochemical proton gradient across the mitochondrial inner membrane (IMM) called ΔΨ. This electrochemical proton gradient allows ATP synthase (ATP-ase) to use the flow of H+ through the enzyme back into the matrix to generate ATP from adenosine diphosphate
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
(ADP) and inorganic phosphate. Complex I (NADH coenzyme Q reductase; labeled I) accepts electrons from the Krebs cycle electron carrier nicotinamide adenine dinucleotide (NADH), and passes them to coenzyme Q (ubiquinone; labeled UQ), which also receives electrons from complex II (succinate dehydrogenase; labeled II). UQ passes electrons to complex III (cytochrome bc1 complex; labeled III), which passes them to cytochrome c
Cytochrome c
The Cytochrome complex, or cyt c is a small heme protein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins. Cytochrome c is a highly soluble protein, unlike other cytochromes, with a solubility of about 100 g/L and is an...
(cyt c). Cyt c passes electrons to Complex IV (cytochrome c oxidase
Cytochrome c oxidase
The enzyme cytochrome c oxidase or Complex IV is a large transmembrane protein complex found in bacteria and the mitochondrion.It is the last enzyme in the respiratory electron transport chain of mitochondria located in the mitochondrial membrane...
; labeled IV), which uses the electrons and hydrogen ions to reduce molecular oxygen to water.
Four membrane-bound complexes have been identified in mitochondria. Each is an extremely complex transmembrane structure that is embedded in the inner membrane. Three of them are proton pumps. The structures are electrically connected by lipid-soluble electron carriers and water-soluble electron carriers. The overall electron transport chain:
NADH → Complex I → Q → Complex III → cytochrome c → Complex IV → O2
↑
Complex II
↑
FADH
Complex I
In Complex I (NADH dehydrogenaseNADH dehydrogenase
NADH dehydrogenase is an enzyme located in the inner mitochondrial membrane that catalyzes the transfer of electrons from NADH to coenzyme Q...
, also called NADH:ubiquinone oxidoreductase; ) two electrons are removed from NADH and transferred to a lipid-soluble carrier, ubiquinone (Q). The reduced product, ubiquinol
Ubiquinol
Ubiquinol is an electron-rich form of coenzyme Q10.The natural ubiquinol form of coenzyme Q10 is 2,3-dimethoxy-5-methyl-6-poly prenyl-1,4-benzoquinol, where the polyprenylated side chain is 9-10 units long in mammals...
(QH2) freely diffuses within the membrane, and Complex I translocates four protons (H+) across the membrane, thus producing a proton gradient. Complex I is one of the main sites at which premature electron leakage to oxygen occurs, thus being one of the main sites of production of harmful superoxide.
The pathway of electrons occurs as follows:
NADH is oxidized to NAD+, by reducing Flavin mononucleotide
Flavin mononucleotide
Flavin mononucleotide , or riboflavin-5′-phosphate, is a biomolecule produced from riboflavin by the enzyme riboflavin kinase and functions as prosthetic group of various oxidoreductases including NADH dehydrogenase as well as cofactor in biological blue-light photo receptors...
to FMNH2 in one two-electron step. FMNH2 is then oxidized in two one-electron steps, through a semiquinone intermediate. Each electron thus transfers from the FMNH2 to an Fe-S cluster
Iron-sulfur cluster
For biological Fe-S clusters, see iron-sulfur proteins.Iron-sulfur clusters are ensembles of iron and sulfide centres. Fe-S clusters are most often discussed in the context of the biological role for iron-sulfur proteins. Many Fe-S clusters are known in the area of organometallic chemistry and as...
, from the Fe-S cluster to ubiquinone (Q). Transfer of the first electron results in the free-radical (semiquinone) form of Q, and transfer of the second electron reduces the semiquinone form to the ubiquinol form, QH2. During this process, four protons are translocated from the mitochondrial matrix to the intermembrane space.
Complex II
In Complex II (succinate dehydrogenase; ) additional electrons are delivered into the quinone pool (Q) originating from succinate and transferred (via FADFAD
In biochemistry, flavin adenine dinucleotide is a redox cofactor involved in several important reactions in metabolism. FAD can exist in two different redox states, which it converts between by accepting or donating electrons. The molecule consists of a riboflavin moiety bound to the phosphate...
) to Q. Complex II consists of four protein subunits: SDHA
SDHA
Succinate dehydrogenase complex, subunit A, flavoprotein variant is a protein that in humans is encoded by the SDHA gene.The succinate dehydrogenase protein complex catalyzes the oxidation of succinate . The SDHA subunit is connected to the SDHB subunit on the hydrophilic, catalytic end of the...
, SDHB
SDHB
Succinate dehydrogenase [ubiquinone] iron-sulfur subunit, mitochondrial also known as iron-sulfur subunit of complex II is a protein that in humans is encoded by the SDHB gene....
, SDHC, and SDHD
SDHD
Succinate dehydrogenase [ubiquinone] cytochrome b small subunit, mitochondrial , also known as succinate dehydrogenase complex subunit D , is a protein that in humans is encoded by the SDHD gene....
. Other electron donors (e.g., fatty acids and glycerol 3-phosphate) also direct electrons into Q (via FAD).
Complex III
In Complex III (cytochrome bc1 complex; ), the Q-cycleQ cycle
- History :The Q cycle describes a series of reactions first proposed by Peter Mitchell that describe how the sequential oxidation and reduction of the lipophilic electron carrier, ubiquinol-ubiquinone , can result in the net pumping of protons across a lipid bilayer...
contributes to the proton gradient by an asymmetric absorption/release of protons. Two electrons are removed from QH2 at the QO site and sequentially transferred to two molecules of cytochrome c
Cytochrome c
The Cytochrome complex, or cyt c is a small heme protein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins. Cytochrome c is a highly soluble protein, unlike other cytochromes, with a solubility of about 100 g/L and is an...
, a water-soluble electron carrier located within the intermembrane space. The two other electrons sequentially pass across the protein to the Qi site where the quinone part of ubiquinone is reduced to quinol. A proton gradient is formed by two quinol (4H+4e-) oxidations at the Qo site to form one quinol (2H+2e-) at the Qi site. (in total six protons are translocated: two protons reduce quinone to quinol and four protons are released from two ubiquinol molecules).
When electron transfer is reduced (by a high membrane potential or respiratory inhibitors such as antimycin A), Complex III may leak electrons to molecular oxygen, resulting in superoxide formation.
Complex IV
In Complex IV (cytochrome c oxidaseCytochrome c oxidase
The enzyme cytochrome c oxidase or Complex IV is a large transmembrane protein complex found in bacteria and the mitochondrion.It is the last enzyme in the respiratory electron transport chain of mitochondria located in the mitochondrial membrane...
; ), sometimes called cytochrome A3, four electrons are removed from four molecules of cytochrome c
Cytochrome c
The Cytochrome complex, or cyt c is a small heme protein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins. Cytochrome c is a highly soluble protein, unlike other cytochromes, with a solubility of about 100 g/L and is an...
and transferred to molecular oxygen (O2), producing two molecules of water. At the same time, four protons are translocated across the membrane, contributing to the proton gradient. The activity of cytochrome c oxidase is inhibited by cyanide
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
.
Coupling with oxidative phosphorylation
According to the chemiosmotic coupling hypothesisChemiosmosis
Chemiosmosis is the movement of ions across a selectively permeable membrane, down their electrochemical gradient. More specifically, it relates to the generation of ATP by the movement of hydrogen ions across a membrane during cellular respiration....
, proposed by Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
winner Peter D. Mitchell
Peter D. Mitchell
Peter Dennis Mitchell, FRS was a British biochemist who was awarded the 1978 Nobel Prize for Chemistry for his discovery of the chemiosmotic mechanism of ATP synthesis.Mitchell was born in Mitcham, Surrey, England....
, the electron transport chain and oxidative phosphorylation
Oxidative phosphorylation
Oxidative phosphorylation is a metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate . Although the many forms of life on earth use a range of different nutrients, almost all aerobic organisms carry out oxidative phosphorylation to produce ATP,...
are coupled by a proton gradient across the inner mitochondrial membrane. The efflux of protons from the mitochondrial matrix creates an electrochemical gradient
Electrochemical gradient
An electrochemical gradient is a spatial variation of both electrical potential and chemical concentration across a membrane; that is, a combination of the membrane potential and the pH gradient...
(proton gradient). This gradient is used by the FOF1 ATP synthase
ATP synthase
right|thumb|300px|Molecular model of ATP synthase by X-ray diffraction methodATP synthase is an important enzyme that provides energy for the cell to use through the synthesis of adenosine triphosphate . ATP is the most commonly used "energy currency" of cells from most organisms...
complex to make ATP via oxidative phosphorylation. ATP synthase is sometimes described as Complex V of the electron transport chain. The FO component of ATP synthase
ATP synthase
right|thumb|300px|Molecular model of ATP synthase by X-ray diffraction methodATP synthase is an important enzyme that provides energy for the cell to use through the synthesis of adenosine triphosphate . ATP is the most commonly used "energy currency" of cells from most organisms...
acts as an ion channel
Ion channel
Ion channels are pore-forming proteins that help establish and control the small voltage gradient across the plasma membrane of cells by allowing the flow of ions down their electrochemical gradient. They are present in the membranes that surround all biological cells...
that provides for a proton flux back into the mitochondrial matrix. This reflux releases free energy produced during the generation of the oxidized forms of the electron carriers (NAD+ and Q). The free energy is used to drive ATP synthesis, catalyzed by the F1 component of the complex.
Coupling with oxidative phosphorylation is a key step for ATP production. However, in specific cases, uncoupling the two processes may be biologically useful. The uncoupling protein, thermogenin
Thermogenin
Thermogenin is an uncoupling protein found in the mitochondria of brown adipose tissue . It is used to generate heat by non-shivering thermogenesis...
—present in the inner mitochondrial membrane of brown adipose tissue
Brown adipose tissue
Brown adipose tissue or brown fat is one of two types of fat or adipose tissue found in mammals....
—provides for an alternative flow of protons back to the inner mitochondrial matrix. This alternative flow results in thermogenesis
Thermogenesis
Thermogenesis is the process of heat production in organisms. It occurs mostly in warm-blooded animals, but a few species of thermogenic plants exist.-Types:...
rather than ATP production and generates heat. Synthetic uncouplers (e.g., 2,4-dinitrophenol
2,4-Dinitrophenol
2,4-Dinitrophenol , C6H4N2O5, is a cellular metabolic poison. It uncouples oxidative phosphorylation by carrying protons across the mitochondrial membrane, leading to a rapid consumption of energy without generation of ATP....
) also exist, and, at high doses, are lethal.
Summary
In the mitochondrial electron transport chain electrons move from an electron donor (NADH or QH2) to a terminal electron acceptor (O2) via a series of redox reactions. These reactions are coupled to the creation of a proton gradient across the mitochondrial inner membrane. There are three proton pumps: I, III, and IV. The resulting transmembrane proton gradient is used to make ATP via ATP synthase.The reactions catalyzed by Complex I and Complex III work roughly at equilibrium. This means that these reactions are readily reversible, by increasing the concentration of the products relative to the concentration of the reactants (for example, by increasing the proton gradient). ATP synthase is also readily reversible. Thus ATP can be used to build a proton gradient, which in turn can be used to make NADH. This process of reverse electron transport is important in many prokaryotic electron transport chains.
Electron transport chains in bacteria
In eukaryotes, NADH is the most important electron donor. The associated electron transport chain isNADH → Complex I → Q → Complex III → cytochrome c → Complex IV → O2
where Complexes I, III and IV are proton pumps, while Q and cytochrome c are mobile electron carriers. The electron acceptor is molecular oxygen.
In prokaryotes (bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
and archaea
Archaea
The Archaea are a group of single-celled microorganisms. A single individual or species from this domain is called an archaeon...
) the situation is more complicated, because there are several different electron donors and several different electron acceptors. The generalized electron transport chain in bacteria is:
Donor Donor Donor
↓ ↓ ↓
dehydrogenase → quinone → bc1 → cytochrome
↓ ↓
oxidase(reductase) oxidase(reductase)
↓ ↓
Acceptor Acceptor
Note that electrons can enter the chain at three levels: at the level of a dehydrogenase
Dehydrogenase
A dehydrogenase is an enzyme that oxidises a substrate by a reduction reaction that transfers one or more hydrides to an electron acceptor, usually NAD+/NADP+ or a flavin coenzyme such as FAD or FMN.-Examples:...
, at the level of the quinone pool, or at the level of a mobile cytochrome
Cytochrome
Cytochromes are, in general, membrane-bound hemoproteins that contain heme groups and carry out electron transport.They are found either as monomeric proteins or as subunits of bigger enzymatic complexes that catalyze redox reactions....
electron carrier. These levels correspond to successively more positive redox potentials, or to successively decreased potential differences relative to the terminal electron acceptor. In other words, they correspond to successively smaller Gibbs free energy changes for the overall redox reaction Donor → Acceptor.
Individual bacteria use multiple electron transport chains, often simultaneously. Bacteria can use a number of different electron donors, a number of different dehydrogenases, a number of different oxidases and reductases, and a number of different electron acceptors. For example, E. coli (when growing aerobically using glucose as an energy source) uses two different NADH dehydrogenases and two different quinol oxidases, for a total of four different electron transport chains operating simultaneously.
A common feature of all electron transport chains is the presence of a proton pump to create a transmembrane proton gradient. Bacterial electron transport chains may contain as many as three proton pumps, like mitochondria, or they may contain only one or two. They always contain at least one proton pump.
Electron donors
In the present day biosphere, the most common electron donors are organic molecules. Organisms that use organic molecules as an energy source are called organotrophs. Organotrophs (animals, fungi, protists) and phototrophs (plants and algae) constitute the vast majority of all familiar life forms.Some prokaryotes can use inorganic matter as an energy source. Such organisms are called lithotrophs ("rock-eaters"). Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. Lithotrophs have been found growing in rock formations thousands of meters below the surface of Earth. Because of their volume of distribution, lithotrophs may actually outnumber organotrophs and phototrophs in our biosphere.
The use of inorganic electron donors as an energy source is of particular interest in the study of evolution. This type of metabolism must logically have preceded the use of organic molecules as an energy source.
Dehydrogenases
Bacteria can use a number of different electron donors. When organic matter is the energy source, the donor may be NADH or succinate, in which case electrons enter the electron transport chain via NADH dehydrogenase (similar to Complex I in mitochondria) or succinate dehydrogenase (similar to Complex II). Other dehydrogenases may be used to process different energy sources: formate dehydrogenase, lactate dehydrogenase, glyceraldehyde-3-phosphate dehydrogenase, H2 dehydrogenase (hydrogenaseHydrogenase
A hydrogenase is an enzyme that catalyses the reversible oxidation of molecular hydrogen . Hydrogenases play a vital role in anaerobic metabolism....
), etc. Some dehydrogenases are also proton pumps; others simply funnel electrons into the quinone pool.
Most of dehydrogenases are synthesized only when needed. Depending on the environment in which they find themselves, bacteria select different enzymes from their DNA library and synthesize only those that are needed for growth.Enzymes that are synthesized only when needed are said to be 'inducible'.
Quinone carriers
Quinones are mobile, lipid-soluble carriers that shuttle electrons (and protons) between large, relatively immobile macromolecular complexes embedded in the membrane. Bacteria use ubiquinone (the same quinone that mitochondria use) and related quinones such as menaquinone.Proton pumps
A proton pump is any process that creates a proton gradient across a membrane. Protons can be physically moved across a membrane; this is seen in mitochondrial Complexes I and IV. The same effect can be produced by moving electrons in the opposite direction. The result is the disappearance of a proton from the cytoplasm and the appearance of a proton in the periplasm. Mitochondrial Complex III uses this second type of proton pump, which is mediated by a quinone (the Q cycleQ cycle
- History :The Q cycle describes a series of reactions first proposed by Peter Mitchell that describe how the sequential oxidation and reduction of the lipophilic electron carrier, ubiquinol-ubiquinone , can result in the net pumping of protons across a lipid bilayer...
).
Some dehydrogenases are proton pumps; others are not. Most oxidases and reductases are proton pumps, but some are not. Cytochrome bc1 is a proton pump found in many, but not all, bacteria (it is not found in E. coli). As the name implies, bacterial bc1 is similar to mitochondrial bc1 (Complex III).
Proton pumps are the heart of the electron transport process. They produce the transmembrane electrochemical gradient that supplies energy to the cell.
Cytochrome electron carriers
Cytochromes are pigments that contain iron. They are found in two very different environments.Some cytochromes are water-soluble carriers that shuttle electrons to and from large, immobile macromolecular structures imbedded in the membrane. The mobile cytochrome electron carrier in mitochondria is cytochrome c. Bacteria use a number of different mobile cytochrome electron carriers.
Other cytochromes are found within macromolecules such as Complex III and Complex IV. They also function as electron carriers, but in a very different, intramolecular, solid-state environment.
Electrons may enter an electron transport chain at the level of a mobile cytochrome or quinone carrier. For example, electrons from inorganic electron donors (nitrite, ferrous iron, etc.) enter the electron transport chain at the cytochrome level. When electrons enter at a redox level greater than NADH, the electron transport chain must operate in reverse to produce this necessary, higher-energy molecule.
Terminal oxidases and reductases
When bacteria grow in aerobic environments, the terminal electron acceptor (O2) is reduced to water by an enzyme called an oxidase. When bacteria grow in anaerobicHypoxia (environmental)
Hypoxia, or oxygen depletion, is a phenomenon that occurs in aquatic environments as dissolved oxygen becomes reduced in concentration to a point where it becomes detrimental to aquatic organisms living in the system...
environments, the terminal electron acceptor is reduced by an enzyme called a reductase.
In mitochondria the terminal membrane complex (Complex IV) is cytochrome oxidase. Aerobic bacteria use a number of different terminal oxidases. For example, E. coli does not have a cytochrome oxidase or a bc1 complex. Under aerobic conditions, it uses two different terminal quinol oxidases (both proton pumps) to reduce oxygen to water.
Anaerobic
Anaerobic organism
An anaerobic organism or anaerobe is any organism that does not require oxygen for growth. It could possibly react negatively and may even die if oxygen is present...
bacteria, which do not use oxygen as a terminal electron acceptor, have terminal reductases individualized to their terminal acceptor. For example, E. coli can use fumarate reductase, nitrate reductase, nitrite reductase, DMSO reductase, or trimethylamine-N-oxide reductase, depending on the availability of these acceptors in the environment.
Most terminal oxidases and reductases are inducible. They are synthesized by the organism as needed, in response to specific environmental conditions.
Electron acceptors
Just as there are a number of different electron donors (organic matter in organotrophs, inorganic matter in lithotrophs), there are a number of different electron acceptors, both organic and inorganic. If oxygen is available, it is invariably used as the terminal electron acceptor, because it generates the greatest Gibbs free energy change and produces the most energy.In anaerobic environments, different electron acceptors are used, including nitrate, nitrite, ferric iron, sulfate, carbon dioxide, and small organic molecules such as fumarate.
Since electron transport chains are redox processes, they can be described as the sum of two redox pairs. For example, the mitochondrial electron transport chain can be described as the sum of the NAD+/NADH redox pair and the O2/H2O redox pair. NADH is the electron donor and O2 is the electron acceptor.
Not every donor-acceptor combination is thermodynamically possible. The redox potential of the acceptor must be more positive than the redox potential of the donor. Furthermore, actual environmental conditions may be far different from standard conditions (1 molar concentrations, 1 atm partial pressures, pH = 7), which apply to standard redox potentials. For example, hydrogen-evolving bacteria grow at an ambient partial pressure of hydrogen gas of 10-4 atm. The associated redox reaction, which is thermodynamically favorable in nature, is thermodynamically impossible under “standard” conditions.
Summary
Bacterial electron transport pathways are, in general, inducible. Depending on their environment, bacteria can synthesize different transmembrane complexes and produce different electron transport chains in their cell membranes. Bacteria select their electron transport chains from a DNA library containing multiple possible dehydrogenases, terminal oxidases and terminal reductases. The situation is often summarized by saying that electron transport chains in bacteria are branched, modular, and inducible.Photosynthetic electron transport chains
In oxidative phosphorylationOxidative phosphorylation
Oxidative phosphorylation is a metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate . Although the many forms of life on earth use a range of different nutrients, almost all aerobic organisms carry out oxidative phosphorylation to produce ATP,...
, electrons are transferred from a high-energy electron donor (e.g., NADH) to an electron acceptor (e.g., O2) through an electron transport chain. In photophosphorylation
Photophosphorylation
The production of ATP using the energy of sunlight is called photophosphorylation. Only two sources of energy are available to living organisms: sunlight and reduction-oxidation reactions...
, the energy of sunlight is used to create a high-energy electron donor and an electron acceptor. Electrons are then transferred from the donor to the acceptor through another electron transport chain.
Photosynthetic electron transport chains have many similarities to the oxidative chains discussed above. They use mobile, lipid-soluble carriers (quinones) and mobile, water-soluble carriers (cytochromes, etc.). They also contain a proton pump. It is remarkable that the proton pump in all photosynthetic chains resembles mitochondrial Complex III.
Photosynthetic electron transport chains are discussed in greater detail in the articles Photophosphorylation
Photophosphorylation
The production of ATP using the energy of sunlight is called photophosphorylation. Only two sources of energy are available to living organisms: sunlight and reduction-oxidation reactions...
, Photosynthesis
Photosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
, Photosynthetic reaction center and Light-dependent reaction.
Summary
Electron transport chains are redox reactions that transfer electrons from an electron donor to an electron acceptor. The transfer of electrons is coupled to the translocation of protons across a membrane, producing a proton gradient. The proton gradient is used to produce useful work.The coupling of thermodynamically favorable to thermodynamically unfavorable biochemical reactions by biological macromolecules is an example of an emergent property – a property that could not have been predicted, even given full knowledge of the primitive geochemical systems from which these macromolecules evolved. It is an open question whether such emergent properties evolve only by chance, or whether they necessarily evolve in any large biogeochemical system, given the underlying laws of physics.
External links
- Khan Academy, video lecture - Complexes with cytochrome b-like domains - Bacterial and mitochondrial cytochrome c oxidases - Photosynthetic reaction centers and photosystems - Cytochrome c family - Cupredoxins - Adrenodoxin reductase - Electron transfer flavoproteins

