
Water (properties)
Encyclopedia
Water
is the most abundant compound on Earth's surface, covering about 70%. In nature, it exists in liquid, solid, and gaseous states. It is in dynamic equilibrium
between the liquid
and gas
states at standard temperature and pressure. At room temperature
, it is a taste
less and odor
less liquid, nearly colorless
with a hint of blue
. Many substances dissolve in water and it is commonly referred to as the universal solvent
. Because of this, water in nature and in use is rarely pure and some of its properties may vary slightly from those of the pure substance. However, there are also many compounds that are essentially, if not completely, insoluble in water. Water is the only common substance found naturally in all three common states of matter and it is essential for all life on Earth. Water usually makes up 55% to 78% of the human body.
can take numerous forms that are broadly categorized by phase of matter
. The liquid phase is the most common among water's phases (within the Earth's atmosphere and surface) and is the form that is generally denoted by the word "water." The solid phase
of water is known as ice
and commonly takes the structure of hard, amalgamated crystals, such as ice cubes, or loosely accumulated granular
crystals, like snow
. For a list of the many different crystalline and amorphous
forms of solid H2O, see the article ice
. The gaseous phase of water is known as water vapor
(or steam
), and is characterized by water assuming the configuration of a transparent cloud
. (Note that the visible steam and clouds are, in fact, water in the liquid form as minute droplets suspended in the air.) The fourth state of water, that of a supercritical fluid
, is much less common than the other three and only rarely occurs in nature, in extremely uninhabitable conditions. When water achieves a specific critical temperature and a specific critical pressure (647 K
and 22.064 MPa
), liquid and gas phase merge to one homogeneous fluid phase, with properties of both gas and liquid. One example of naturally occurring supercritical water is found in the hottest parts of deep water hydrothermal vents, in which water is heated to the critical temperature by scalding volcanic
plumes
and achieves the critical pressure because of the crushing weight of the ocean at the extreme depths at which the vents are located. Additionally, anywhere there is volcanic activity below a depth of 2.25 km (1.4 mi) can be expected to have water in the supercritical phase.
Vienna Standard Mean Ocean Water is the current international standard for water isotopes. Naturally occurring water is almost completely composed of the neutron-less hydrogen isotope protium. Only 155 ppm
include deuterium
( or D), a hydrogen isotope with one neutron, and less than 20 parts per quintillion include tritium
( or T), which has two.
Heavy water
is water with a higher-than-average deuterium content, up to 100%. Chemically, it is similar but not identical to normal water. This is because the nucleus of deuterium is twice as heavy as protium, and this causes noticeable differences in bonding energies. Because water molecules exchange hydrogen atoms with one another, hydrogen deuterium oxide (DOH) is much more common in low-purity heavy water than pure dideuterium monoxide (D2O). Humans are generally unaware of taste differences, but sometimes report a burning sensation or sweet flavor. Rats, however, are able to avoid heavy water by smell. Toxic to many animals, heavy water is used in the nuclear reactor
industry to moderate
(slow down) neutron
s. Light water reactors are also common, where "light" simply designates normal water.
Light water
more specifically refers to deuterium-depleted water (DDW), water in which the deuterium content has been reduced below the standard 155ppm level. Light water has been found to be beneficial for improving cancer survival rates in mice and humans undergoing chemotherapy.
with chemical formula
: one molecule
of water has two hydrogen atom
s covalently bonded
to a single oxygen
atom.
Water is a tasteless, odorless liquid at ambient temperature and pressure, and appears colorless in small quantities, although it has its own intrinsic very light blue hue. Ice also appears colorless, and water vapor is essentially invisible as a gas.
Water is primarily a liquid under standard conditions, which is not predicted from its relationship to other analogous hydrides of the oxygen family
in the periodic table
, which are gases such as hydrogen sulfide
. The elements surrounding oxygen in the periodic table
, nitrogen
, fluorine
, phosphorus
, sulfur
and chlorine
, all combine with hydrogen to produce gases under standard conditions. The reason that water forms a liquid is that oxygen is more electronegative than all of these elements with the exception of fluorine. Oxygen attracts electrons much more strongly than hydrogen, resulting in a net positive charge on the hydrogen atoms, and a net negative charge on the oxygen atom. The presence of a charge on each of these atoms gives each water molecule a net dipole moment. Electrical attraction between water molecules due to this dipole pulls individual molecules closer together, making it more difficult to separate the molecules and therefore raising the boiling point. This attraction is known as hydrogen bonding. The molecules of water are constantly moving in relation to each other, and the hydrogen bonds are continually breaking and reforming at timescales faster than 200 femtoseconds. However, this bond is sufficiently strong to create many of the peculiar properties of water, such as those that make it integral to life. Water can be described as a polar liquid that slightly dissociates disproportionately into the hydronium
ion ((aq)) and an associated hydroxide
ion ((aq)).
The dissociation constant
for this dissociation is commonly symbolized as Kw and has a value of about 10−14 at 25 °C; see "Water (data page)
" and "Self-ionization of water
" for more information.
, as well as a high heat of vaporization (40.65 kJ/mol or 2257 kJ/kg at the normal boiling point), both of which are a result of the extensive hydrogen bond
ing between its molecules. These two unusual properties allow water to moderate Earth's climate
by buffering large fluctuations in temperature. According to Josh Willis, of NASA
's Jet Propulsion Laboratory
, the oceans absorb one thousand times more heat than the atmosphere (air) and are holding 80 to 90% of global warming
heat.
The specific enthalpy of fusion
of water is 333.55 kJ/kg at 0 °C. Of common substances, only that of ammonia is higher. This property confers resistance to melting on the ice of glacier
s and drift ice
. Before and since the advent of mechanical refrigeration
, ice was and still is in common use for retarding food spoilage.
Note that the specific heat capacity of ice at −10 °C is about 2.05 J/(g·K) and that the heat capacity of steam at 100 °C is about 2.080 J/(g·K).
The density of water is approximately one gram per cubic centimeter. More precisely, it is dependent on its temperature, but the relation is not linear and is unimodal rather than monotonic
(see right-hand table). When cooled from room temperature
liquid water becomes increasingly dense, just like other substances. But at approximately 4 °C, pure water reaches its maximum density. As it is cooled further, it expands to become less dense. This unusual negative thermal expansion is attributed to strong, orientation-dependent, intermolecular interactions and is also observed in molten silica.
The solid form of most substances is denser
than the liquid phase
; thus, a block of most solids will sink in the liquid. However, a block of ice floats in liquid water because ice is less dense. Upon freezing, the density of water decreases by about 9%. The reason for this is the 'cooling' of intermolecular vibrations allowing the molecules to form steady hydrogen bonds with their neighbors and thereby gradually locking into positions reminiscent of the hexagonal packing achieved upon freezing to ice Ih
. Whereas the hydrogen bonds are shorter in the crystal than in the liquid, this locking effect reduces the average coordination number of molecules as the liquid approaches nucleation. Other substances that expand on freezing are silicon
, gallium
, germanium
, antimony
, bismuth
, plutonium
and other compounds that form spacious crystal lattices with tetrahedral coordination.
Only ordinary hexagonal ice is less dense than the liquid. Under increasing pressure, ice undergoes a number of transitions to other allotropic forms
with higher density than liquid water, such as high density amorphous ice (HDA) and very high density amorphous ice (VHDA).
Water also expands significantly as the temperature increases. Its density decreases by 4% from its highest value when approaching its boiling point.
The melting point of ice is 0 °C (32 °F, 273 K) at standard pressure, however, pure liquid water can be supercooled well below that temperature without freezing if the liquid is not mechanically disturbed. It can remain in a fluid state down to its homogeneous nucleation
point of approximately 231 K (−42 °C). The melting point of ordinary hexagonal ice falls slightly under moderately high pressures, but as ice transforms into its allotropes (see crystalline states of ice) above 209.9 MPa (2,071.6 atm), the melting point increases markedly with pressure, i.e., reaching 355 kelvins (81.9 °C) at 2.216 GPa (21,870.2 atm) (triple point of Ice VII
).
A significant increase of pressure is required to lower the melting point of ordinary ice—the pressure exerted by an ice skater on the ice only reduces the melting point by approximately 0.09 °C (0.16 °F).
These properties of water have important consequences in its role in the ecosystem
of Earth. Water at a temperature of 4 °C will always accumulate at the bottom of fresh water lakes, irrespective of the temperature in the atmosphere. Since water and ice are poor conductors of heat (good insulators) it is unlikely that sufficiently deep lakes will freeze completely, unless stirred by strong currents that mix cooler and warmer water and accelerate the cooling. In warming weather, chunks of ice float, rather than sink to the bottom where they might melt extremely slowly. These phenomena thus may help to preserve aquatic life.
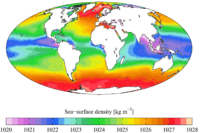 The density of water is dependent on the dissolved salt content as well as the temperature of the water. Ice still floats in the oceans, otherwise they would freeze from the bottom up. However, the salt content of oceans lowers the freezing point by about 2 °C (see following paragraph for explanation) and lowers the temperature of the density maximum of water to the freezing point. This is why, in ocean water, the downward convection of colder water is not blocked by an expansion of water as it becomes colder near the freezing point. The oceans' cold water near the freezing point continues to sink. For this reason, any creature attempting to survive at the bottom of such cold water as the Arctic Ocean
The density of water is dependent on the dissolved salt content as well as the temperature of the water. Ice still floats in the oceans, otherwise they would freeze from the bottom up. However, the salt content of oceans lowers the freezing point by about 2 °C (see following paragraph for explanation) and lowers the temperature of the density maximum of water to the freezing point. This is why, in ocean water, the downward convection of colder water is not blocked by an expansion of water as it becomes colder near the freezing point. The oceans' cold water near the freezing point continues to sink. For this reason, any creature attempting to survive at the bottom of such cold water as the Arctic Ocean
generally lives in water that is 4 °C colder than the temperature at the bottom of frozen-over fresh water
lakes and rivers in the winter.
In cold countries, when the temperature of fresh water reaches 4 °C, the layers of water near the top in contact with cold air continue to lose heat energy and their temperature falls below 4 °C. On cooling below 4 °C, these layers do not sink but may rise up as fresh water has a maximum density at 4 °C. (Refer: Polarity and hydrogen bonding) Due to this, the layer of water at 4 °C remains at the bottom and above this layers of water 3 °C, 2 °C, 1 °C and 0 °C are formed. Since ice is a poor conductor of heat, it does not absorb heat energy from the water beneath the layer of ice which prevents the water freezing. Thus, aquatic creatures survive in such places.
As the surface
of salt water begins to freeze (at −1.9°C for normal salinity seawater
, 3.5%) the ice that forms is essentially salt free with a density approximately equal to that of freshwater ice. This ice floats on the surface and the salt that is "frozen out" adds to the salinity
and density of the seawater just below it, in a process known as brine
rejection. This denser saltwater sinks by convection and the replacing seawater is subject to the same process. This provides essentially freshwater ice at −1.9°C on the surface. The increased density of the seawater beneath the forming ice causes it to sink towards the bottom. On a large scale, the process of brine rejection and sinking cold salty water results in ocean currents forming to transport such water away from the Poles, leading to a global system of currents called the thermohaline circulation
. One potential consequence of global warming
is that the loss of Arctic and Antarctic ice could result in the loss of these currents as well, which could have unforeseeable consequences on near and distant climates.
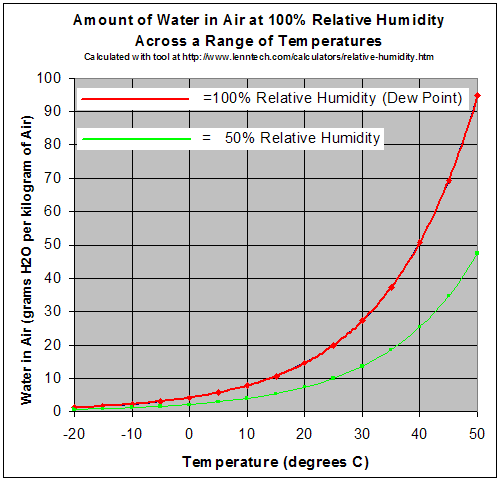
Water is miscible with many liquids, for example ethanol
in all proportions, forming a single homogeneous liquid. On the other hand, water and most oil
s are immiscible usually forming layers according to increasing density from the top.
As a gas, water vapor is completely miscible with air. On the other hand the maximum water vapor pressure that is thermodynamically stable with the liquid (or solid) at a given temperature is relatively low compared with total atmospheric pressure.
For example, if the vapor partial pressure
is 2% of atmospheric pressure and the air is cooled from 25 °C, starting at about 22 °C water will start to condense, defining the dew point
, and creating fog
or dew
. The reverse process accounts for the fog burning off in the morning.
If the humidity is increased at room temperature, for example, by running a hot shower or a bath, and the temperature stays about the same, the vapor soon reaches the pressure for phase change, and then condenses out as minute water droplets, commonly referred to as steam.
A gas in this context is referred to as saturated or 100% relative humidity, when the vapor pressure of water in the air is at the equilibrium with vapor pressure due to (liquid) water; water (or ice, if cool enough) will fail to lose mass through evaporation when exposed to saturated air. Because the amount of water vapor in air is small, relative humidity, the ratio of the partial pressure due to the water vapor to the saturated partial vapor pressure, is much more useful.
Water vapor pressure above 100% relative humidity is called super-saturated and can occur if air is rapidly cooled, for example, by rising suddenly in an updraft.
The bulk modulus
of water is 2.2 GPa. The low compressibility of non-gases, and of water in particular, leads to their often being assumed as incompressible. The low compressibility of water means that even in the deep ocean
s at 4 km depth, where pressures are 40 MPa, there is only a 1.8% decrease in volume.
The temperature
and pressure
at which solid, liquid, and gaseous water
coexist in equilibrium is called the triple point
of water. This point is used to define the units of temperature (the kelvin
, the SI unit of thermodynamic temperature and, indirectly, the degree Celsius
and even the degree Fahrenheit
).
As a consequence, water's triple point temperature is a prescribed value rather than a measured quantity.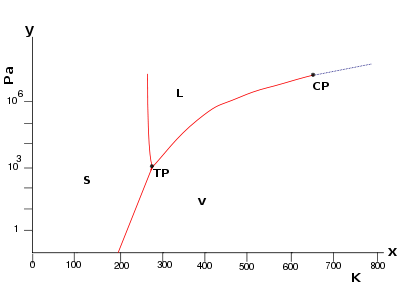 The triple point is at a temperature of 273.16 K (0.01 °C) by convention, and at a pressure of 611.73 Pa
The triple point is at a temperature of 273.16 K (0.01 °C) by convention, and at a pressure of 611.73 Pa
. This pressure is quite low, about of the normal sea level barometric pressure of 101,325 Pa. The atmospheric surface pressure on planet Mars
is remarkably close to the triple point pressure, and the zero-elevation or "sea level" of Mars is defined by the height at which the atmospheric pressure corresponds to the triple point of water.
Although it is commonly named as "the triple point of water", the stable combination of liquid water, ice I, and water vapor is but one of several triple points on the phase diagram
of water. Gustav Heinrich Johann Apollon Tammann in Göttingen produced data on several other triple points in the early 20th century. Kamb and others documented further triple points in the 1960s.
, but not even "deionized" water is completely free of ions. Water undergoes auto-ionization
in the liquid state. Further, because water is such a good solvent, it almost always has some solute
dissolved in it, most frequently a salt. If water has even a tiny amount of such an impurity, then it can conduct electricity readily, as impurities such as salt separate into free ion
s in aqueous solution by which an electric current can flow.
It is known that the theoretical maximum electrical resistivity for water is approximately 182 kΩ·m at 25 °C. This figure agrees well with what is typically seen on reverse osmosis
, ultra-filtered
and deionized ultra-pure water systems used, for instance, in semiconductor manufacturing plants. A salt or acid contaminant level exceeding even 100 parts per trillion (ppt) in otherwise ultra-pure water begins to noticeably lower its resistivity by up to several kΩ·m.
The electrical conductivity of water increases significantly upon solvation of a small amount of ionic material, such as hydrogen chloride
or any salt.
Any electrical conductivity observable in water is the result of ion
s of mineral salts and carbon dioxide
dissolved in it. Carbon dioxide forms carbonate
ions in water. Water self-ionizes
, when two water molecules form one hydroxide
anion (OH−) and one hydronium
cation , but not enough to carry sufficient electric current
to do any work or harm for most operations. In pure water, sensitive equipment can detect a very slight electrical conductivity of 0.055 µS
/cm at 25 °C. Water can also be electrolyzed
into oxygen and hydrogen gases but in the absence of dissolved ions this is a very slow process, as very little current is conducted. While electrons are the primary charge carriers in water (and metals), in ice the primary charge carriers are protons (see proton conductor
).
. Water molecules naturally dissociate into and ions, which are attracted toward the cathode
and anode
, respectively. At the cathode, two ions pick up electrons and form gas. At the anode, four ions combine and release gas, molecular water, and four electrons. The gases produced bubble to the surface, where they can be collected. The standard potential of the water electrolysis cell is 1.23 V at 25 °C.
than hydrogen, the side of the molecule with the oxygen atom has a partial negative charge. An object with such a charge difference is called a dipole
meaning two poles. The oxygen end is partially negative and the hydrogen end is partially positive, because of this the direction of the dipole moment
points towards the oxygen. The charge differences cause water molecules to be attracted to each other (the relatively positive areas being attracted to the relatively negative areas) and to other polar molecules. This attraction contributes to hydrogen bond
ing, and explains many of the properties of water, such as solvent action.
A water molecule can form a maximum of four hydrogen bond
s because it can accept two and donate two hydrogen atoms. Other molecules like hydrogen fluoride
, ammonia
, methanol
form hydrogen bonds but they do not show anomalous behavior of thermodynamic
, kinetic
or structural properties like those observed in water. The answer to the apparent difference between water and other hydrogen bonding liquids lies in the fact that apart from water none of the hydrogen bonding molecules can form four hydrogen bonds, either due to an inability to donate/accept hydrogens or due to steric effects in bulky residues. In water, local tetrahedral order due to the four hydrogen bonds gives rise to an open structure and a 3-dimensional bonding network, resulting in the anomalous decrease of density when cooled below 4 °C.
Although hydrogen bonding is a relatively weak attraction compared to the covalent bonds within the water molecule itself, it is responsible for a number of water's physical properties. One such property is its relatively high melting
and boiling point
temperatures; more energy is required to break the hydrogen bonds between molecules. The similar compound hydrogen sulfide , which has much weaker hydrogen bonding, is a gas at room temperature
even though it has twice the molecular mass of water. The extra bonding between water molecules also gives liquid water a large specific heat capacity. This high heat capacity makes water a good heat storage medium (coolant) and heat shield.
 Water molecules stay close to each other (cohesion
Water molecules stay close to each other (cohesion
), due to the collective action of hydrogen bonds between water molecules. These hydrogen bonds are constantly breaking, with new bonds being formed with different water molecules; but at any given time in a sample of liquid water, a large portion of the molecules are held together by such bonds.
Water also has high adhesion
properties because of its polar nature. On extremely clean/smooth glass
the water may form a thin film because the molecular forces between glass and water molecules (adhesive forces) are stronger than the cohesive forces.
In biological cells and organelle
s, water is in contact with membrane and protein surfaces that are hydrophilic; that is, surfaces that have a strong attraction to water. Irving Langmuir
observed a strong repulsive force between hydrophilic surfaces. To dehydrate hydrophilic surfaces—to remove the strongly held layers of water of hydration—requires doing substantial work against these forces, called hydration forces. These forces are very large but decrease rapidly over a nanometer or less. They are important in biology, particularly when cells are dehydrated by exposure to dry atmospheres or to extracellular freezing.
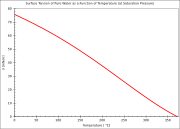 Water has a high surface tension
Water has a high surface tension
of 72.8 mN/m at room temperature
, caused by the strong cohesion between water molecules, the highest of the non-metallic liquids. This can be seen when small quantities of water are placed onto a sorption
-free (non-adsorbent and non-absorbent) surface, such as polyethylene
or Teflon, and the water stays together as drops. Just as significantly, air trapped in surface disturbances forms bubbles, which sometimes last long enough to transfer gas molecules to the water.
Another surface tension effect is capillary wave
s, which are the surface ripples that form around the impacts of drops on water surfaces, and sometimes occur with strong subsurface currents flowing to the water surface. The apparent elasticity caused by surface tension drives the waves.
whereby water rises into a narrow tube against the force of gravity. Water adheres to the inside wall of the tube and surface tension tends to straighten the surface causing a surface rise and more water is pulled up through cohesion. The process continues as the water flows up the tube until there is enough water such that gravity balances the adhesive force.
Surface tension and capillary action are important in biology. For example, when water is carried through xylem
up stems in plants, the strong intermolecular attractions (cohesion) hold the water column together and adhesive properties maintain the water attachment to the xylem and prevent tension rupture caused by transpiration pull.
 Water is also a good solvent
Water is also a good solvent
due to its polarity. Substances that will mix well and dissolve in water (e.g. salts) are known as hydrophilic ("water-loving") substances, while those that do not mix well with water (e.g. fats and oils), are known as hydrophobic ("water-fearing") substances. The ability of a substance to dissolve in water is determined by whether or not the substance can match or better the strong attractive forces that water molecules generate between other water molecules. If a substance has properties that do not allow it to overcome these strong intermolecular forces, the molecules are "pushed out
" from the water, and do not dissolve. Contrary to the common misconception, water and hydrophobic substances do not "repel", and the hydration of a hydrophobic surface is energetically, but not entropically, favorable.
When an ionic or polar compound enters water, it is surrounded by water molecules (Hydration
). The relatively small size of water molecules typically allows many water molecules to surround one molecule of solute
. The partially negative dipole ends of the water are attracted to positively charged components of the solute, and vice versa for the positive dipole ends.
In general, ionic and polar substances such as acid
s, alcohol
s, and salt
s are relatively soluble in water, and non-polar substances such as fats and oils are not. Non-polar molecules stay together in water because it is energetically more favorable for the water molecules to hydrogen bond to each other than to engage in van der Waals interactions
with non-polar molecules.
An example of an ionic solute is table salt
; the sodium chloride, NaCl, separates into cations and anions, each being surrounded by water molecules. The ions are then easily transported away from their crystalline lattice into solution. An example of a nonionic solute is table sugar
. The water dipoles make hydrogen bonds with the polar regions of the sugar molecule (OH groups) and allow it to be carried away into solution.
or a base
in chemical reactions. According to the Brønsted-Lowry
definition, an acid is defined as a species which donates a proton (a ion) in a reaction, and a base as one which receives a proton. When reacting with a stronger acid, water acts as a base; when reacting with a stronger base, it acts as an acid. For instance, water receives an ion from HCl when hydrochloric acid
is formed:
In the reaction with ammonia
, , water donates a ion, and is thus acting as an acid:
Because the oxygen atom in water has two lone pair
s, water often acts as a Lewis base, or electron pair donor, in reactions with Lewis acid
s, although it can also react with Lewis bases, forming hydrogen bonds between the electron pair donors and the hydrogen atoms of water. HSAB theory
describes water as both a weak hard acid and a weak hard base, meaning that it reacts preferentially with other hard species:
When a salt of a weak acid or of a weak base is dissolved in water, water can partially hydrolyze
the salt, producing the corresponding base or acid, which gives aqueous solutions of soap
and baking soda their basic pH:
in transition metal
complexes, examples of which range from solvated ions, such as , to perrhenic acid
, which contains two water molecules coordinated to a rhenium
atom, to various solid hydrates, such as . Water is typically a monodentate ligand, it forms only one bond with the central atom.
s, for example in hydration reaction
, in which a hydroxyl group and an acidic proton are added to the two carbon atoms bonded together in the carbon-carbon double bond, resulting in an alcohol. When addition of water to an organic molecule cleaves the molecule in two, hydrolysis
is said to occur. Notable examples of hydrolysis are saponification
of fats and digestion
of proteins and polysaccharides. Water can also be a leaving group
in SN2 substitution
and E2 elimination
reactions, the latter is then known as dehydration reaction
.
ions equal to that of the hydronium
or hydrogen ions, which gives pH
of 7 at 298 K. In practice, pure water is very difficult to produce. Water left exposed to air for any length of time will dissolve carbon dioxide
, forming a dilute solution of carbonic acid
, with a limiting pH of about 5.7. As cloud droplets form in the atmosphere and as raindrops fall through the air minor amounts of are absorbed, and thus most rain is slightly acidic. If high amounts of nitrogen
and sulfur
oxides are present in the air, they too will dissolve into the cloud and rain drops, producing acid rain
.
+1 and oxygen in oxidation state −2. Because of that, water oxidizes chemicals with reduction potential
below the potential of /, such as hydrides, alkali
and alkaline earth
metals (except for beryllium
), etc. Some other reactive metals, such as aluminum, are oxidized by water as well, but their oxides are not soluble, and the reaction stops because of passivation
. Note, however, that rusting of iron
is a reaction between iron and oxygen, dissolved in water, not between iron and water.
Water can be oxidized itself, emitting oxygen gas, but very few oxidants react with water even if their reduction potential is greater than the potential of . Almost all such reactions require a catalyst
and water erosion, physical processes that convert solid rocks and minerals into soil and sediment, but under some conditions chemical reactions with water occur as well, resulting in metasomatism
or mineral hydration
, a type of chemical alteration of a rock which produces clay minerals
in nature and also occurs when Portland cement
hardens.
Water ice can form clathrate compounds, known as clathrate hydrates, with a variety of small molecules that can be embedded in its spacious crystal lattice. The most notable of these is methane clathrate
, 4, naturally found in large quantities on the ocean floor.
light, but it absorbs most ultraviolet light, infrared light, and microwave
s. Most photoreceptor
s and photosynthetic pigment
s utilize the portion of the light spectrum that is transmitted well through water. Microwave ovens take advantage of water's opacity to microwave radiation to heat the water inside of foods. The very weak onset of absorption in the red end of the visible spectrum lends water its intrinsic blue hue (see Color of water
).
s of both hydrogen and oxygen exist, giving rise to several known isotopologue
s of water.
Hydrogen occurs naturally in three isotopes. The most common (1H) accounting for more than 99.98% of hydrogen in water, consists of only a single proton in its nucleus. A second, stable isotope, deuterium
(chemical symbol D or 2H), has an additional neutron. Deuterium oxide, , is also known as heavy water
because of its higher density. It is used in nuclear reactor
s as a neutron moderator
. The third isotope, tritium
, has 1 proton and 2 neutrons, and is radioactive, decaying with a half-life
of 4500 days. exists in nature only in minute quantities, being produced primarily via cosmic ray-induced nuclear reactions in the atmosphere. Water with one deuterium atom occurs naturally in ordinary water in low concentrations (~0.03%) and in far lower amounts (0.000003%).
The most notable physical differences between and , other than the simple difference in specific mass, involve properties that are affected by hydrogen bonding, such as freezing and boiling, and other kinetic effects. The difference in boiling points allows the isotopologues to be separated.
Consumption of pure isolated may affect biochemical processes – ingestion of large amounts impairs kidney and central nervous system function. Small quantities can be consumed without any ill-effects, and even very large amounts of heavy water must be consumed for any toxicity to become apparent.
Oxygen also has three stable isotopes, with present in 99.76%, in 0.04%, and in 0.2% of water molecules.
surfaces, water exists in a liquid crystal
state. This liquid crystal state has the following properties:
Gerald Pollack speculated that this liquid crystal zone remained relatively unexplored recently, despite extensive writing on this topic up through 1949, because of the polywater
and water memory
debacles.
, was done in 1800 by an English chemist William Nicholson
. In 1805, Joseph Louis Gay-Lussac
and Alexander von Humboldt
showed that water is composed of two parts hydrogen and one part oxygen.
Gilbert Newton Lewis isolated the first sample of pure heavy water
in 1933.
The properties of water have historically been used to define various temperature scales. Notably, the Kelvin
, Celsius
, Rankine, and Fahrenheit
scales were, or currently are, defined by the freezing and boiling points of water. The less common scales of Delisle
, Newton
, Réaumur and Rømer
were defined similarly. The triple point
of water is a more commonly used standard point today.
name of water is oxidane or simply water, or its equivalent in different languages, although there are other systematic names which can be used to describe the molecule.
The simplest systematic name of water is hydrogen oxide. This is analogous to related compounds such as hydrogen peroxide
, hydrogen sulfide
, and deuterium oxide (heavy water). Another systematic name, oxidane, is accepted by IUPAC as a parent name for the systematic naming of oxygen-based substituent groups, although even these commonly have other recommended names. For example, the name hydroxyl
is recommended over oxidanyl for the –OH group. The name oxane is explicitly mentioned by the IUPAC as being unsuitable for this purpose, since it is already the name of a cyclic ether also known as tetrahydropyran
.
The polarized form of the water molecule, H+OH−, is also called hydron hydroxide by IUPAC nomenclature.
Dihydrogen monoxide (DHMO) is a rarely used name of water. This term has been used in various hoaxes that call for this "lethal chemical" to be banned, such as in the dihydrogen monoxide hoax
. Other systematic names for water include hydroxic acid, hydroxylic acid, and hydrogen hydroxide. Both acid and alkali names exist for water because it is amphoteric
(able to react both as an acid or an alkali). None of these exotic names are used widely.
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
is the most abundant compound on Earth's surface, covering about 70%. In nature, it exists in liquid, solid, and gaseous states. It is in dynamic equilibrium
Dynamic equilibrium
A dynamic equilibrium exists once a reversible reaction ceases to change its ratio of reactants/products, but substances move between the chemicals at an equal rate, meaning there is no net change. It is a particular example of a system in a steady state...
between the liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
and gas
Water vapor
Water vapor or water vapour , also aqueous vapor, is the gas phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Under typical atmospheric conditions, water vapor is continuously...
states at standard temperature and pressure. At room temperature
Room temperature
-Comfort levels:The American Society of Heating, Refrigerating and Air-Conditioning Engineers has listings for suggested temperatures and air flow rates in different types of buildings and different environmental circumstances. For example, a single office in a building has an occupancy ratio per...
, it is a taste
Taste
Taste is one of the traditional five senses. It refers to the ability to detect the flavor of substances such as food, certain minerals, and poisons, etc....
less and odor
Odor
An odor or odour is caused by one or more volatilized chemical compounds, generally at a very low concentration, that humans or other animals perceive by the sense of olfaction. Odors are also commonly called scents, which can refer to both pleasant and unpleasant odors...
less liquid, nearly colorless
Transparency and translucency
In the field of optics, transparency is the physical property of allowing light to pass through a material; translucency only allows light to pass through diffusely. The opposite property is opacity...
with a hint of blue
Color of water
The color of water is a subject of both scientific study and popular misconception. While relatively small quantities of water are observed by humans to be colorless, pure water has a slight blue color that becomes a deeper blue as the thickness of the observed sample increases...
. Many substances dissolve in water and it is commonly referred to as the universal solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
. Because of this, water in nature and in use is rarely pure and some of its properties may vary slightly from those of the pure substance. However, there are also many compounds that are essentially, if not completely, insoluble in water. Water is the only common substance found naturally in all three common states of matter and it is essential for all life on Earth. Water usually makes up 55% to 78% of the human body.
Forms of water
Like many substances, waterWater
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
can take numerous forms that are broadly categorized by phase of matter
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
. The liquid phase is the most common among water's phases (within the Earth's atmosphere and surface) and is the form that is generally denoted by the word "water." The solid phase
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
of water is known as ice
Ice
Ice is water frozen into the solid state. Usually ice is the phase known as ice Ih, which is the most abundant of the varying solid phases on the Earth's surface. It can appear transparent or opaque bluish-white color, depending on the presence of impurities or air inclusions...
and commonly takes the structure of hard, amalgamated crystals, such as ice cubes, or loosely accumulated granular
Granular material
A granular material is a conglomeration of discrete solid, macroscopic particles characterized by a loss of energy whenever the particles interact . The constituents that compose granular material must be large enough such that they are not subject to thermal motion fluctuations...
crystals, like snow
Snow
Snow is a form of precipitation within the Earth's atmosphere in the form of crystalline water ice, consisting of a multitude of snowflakes that fall from clouds. Since snow is composed of small ice particles, it is a granular material. It has an open and therefore soft structure, unless packed by...
. For a list of the many different crystalline and amorphous
Amorphous solid
In condensed matter physics, an amorphous or non-crystalline solid is a solid that lacks the long-range order characteristic of a crystal....
forms of solid H2O, see the article ice
Ice
Ice is water frozen into the solid state. Usually ice is the phase known as ice Ih, which is the most abundant of the varying solid phases on the Earth's surface. It can appear transparent or opaque bluish-white color, depending on the presence of impurities or air inclusions...
. The gaseous phase of water is known as water vapor
Water vapor
Water vapor or water vapour , also aqueous vapor, is the gas phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Under typical atmospheric conditions, water vapor is continuously...
(or steam
Steam
Steam is the technical term for water vapor, the gaseous phase of water, which is formed when water boils. In common language it is often used to refer to the visible mist of water droplets formed as this water vapor condenses in the presence of cooler air...
), and is characterized by water assuming the configuration of a transparent cloud
Vapor
A vapor or vapour is a substance in the gas phase at a temperature lower than its critical point....
. (Note that the visible steam and clouds are, in fact, water in the liquid form as minute droplets suspended in the air.) The fourth state of water, that of a supercritical fluid
Supercritical fluid
A supercritical fluid is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist. It can effuse through solids like a gas, and dissolve materials like a liquid...
, is much less common than the other three and only rarely occurs in nature, in extremely uninhabitable conditions. When water achieves a specific critical temperature and a specific critical pressure (647 K
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
and 22.064 MPa
Pascal (unit)
The pascal is the SI derived unit of pressure, internal pressure, stress, Young's modulus and tensile strength, named after the French mathematician, physicist, inventor, writer, and philosopher Blaise Pascal. It is a measure of force per unit area, defined as one newton per square metre...
), liquid and gas phase merge to one homogeneous fluid phase, with properties of both gas and liquid. One example of naturally occurring supercritical water is found in the hottest parts of deep water hydrothermal vents, in which water is heated to the critical temperature by scalding volcanic
Submarine volcano
Submarine volcanoes are underwater fissures in the Earth's surface from which magma can erupt. They are estimated to account for 75% of annual magma output. The vast majority are located near areas of tectonic plate movement, known as ocean ridges...
plumes
Plume (hydrodynamics)
In hydrodynamics, a plume is a column of one fluid or gas moving through another. Several effects control the motion of the fluid, including momentum, diffusion, and buoyancy...
and achieves the critical pressure because of the crushing weight of the ocean at the extreme depths at which the vents are located. Additionally, anywhere there is volcanic activity below a depth of 2.25 km (1.4 mi) can be expected to have water in the supercritical phase.
Vienna Standard Mean Ocean Water is the current international standard for water isotopes. Naturally occurring water is almost completely composed of the neutron-less hydrogen isotope protium. Only 155 ppm
Parts-per notation
In science and engineering, the parts-per notation is a set of pseudo units to describe small values of miscellaneous dimensionless quantities, e.g. mole fraction or mass fraction. Since these fractions are quantity-per-quantity measures, they are pure numbers with no associated units of measurement...
include deuterium
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
( or D), a hydrogen isotope with one neutron, and less than 20 parts per quintillion include tritium
Tritium
Tritium is a radioactive isotope of hydrogen. The nucleus of tritium contains one proton and two neutrons, whereas the nucleus of protium contains one proton and no neutrons...
( or T), which has two.
Heavy water
Heavy water
Heavy water is water highly enriched in the hydrogen isotope deuterium; e.g., heavy water used in CANDU reactors is 99.75% enriched by hydrogen atom-fraction...
is water with a higher-than-average deuterium content, up to 100%. Chemically, it is similar but not identical to normal water. This is because the nucleus of deuterium is twice as heavy as protium, and this causes noticeable differences in bonding energies. Because water molecules exchange hydrogen atoms with one another, hydrogen deuterium oxide (DOH) is much more common in low-purity heavy water than pure dideuterium monoxide (D2O). Humans are generally unaware of taste differences, but sometimes report a burning sensation or sweet flavor. Rats, however, are able to avoid heavy water by smell. Toxic to many animals, heavy water is used in the nuclear reactor
Nuclear reactor
A nuclear reactor is a device to initiate and control a sustained nuclear chain reaction. Most commonly they are used for generating electricity and for the propulsion of ships. Usually heat from nuclear fission is passed to a working fluid , which runs through turbines that power either ship's...
industry to moderate
Neutron moderator
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, thereby turning them into thermal neutrons capable of sustaining a nuclear chain reaction involving uranium-235....
(slow down) neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
s. Light water reactors are also common, where "light" simply designates normal water.
Light water
Deuterium-depleted water
Deuterium-depleted water , or "light water", is water which has a lower concentration than naturally occurs of deuterium, a heavier isotope of hydrogen which has, in addition to its one proton, a neutron, roughly doubling its mass....
more specifically refers to deuterium-depleted water (DDW), water in which the deuterium content has been reduced below the standard 155ppm level. Light water has been found to be beneficial for improving cancer survival rates in mice and humans undergoing chemotherapy.
Physics and chemistry
Water is the chemical substanceChemical substance
In chemistry, a chemical substance is a form of matter that has constant chemical composition and characteristic properties. It cannot be separated into components by physical separation methods, i.e. without breaking chemical bonds. They can be solids, liquids or gases.Chemical substances are...
with chemical formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
: one molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
of water has two hydrogen atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s covalently bonded
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
to a single oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
atom.
Water is a tasteless, odorless liquid at ambient temperature and pressure, and appears colorless in small quantities, although it has its own intrinsic very light blue hue. Ice also appears colorless, and water vapor is essentially invisible as a gas.
Water is primarily a liquid under standard conditions, which is not predicted from its relationship to other analogous hydrides of the oxygen family
Chalcogen
The chalcogens are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family...
in the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
, which are gases such as hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
. The elements surrounding oxygen in the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
, nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
, fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
, phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
, sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
and chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
, all combine with hydrogen to produce gases under standard conditions. The reason that water forms a liquid is that oxygen is more electronegative than all of these elements with the exception of fluorine. Oxygen attracts electrons much more strongly than hydrogen, resulting in a net positive charge on the hydrogen atoms, and a net negative charge on the oxygen atom. The presence of a charge on each of these atoms gives each water molecule a net dipole moment. Electrical attraction between water molecules due to this dipole pulls individual molecules closer together, making it more difficult to separate the molecules and therefore raising the boiling point. This attraction is known as hydrogen bonding. The molecules of water are constantly moving in relation to each other, and the hydrogen bonds are continually breaking and reforming at timescales faster than 200 femtoseconds. However, this bond is sufficiently strong to create many of the peculiar properties of water, such as those that make it integral to life. Water can be described as a polar liquid that slightly dissociates disproportionately into the hydronium
Hydronium
In chemistry, a hydronium ion is the cation , a type of oxonium ion produced by protonation of water. This cation is often used to represent the nature of the proton in aqueous solution, where the proton is highly solvated...
ion ((aq)) and an associated hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
ion ((aq)).
- 2 (l) (aq) + (aq)
The dissociation constant
Dissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant is a specific type of equilibrium constant that measures the propensity of a larger object to separate reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into...
for this dissociation is commonly symbolized as Kw and has a value of about 10−14 at 25 °C; see "Water (data page)
Water (data page)
This page provides supplementary data of the properties of water.Further comprehensive authoritative data can be found at the page on thermophysical properties of fluids.-Structure and properties:-Thermodynamic properties:-Liquid physical properties:...
" and "Self-ionization of water
Self-ionization of water
The self-ionization of water is the chemical reaction in which a proton is transferred from one water molecule to another, in pure water or an aqueous solution, to create the two ions, hydronium, H3O+ and hydroxide, OH−...
" for more information.
Heat capacity and heats of vaporization and fusion
Water has the second highest specific heat capacity of all known substances, after ammoniaAmmonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
, as well as a high heat of vaporization (40.65 kJ/mol or 2257 kJ/kg at the normal boiling point), both of which are a result of the extensive hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
ing between its molecules. These two unusual properties allow water to moderate Earth's climate
Climate
Climate encompasses the statistics of temperature, humidity, atmospheric pressure, wind, rainfall, atmospheric particle count and other meteorological elemental measurements in a given region over long periods...
by buffering large fluctuations in temperature. According to Josh Willis, of NASA
NASA
The National Aeronautics and Space Administration is the agency of the United States government that is responsible for the nation's civilian space program and for aeronautics and aerospace research...
's Jet Propulsion Laboratory
Jet Propulsion Laboratory
Jet Propulsion Laboratory is a federally funded research and development center and NASA field center located in the San Gabriel Valley area of Los Angeles County, California, United States. The facility is headquartered in the city of Pasadena on the border of La Cañada Flintridge and Pasadena...
, the oceans absorb one thousand times more heat than the atmosphere (air) and are holding 80 to 90% of global warming
Global warming
Global warming refers to the rising average temperature of Earth's atmosphere and oceans and its projected continuation. In the last 100 years, Earth's average surface temperature increased by about with about two thirds of the increase occurring over just the last three decades...
heat.
The specific enthalpy of fusion
Enthalpy of fusion
The enthalpy of fusion is the change in enthalpy resulting from heating one mole of a substance to change its state from a solid to a liquid. The temperature at which this occurs is the melting point....
of water is 333.55 kJ/kg at 0 °C. Of common substances, only that of ammonia is higher. This property confers resistance to melting on the ice of glacier
Glacier
A glacier is a large persistent body of ice that forms where the accumulation of snow exceeds its ablation over many years, often centuries. At least 0.1 km² in area and 50 m thick, but often much larger, a glacier slowly deforms and flows due to stresses induced by its weight...
s and drift ice
Drift ice
Drift ice is ice that floats on the surface of the water in cold regions, as opposed to fast ice, which is attached to a shore. Usually drift ice is carried along by winds and sea currents, hence its name, "drift ice"....
. Before and since the advent of mechanical refrigeration
Refrigeration
Refrigeration is a process in which work is done to move heat from one location to another. This work is traditionally done by mechanical work, but can also be done by magnetism, laser or other means...
, ice was and still is in common use for retarding food spoilage.
| Temperature (°C) | Cp (J/(g·K) at 100 kPa) |
|---|---|
| 0 | 4.2176 |
| 10 | 4.1921 |
| 20 | 4.1818 |
| 30 | 4.1784 |
| 40 | 4.1785 |
| 50 | 4.1806 |
| 60 | 4.1843 |
| 70 | 4.1895 |
| 80 | 4.1963 |
| 90 | 4.205 |
| 100 | 4.2159 |
Note that the specific heat capacity of ice at −10 °C is about 2.05 J/(g·K) and that the heat capacity of steam at 100 °C is about 2.080 J/(g·K).
Density of water and ice
| Temp (°C) | Density (kg/m3) |
|---|---|
| +100 | 958.4 |
| +80 | 971.8 |
| +60 | 983.2 |
| +40 | 992.2 |
| +30 | 995.6502 |
| +25 | 997.0479 |
| +22 | 997.7735 |
| +20 | 998.2071 |
| +15 | 999.1026 |
| +10 | 999.7026 |
| +4 | 999.9720 |
| 0 | 999.8395 |
| −10 | 998.117 |
| −20 | 993.547 |
| −30 | 983.854 |
| The values below 0 °C refer to supercooled Supercooling Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid.... water. |
|
The density of water is approximately one gram per cubic centimeter. More precisely, it is dependent on its temperature, but the relation is not linear and is unimodal rather than monotonic
Monotonic function
In mathematics, a monotonic function is a function that preserves the given order. This concept first arose in calculus, and was later generalized to the more abstract setting of order theory....
(see right-hand table). When cooled from room temperature
Room temperature
-Comfort levels:The American Society of Heating, Refrigerating and Air-Conditioning Engineers has listings for suggested temperatures and air flow rates in different types of buildings and different environmental circumstances. For example, a single office in a building has an occupancy ratio per...
liquid water becomes increasingly dense, just like other substances. But at approximately 4 °C, pure water reaches its maximum density. As it is cooled further, it expands to become less dense. This unusual negative thermal expansion is attributed to strong, orientation-dependent, intermolecular interactions and is also observed in molten silica.
The solid form of most substances is denser
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
than the liquid phase
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
; thus, a block of most solids will sink in the liquid. However, a block of ice floats in liquid water because ice is less dense. Upon freezing, the density of water decreases by about 9%. The reason for this is the 'cooling' of intermolecular vibrations allowing the molecules to form steady hydrogen bonds with their neighbors and thereby gradually locking into positions reminiscent of the hexagonal packing achieved upon freezing to ice Ih
Ice Ih
thumb|Photograph showing details of an ice cube under magnification. Ice Ih is the form of ice commonly seen on earth.Ice Ih is the hexagonal crystal form of ordinary ice, or frozen water. Virtually all ice in the biosphere is ice Ih, with the exception only of a small amount of ice Ic which is...
. Whereas the hydrogen bonds are shorter in the crystal than in the liquid, this locking effect reduces the average coordination number of molecules as the liquid approaches nucleation. Other substances that expand on freezing are silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
, gallium
Gallium
Gallium is a chemical element that has the symbol Ga and atomic number 31. Elemental gallium does not occur in nature, but as the gallium salt in trace amounts in bauxite and zinc ores. A soft silvery metallic poor metal, elemental gallium is a brittle solid at low temperatures. As it liquefies...
, germanium
Germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. The isolated element is a semiconductor, with an appearance most similar to elemental silicon....
, antimony
Antimony
Antimony is a toxic chemical element with the symbol Sb and an atomic number of 51. A lustrous grey metalloid, it is found in nature mainly as the sulfide mineral stibnite...
, bismuth
Bismuth
Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead...
, plutonium
Plutonium
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
and other compounds that form spacious crystal lattices with tetrahedral coordination.
Only ordinary hexagonal ice is less dense than the liquid. Under increasing pressure, ice undergoes a number of transitions to other allotropic forms
Allotropy
Allotropy or allotropism is the property of some chemical elements to exist in two or more different forms, known as allotropes of these elements...
with higher density than liquid water, such as high density amorphous ice (HDA) and very high density amorphous ice (VHDA).
Water also expands significantly as the temperature increases. Its density decreases by 4% from its highest value when approaching its boiling point.
The melting point of ice is 0 °C (32 °F, 273 K) at standard pressure, however, pure liquid water can be supercooled well below that temperature without freezing if the liquid is not mechanically disturbed. It can remain in a fluid state down to its homogeneous nucleation
Nucleation
Nucleation is the extremely localized budding of a distinct thermodynamic phase. Some examples of phases that may form by way of nucleation in liquids are gaseous bubbles, crystals or glassy regions. Creation of liquid droplets in saturated vapor is also characterized by nucleation...
point of approximately 231 K (−42 °C). The melting point of ordinary hexagonal ice falls slightly under moderately high pressures, but as ice transforms into its allotropes (see crystalline states of ice) above 209.9 MPa (2,071.6 atm), the melting point increases markedly with pressure, i.e., reaching 355 kelvins (81.9 °C) at 2.216 GPa (21,870.2 atm) (triple point of Ice VII
Ice VII
Ice VII is a cubic crystalline form of ice. It has a triple point with liquid water and Ice VI at 355 K and 2.216 GPa, with the melt line extending to at least 715 K and 10 GPa. It can also be reached in the solid state by increasing the pressure on ice VI at ambient temperature. Like the majority...
).
A significant increase of pressure is required to lower the melting point of ordinary ice—the pressure exerted by an ice skater on the ice only reduces the melting point by approximately 0.09 °C (0.16 °F).
These properties of water have important consequences in its role in the ecosystem
Ecosystem
An ecosystem is a biological environment consisting of all the organisms living in a particular area, as well as all the nonliving , physical components of the environment with which the organisms interact, such as air, soil, water and sunlight....
of Earth. Water at a temperature of 4 °C will always accumulate at the bottom of fresh water lakes, irrespective of the temperature in the atmosphere. Since water and ice are poor conductors of heat (good insulators) it is unlikely that sufficiently deep lakes will freeze completely, unless stirred by strong currents that mix cooler and warmer water and accelerate the cooling. In warming weather, chunks of ice float, rather than sink to the bottom where they might melt extremely slowly. These phenomena thus may help to preserve aquatic life.
Density of saltwater and ice

Arctic Ocean
The Arctic Ocean, located in the Northern Hemisphere and mostly in the Arctic north polar region, is the smallest and shallowest of the world's five major oceanic divisions...
generally lives in water that is 4 °C colder than the temperature at the bottom of frozen-over fresh water
Fresh Water
Fresh Water is the debut album by Australian rock and blues singer Alison McCallum, released in 1972. Rare for an Australian artist at the time, it came in a gatefold sleeve...
lakes and rivers in the winter.
In cold countries, when the temperature of fresh water reaches 4 °C, the layers of water near the top in contact with cold air continue to lose heat energy and their temperature falls below 4 °C. On cooling below 4 °C, these layers do not sink but may rise up as fresh water has a maximum density at 4 °C. (Refer: Polarity and hydrogen bonding) Due to this, the layer of water at 4 °C remains at the bottom and above this layers of water 3 °C, 2 °C, 1 °C and 0 °C are formed. Since ice is a poor conductor of heat, it does not absorb heat energy from the water beneath the layer of ice which prevents the water freezing. Thus, aquatic creatures survive in such places.
As the surface
Surface
In mathematics, specifically in topology, a surface is a two-dimensional topological manifold. The most familiar examples are those that arise as the boundaries of solid objects in ordinary three-dimensional Euclidean space R3 — for example, the surface of a ball...
of salt water begins to freeze (at −1.9°C for normal salinity seawater
Seawater
Seawater is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% . This means that every kilogram of seawater has approximately of dissolved salts . The average density of seawater at the ocean surface is 1.025 g/ml...
, 3.5%) the ice that forms is essentially salt free with a density approximately equal to that of freshwater ice. This ice floats on the surface and the salt that is "frozen out" adds to the salinity
Salinity
Salinity is the saltiness or dissolved salt content of a body of water. It is a general term used to describe the levels of different salts such as sodium chloride, magnesium and calcium sulfates, and bicarbonates...
and density of the seawater just below it, in a process known as brine
Brine
Brine is water, saturated or nearly saturated with salt .Brine is used to preserve vegetables, fruit, fish, and meat, in a process known as brining . Brine is also commonly used to age Halloumi and Feta cheeses, or for pickling foodstuffs, as a means of preserving them...
rejection. This denser saltwater sinks by convection and the replacing seawater is subject to the same process. This provides essentially freshwater ice at −1.9°C on the surface. The increased density of the seawater beneath the forming ice causes it to sink towards the bottom. On a large scale, the process of brine rejection and sinking cold salty water results in ocean currents forming to transport such water away from the Poles, leading to a global system of currents called the thermohaline circulation
Thermohaline circulation
The term thermohaline circulation refers to a part of the large-scale ocean circulation that is driven by global density gradients created by surface heat and freshwater fluxes....
. One potential consequence of global warming
Global warming
Global warming refers to the rising average temperature of Earth's atmosphere and oceans and its projected continuation. In the last 100 years, Earth's average surface temperature increased by about with about two thirds of the increase occurring over just the last three decades...
is that the loss of Arctic and Antarctic ice could result in the loss of these currents as well, which could have unforeseeable consequences on near and distant climates.
Miscibility and condensation

Water is miscible with many liquids, for example ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
in all proportions, forming a single homogeneous liquid. On the other hand, water and most oil
Oil
An oil is any substance that is liquid at ambient temperatures and does not mix with water but may mix with other oils and organic solvents. This general definition includes vegetable oils, volatile essential oils, petrochemical oils, and synthetic oils....
s are immiscible usually forming layers according to increasing density from the top.
As a gas, water vapor is completely miscible with air. On the other hand the maximum water vapor pressure that is thermodynamically stable with the liquid (or solid) at a given temperature is relatively low compared with total atmospheric pressure.
For example, if the vapor partial pressure
Partial pressure
In a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
is 2% of atmospheric pressure and the air is cooled from 25 °C, starting at about 22 °C water will start to condense, defining the dew point
Dew point
The dew point is the temperature to which a given parcel of humid air must be cooled, at constant barometric pressure, for water vapor to condense into liquid water. The condensed water is called dew when it forms on a solid surface. The dew point is a saturation temperature.The dew point is...
, and creating fog
Fog
Fog is a collection of water droplets or ice crystals suspended in the air at or near the Earth's surface. While fog is a type of stratus cloud, the term "fog" is typically distinguished from the more generic term "cloud" in that fog is low-lying, and the moisture in the fog is often generated...
or dew
Dew
[Image:Dew on a flower.jpg|right|220px|thumb|Some dew on an iris in Sequoia National Park]]Dew is water in the form of droplets that appears on thin, exposed objects in the morning or evening...
. The reverse process accounts for the fog burning off in the morning.
If the humidity is increased at room temperature, for example, by running a hot shower or a bath, and the temperature stays about the same, the vapor soon reaches the pressure for phase change, and then condenses out as minute water droplets, commonly referred to as steam.
A gas in this context is referred to as saturated or 100% relative humidity, when the vapor pressure of water in the air is at the equilibrium with vapor pressure due to (liquid) water; water (or ice, if cool enough) will fail to lose mass through evaporation when exposed to saturated air. Because the amount of water vapor in air is small, relative humidity, the ratio of the partial pressure due to the water vapor to the saturated partial vapor pressure, is much more useful.
Water vapor pressure above 100% relative humidity is called super-saturated and can occur if air is rapidly cooled, for example, by rising suddenly in an updraft.
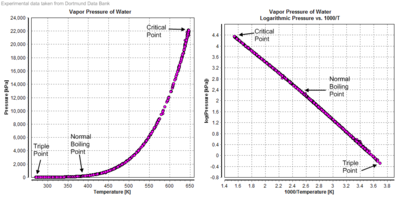
Vapor pressure

| Temperature | Pressure | ||||||
|---|---|---|---|---|---|---|---|
| °C | K | °F | Pa | atm | torr | in Hg | psi |
| 0 | 611 | 0.00603010115963484 | 4.58 torr | ||||
| 5 | 872 | 0.00860597088576363 | 6.54 torr | ||||
| 10 | 1228 | 0.0121194177152726 | 9.21 torr | ||||
| 12 | 1403 | 0.0138465334320257 | 10.52 torr | ||||
| 14 | 1599 | 0.015780903034789 | 11.99 torr | ||||
| 16 | 1817 | 0.01793239575623 | 13.63 torr | ||||
| 17 | 1937 | 0.0191167036762892 | 14.53 torr | ||||
| 18 | 2064 | 0.0203700962250185 | 15.48 torr | ||||
| 19 | 2197 | 0.0216827041697508 | 16.48 torr | ||||
| 20 | 2338 | 0.0230742659758204 | 17.54 torr | ||||
| 21 | 2486 | 0.0245349124105601 | 18.65 torr | ||||
| 22 | 2644 | 0.0260942511719714 | 19.83 torr | ||||
| 23 | 2809 | 0.0277226745620528 | 21.07 torr | ||||
| 24 | 2984 | 0.0294497902788058 | 22.38 torr | ||||
| 25 | 3168 | 0.0312657290895633 | 23.76 torr | ||||
Compressibility
The compressibility of water is a function of pressure and temperature. At 0 °C, at the limit of zero pressure, the compressibility is . At the zero-pressure limit, the compressibility reaches a minimum of around 45 °C before increasing again with increasing temperature. As the pressure is increased, the compressibility decreases, being at 0 °C and 100 MPa.The bulk modulus
Bulk modulus
The bulk modulus of a substance measures the substance's resistance to uniform compression. It is defined as the pressure increase needed to decrease the volume by a factor of 1/e...
of water is 2.2 GPa. The low compressibility of non-gases, and of water in particular, leads to their often being assumed as incompressible. The low compressibility of water means that even in the deep ocean
Ocean
An ocean is a major body of saline water, and a principal component of the hydrosphere. Approximately 71% of the Earth's surface is covered by ocean, a continuous body of water that is customarily divided into several principal oceans and smaller seas.More than half of this area is over 3,000...
s at 4 km depth, where pressures are 40 MPa, there is only a 1.8% decrease in volume.
Triple point
| Phases in stable equilibrium | Pressure | Temperature |
|---|---|---|
| liquid water, ice Ih Ice Ih thumb|Photograph showing details of an ice cube under magnification. Ice Ih is the form of ice commonly seen on earth.Ice Ih is the hexagonal crystal form of ordinary ice, or frozen water. Virtually all ice in the biosphere is ice Ih, with the exception only of a small amount of ice Ic which is... , and water vapor |
611.73 Pa | 273.16 K (0.01 °C) |
| liquid water, ice Ih, and ice III Ice III Ice III is a form of solid matter which consists of tetragonal crystalline ice, formed by cooling water down to at . It is the least dense of the high-pressure water phases, with a density of . The proton-ordered form of is ice IX.... |
209.9 MPa | 251 K (−22 °C) |
| liquid water, ice III, and ice V | 350.1 MPa | −17.0 °C |
| liquid water, ice V, and ice VI | 632.4 MPa | 0.16 °C |
| ice Ih, Ice II Ice II Ice II is a rhombohedral crystalline form of ice with highly ordered structure. It is formed from ice Ih by compressing it at temperature of 198 K at 300 MPa or by decompressing ice V. When heated it undergoes transformation to ice III.... , and ice III |
213 MPa | −35 °C |
| ice II, ice III, and ice V | 344 MPa | −24 °C |
| ice II, ice V, and ice VI | 626 MPa | −70 °C |
The temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
and pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
at which solid, liquid, and gaseous water
Water vapor
Water vapor or water vapour , also aqueous vapor, is the gas phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Under typical atmospheric conditions, water vapor is continuously...
coexist in equilibrium is called the triple point
Triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases of that substance coexist in thermodynamic equilibrium...
of water. This point is used to define the units of temperature (the kelvin
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
, the SI unit of thermodynamic temperature and, indirectly, the degree Celsius
Celsius
Celsius is a scale and unit of measurement for temperature. It is named after the Swedish astronomer Anders Celsius , who developed a similar temperature scale two years before his death...
and even the degree Fahrenheit
Fahrenheit
Fahrenheit is the temperature scale proposed in 1724 by, and named after, the German physicist Daniel Gabriel Fahrenheit . Within this scale, the freezing of water into ice is defined at 32 degrees, while the boiling point of water is defined to be 212 degrees...
).
As a consequence, water's triple point temperature is a prescribed value rather than a measured quantity.

Pascal (unit)
The pascal is the SI derived unit of pressure, internal pressure, stress, Young's modulus and tensile strength, named after the French mathematician, physicist, inventor, writer, and philosopher Blaise Pascal. It is a measure of force per unit area, defined as one newton per square metre...
. This pressure is quite low, about of the normal sea level barometric pressure of 101,325 Pa. The atmospheric surface pressure on planet Mars
Mars
Mars is the fourth planet from the Sun in the Solar System. The planet is named after the Roman god of war, Mars. It is often described as the "Red Planet", as the iron oxide prevalent on its surface gives it a reddish appearance...
is remarkably close to the triple point pressure, and the zero-elevation or "sea level" of Mars is defined by the height at which the atmospheric pressure corresponds to the triple point of water.
Although it is commonly named as "the triple point of water", the stable combination of liquid water, ice I, and water vapor is but one of several triple points on the phase diagram
Phase diagram
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases can occur at equilibrium...
of water. Gustav Heinrich Johann Apollon Tammann in Göttingen produced data on several other triple points in the early 20th century. Kamb and others documented further triple points in the 1960s.
Electrical conductivity
Pure water containing no ions is an excellent insulatorElectrical insulation
thumb|250px|[[Coaxial Cable]] with dielectric insulator supporting a central coreThis article refers to electrical insulation. For insulation of heat, see Thermal insulation...
, but not even "deionized" water is completely free of ions. Water undergoes auto-ionization
Self-ionization of water
The self-ionization of water is the chemical reaction in which a proton is transferred from one water molecule to another, in pure water or an aqueous solution, to create the two ions, hydronium, H3O+ and hydroxide, OH−...
in the liquid state. Further, because water is such a good solvent, it almost always has some solute
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
dissolved in it, most frequently a salt. If water has even a tiny amount of such an impurity, then it can conduct electricity readily, as impurities such as salt separate into free ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s in aqueous solution by which an electric current can flow.
It is known that the theoretical maximum electrical resistivity for water is approximately 182 kΩ·m at 25 °C. This figure agrees well with what is typically seen on reverse osmosis
Reverse osmosis
Reverse osmosis is a membrane technical filtration method that removes many types of large molecules and ions from solutions by applying pressure to the solution when it is on one side of a selective membrane. The result is that the solute is retained on the pressurized side of the membrane and...
, ultra-filtered
Ultrafiltration
Ultrafiltration is a variety of membrane filtration in which hydrostatic pressure forces a liquid against a semipermeable membrane. Suspended solids and solutes of high molecular weight are retained, while water and low molecular weight solutes pass through the membrane...
and deionized ultra-pure water systems used, for instance, in semiconductor manufacturing plants. A salt or acid contaminant level exceeding even 100 parts per trillion (ppt) in otherwise ultra-pure water begins to noticeably lower its resistivity by up to several kΩ·m.
The electrical conductivity of water increases significantly upon solvation of a small amount of ionic material, such as hydrogen chloride
Hydrogen chloride
The compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
or any salt.
Any electrical conductivity observable in water is the result of ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s of mineral salts and carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
dissolved in it. Carbon dioxide forms carbonate
Carbonate
In chemistry, a carbonate is a salt of carbonic acid, characterized by the presence of the carbonate ion, . The name may also mean an ester of carbonic acid, an organic compound containing the carbonate group C2....
ions in water. Water self-ionizes
Self-ionization of water
The self-ionization of water is the chemical reaction in which a proton is transferred from one water molecule to another, in pure water or an aqueous solution, to create the two ions, hydronium, H3O+ and hydroxide, OH−...
, when two water molecules form one hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
anion (OH−) and one hydronium
Hydronium
In chemistry, a hydronium ion is the cation , a type of oxonium ion produced by protonation of water. This cation is often used to represent the nature of the proton in aqueous solution, where the proton is highly solvated...
cation , but not enough to carry sufficient electric current
Electric current
Electric current is a flow of electric charge through a medium.This charge is typically carried by moving electrons in a conductor such as wire...
to do any work or harm for most operations. In pure water, sensitive equipment can detect a very slight electrical conductivity of 0.055 µS
Siemens (unit)
The siemens is the SI derived unit of electric conductance and electric admittance. Conductance and admittance are the reciprocals of resistance and impedance respectively, hence one siemens is equal to the reciprocal of one ohm, and is sometimes referred to as the mho. In English, the term...
/cm at 25 °C. Water can also be electrolyzed
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
into oxygen and hydrogen gases but in the absence of dissolved ions this is a very slow process, as very little current is conducted. While electrons are the primary charge carriers in water (and metals), in ice the primary charge carriers are protons (see proton conductor
Proton conductor
A proton conductor is an electrolyte, typically a solid electrolyte, in which H+ are the primary charge carriers.-Composition:For practical applications, proton conductors are usually solid materials. Typical materials are polymers or ceramic. Typically the pores in practical materials are small...
).
Electrolysis
Water can be split into its constituent elements, hydrogen and oxygen, by passing an electric current through it. This process is called electrolysisElectrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
. Water molecules naturally dissociate into and ions, which are attracted toward the cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
and anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
, respectively. At the cathode, two ions pick up electrons and form gas. At the anode, four ions combine and release gas, molecular water, and four electrons. The gases produced bubble to the surface, where they can be collected. The standard potential of the water electrolysis cell is 1.23 V at 25 °C.
Static dielectric constant
| temperature /°C | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ε | 87.9 | 83.95 | 80.18 | 76.58 | 73.18 | 69.88 | 66.76 | 63.78 | 60.93 | 58.2 | 55.58 |
Polarity and hydrogen bonding
An important feature of water is its polar nature. The water molecule forms an angle, with hydrogen atoms at the tips and oxygen at the vertex. Since oxygen has a higher electronegativityElectronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
than hydrogen, the side of the molecule with the oxygen atom has a partial negative charge. An object with such a charge difference is called a dipole
Dipole
In physics, there are several kinds of dipoles:*An electric dipole is a separation of positive and negative charges. The simplest example of this is a pair of electric charges of equal magnitude but opposite sign, separated by some distance. A permanent electric dipole is called an electret.*A...
meaning two poles. The oxygen end is partially negative and the hydrogen end is partially positive, because of this the direction of the dipole moment
Dipole moment
Dipole moment can be defined as the product of magnitude of charge & distance of separation between the charges.Dipole moment may refer to:*Electric dipole moment, the measure of the electrical polarity of a system of charges...
points towards the oxygen. The charge differences cause water molecules to be attracted to each other (the relatively positive areas being attracted to the relatively negative areas) and to other polar molecules. This attraction contributes to hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
ing, and explains many of the properties of water, such as solvent action.
A water molecule can form a maximum of four hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
s because it can accept two and donate two hydrogen atoms. Other molecules like hydrogen fluoride
Hydrogen fluoride
Hydrogen fluoride is a chemical compound with the formula HF. This colorless gas is the principal industrial source of fluorine, often in the aqueous form as hydrofluoric acid, and thus is the precursor to many important compounds including pharmaceuticals and polymers . HF is widely used in the...
, ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
, methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
form hydrogen bonds but they do not show anomalous behavior of thermodynamic
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
, kinetic
Kinetic theory
The kinetic theory of gases describes a gas as a large number of small particles , all of which are in constant, random motion. The rapidly moving particles constantly collide with each other and with the walls of the container...
or structural properties like those observed in water. The answer to the apparent difference between water and other hydrogen bonding liquids lies in the fact that apart from water none of the hydrogen bonding molecules can form four hydrogen bonds, either due to an inability to donate/accept hydrogens or due to steric effects in bulky residues. In water, local tetrahedral order due to the four hydrogen bonds gives rise to an open structure and a 3-dimensional bonding network, resulting in the anomalous decrease of density when cooled below 4 °C.
Although hydrogen bonding is a relatively weak attraction compared to the covalent bonds within the water molecule itself, it is responsible for a number of water's physical properties. One such property is its relatively high melting
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
and boiling point
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
temperatures; more energy is required to break the hydrogen bonds between molecules. The similar compound hydrogen sulfide , which has much weaker hydrogen bonding, is a gas at room temperature
Room temperature
-Comfort levels:The American Society of Heating, Refrigerating and Air-Conditioning Engineers has listings for suggested temperatures and air flow rates in different types of buildings and different environmental circumstances. For example, a single office in a building has an occupancy ratio per...
even though it has twice the molecular mass of water. The extra bonding between water molecules also gives liquid water a large specific heat capacity. This high heat capacity makes water a good heat storage medium (coolant) and heat shield.
Cohesion and adhesion

Cohesion (chemistry)
Cohesion or cohesive attraction or cohesive force is the action or property of like molecules sticking together, being mutually attractive...
), due to the collective action of hydrogen bonds between water molecules. These hydrogen bonds are constantly breaking, with new bonds being formed with different water molecules; but at any given time in a sample of liquid water, a large portion of the molecules are held together by such bonds.
Water also has high adhesion
Adhesion
Adhesion is any attraction process between dissimilar molecular species that can potentially bring them in close contact. By contrast, cohesion takes place between similar molecules....
properties because of its polar nature. On extremely clean/smooth glass
Glass
Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
the water may form a thin film because the molecular forces between glass and water molecules (adhesive forces) are stronger than the cohesive forces.
In biological cells and organelle
Organelle
In cell biology, an organelle is a specialized subunit within a cell that has a specific function, and is usually separately enclosed within its own lipid bilayer....
s, water is in contact with membrane and protein surfaces that are hydrophilic; that is, surfaces that have a strong attraction to water. Irving Langmuir
Irving Langmuir
Irving Langmuir was an American chemist and physicist. His most noted publication was the famous 1919 article "The Arrangement of Electrons in Atoms and Molecules" in which, building on Gilbert N. Lewis's cubical atom theory and Walther Kossel's chemical bonding theory, he outlined his...
observed a strong repulsive force between hydrophilic surfaces. To dehydrate hydrophilic surfaces—to remove the strongly held layers of water of hydration—requires doing substantial work against these forces, called hydration forces. These forces are very large but decrease rapidly over a nanometer or less. They are important in biology, particularly when cells are dehydrated by exposure to dry atmospheres or to extracellular freezing.
Surface tension

Surface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
of 72.8 mN/m at room temperature
Room temperature
-Comfort levels:The American Society of Heating, Refrigerating and Air-Conditioning Engineers has listings for suggested temperatures and air flow rates in different types of buildings and different environmental circumstances. For example, a single office in a building has an occupancy ratio per...
, caused by the strong cohesion between water molecules, the highest of the non-metallic liquids. This can be seen when small quantities of water are placed onto a sorption
Sorption
Sorption refers to the action of absorption* Absorption is the incorporation of a substance in one state into another of a different state ....
-free (non-adsorbent and non-absorbent) surface, such as polyethylene
Polyethylene
Polyethylene or polythene is the most widely used plastic, with an annual production of approximately 80 million metric tons...
or Teflon, and the water stays together as drops. Just as significantly, air trapped in surface disturbances forms bubbles, which sometimes last long enough to transfer gas molecules to the water.
Another surface tension effect is capillary wave
Capillary wave
A capillary wave is a wave traveling along the phase boundary of a fluid, whose dynamics are dominated by the effects of surface tension.Capillary waves are common in nature and the home, and are often referred to as ripples...
s, which are the surface ripples that form around the impacts of drops on water surfaces, and sometimes occur with strong subsurface currents flowing to the water surface. The apparent elasticity caused by surface tension drives the waves.
Capillary action
Due to an interplay of the forces of adhesion and surface tension, water exhibits capillary actionCapillary action
Capillary action, or capilarity, is the ability of a liquid to flow against gravity where liquid spontanously rise in a narrow space such as between the hair of a paint-brush, in a thin tube, or in porous material such as paper or in some non-porous material such as liquified carbon fiber, or in a...
whereby water rises into a narrow tube against the force of gravity. Water adheres to the inside wall of the tube and surface tension tends to straighten the surface causing a surface rise and more water is pulled up through cohesion. The process continues as the water flows up the tube until there is enough water such that gravity balances the adhesive force.
Surface tension and capillary action are important in biology. For example, when water is carried through xylem
Xylem
Xylem is one of the two types of transport tissue in vascular plants. . The word xylem is derived from the Classical Greek word ξυλον , meaning "wood"; the best-known xylem tissue is wood, though it is found throughout the plant...
up stems in plants, the strong intermolecular attractions (cohesion) hold the water column together and adhesive properties maintain the water attachment to the xylem and prevent tension rupture caused by transpiration pull.
Water as a solvent

Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
due to its polarity. Substances that will mix well and dissolve in water (e.g. salts) are known as hydrophilic ("water-loving") substances, while those that do not mix well with water (e.g. fats and oils), are known as hydrophobic ("water-fearing") substances. The ability of a substance to dissolve in water is determined by whether or not the substance can match or better the strong attractive forces that water molecules generate between other water molecules. If a substance has properties that do not allow it to overcome these strong intermolecular forces, the molecules are "pushed out
Precipitation (chemistry)
Precipitation is the formation of a solid in a solution or inside anothersolid during a chemical reaction or by diffusion in a solid. When the reaction occurs in a liquid, the solid formed is called the precipitate, or when compacted by a centrifuge, a pellet. The liquid remaining above the solid...
" from the water, and do not dissolve. Contrary to the common misconception, water and hydrophobic substances do not "repel", and the hydration of a hydrophobic surface is energetically, but not entropically, favorable.
When an ionic or polar compound enters water, it is surrounded by water molecules (Hydration
Solvation
Solvation, also sometimes called dissolution, is the process of attraction and association of molecules of a solvent with molecules or ions of a solute...
). The relatively small size of water molecules typically allows many water molecules to surround one molecule of solute
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
. The partially negative dipole ends of the water are attracted to positively charged components of the solute, and vice versa for the positive dipole ends.
In general, ionic and polar substances such as acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
s, alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s, and salt
Salt
In chemistry, salts are ionic compounds that result from the neutralization reaction of an acid and a base. They are composed of cations and anions so that the product is electrically neutral...
s are relatively soluble in water, and non-polar substances such as fats and oils are not. Non-polar molecules stay together in water because it is energetically more favorable for the water molecules to hydrogen bond to each other than to engage in van der Waals interactions
Van der Waals force
In physical chemistry, the van der Waals force , named after Dutch scientist Johannes Diderik van der Waals, is the sum of the attractive or repulsive forces between molecules other than those due to covalent bonds or to the electrostatic interaction of ions with one another or with neutral...
with non-polar molecules.
An example of an ionic solute is table salt
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
; the sodium chloride, NaCl, separates into cations and anions, each being surrounded by water molecules. The ions are then easily transported away from their crystalline lattice into solution. An example of a nonionic solute is table sugar
Sucrose
Sucrose is the organic compound commonly known as table sugar and sometimes called saccharose. A white, odorless, crystalline powder with a sweet taste, it is best known for its role in human nutrition. The molecule is a disaccharide composed of glucose and fructose with the molecular formula...
. The water dipoles make hydrogen bonds with the polar regions of the sugar molecule (OH groups) and allow it to be carried away into solution.
Water in acid-base reactions
Chemically, water is amphoteric: it can act as either an acidAcid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
or a base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
in chemical reactions. According to the Brønsted-Lowry
Brønsted-Lowry
In chemistry, the Brønsted–Lowry theory is an acid-base theory, proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923...
definition, an acid is defined as a species which donates a proton (a ion) in a reaction, and a base as one which receives a proton. When reacting with a stronger acid, water acts as a base; when reacting with a stronger base, it acts as an acid. For instance, water receives an ion from HCl when hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
is formed:
- HCl (acid) + (base) +
In the reaction with ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
, , water donates a ion, and is thus acting as an acid:
- (base) + (acid) +
Because the oxygen atom in water has two lone pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
s, water often acts as a Lewis base, or electron pair donor, in reactions with Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
s, although it can also react with Lewis bases, forming hydrogen bonds between the electron pair donors and the hydrogen atoms of water. HSAB theory
HSAB theory
The HSAB concept is an acronym for 'hard and soft acids and bases. Also known as the Pearson acid base concept, HSAB is widely used in chemistry for explaining stability of compounds, reaction mechanisms and pathways....
describes water as both a weak hard acid and a weak hard base, meaning that it reacts preferentially with other hard species:
- (Lewis acid) + (Lewis base) →
- (Lewis acid) + (Lewis base) →
- (Lewis base) + (Lewis acid) →
When a salt of a weak acid or of a weak base is dissolved in water, water can partially hydrolyze
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
the salt, producing the corresponding base or acid, which gives aqueous solutions of soap
Soap
In chemistry, soap is a salt of a fatty acid.IUPAC. "" Compendium of Chemical Terminology, 2nd ed. . Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford . XML on-line corrected version: created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. ISBN...
and baking soda their basic pH:
- + NaOH +
Ligand chemistry
Water's Lewis base character makes it a common ligandLigand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
in transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
complexes, examples of which range from solvated ions, such as , to perrhenic acid
Perrhenic acid
Perrhenic acid is the chemical compound with the formula Re2O72. It is obtained by evaporating aqueous solutions of Re2O7. Conventionally, perrhenic acid is considered to have the formula HReO4, and a species of this formula forms when rhenium oxide sublimes in the presence of water or steam...
, which contains two water molecules coordinated to a rhenium
Rhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-white, heavy, third-row transition metal in group 7 of the periodic table. With an average concentration of 1 part per billion , rhenium is one of the rarest elements in the Earth's crust. The free element has...
atom, to various solid hydrates, such as . Water is typically a monodentate ligand, it forms only one bond with the central atom.
Organic chemistry
As a hard base, water reacts readily with organic carbocationCarbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
s, for example in hydration reaction
Hydration reaction
In organic chemistry, a hydration reaction is a chemical reaction in which a hydroxyl group and a hydrogen cation are added to the two carbon atoms bonded together in the carbon-carbon double bond which makes up an alkene functional group. The reaction usually runs in a strong acidic, aqueous...
, in which a hydroxyl group and an acidic proton are added to the two carbon atoms bonded together in the carbon-carbon double bond, resulting in an alcohol. When addition of water to an organic molecule cleaves the molecule in two, hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
is said to occur. Notable examples of hydrolysis are saponification
Saponification
Saponification is a process that produces soap, usually from fats and lye. In technical terms, saponification involves base hydrolysis of triglycerides, which are esters of fatty acids, to form the sodium salt of a carboxylate. In addition to soap, such traditional saponification processes...
of fats and digestion
Digestion
Digestion is the mechanical and chemical breakdown of food into smaller components that are more easily absorbed into a blood stream, for instance. Digestion is a form of catabolism: a breakdown of large food molecules to smaller ones....
of proteins and polysaccharides. Water can also be a leaving group
Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
in SN2 substitution
SN2 reaction
The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
and E2 elimination
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
reactions, the latter is then known as dehydration reaction
Dehydration reaction
In chemistry and the biological sciences, a dehydration reaction is usually defined as a chemical reaction that involves the loss of water from the reacting molecule. Dehydration reactions are a subset of elimination reactions...
.
Acidity in nature
Pure water has the concentration of hydroxideHydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
ions equal to that of the hydronium
Hydronium
In chemistry, a hydronium ion is the cation , a type of oxonium ion produced by protonation of water. This cation is often used to represent the nature of the proton in aqueous solution, where the proton is highly solvated...
or hydrogen ions, which gives pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
of 7 at 298 K. In practice, pure water is very difficult to produce. Water left exposed to air for any length of time will dissolve carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, forming a dilute solution of carbonic acid
Carbonic acid
Carbonic acid is the inorganic compound with the formula H2CO3 . It is also a name sometimes given to solutions of carbon dioxide in water, because such solutions contain small amounts of H2CO3. Carbonic acid forms two kinds of salts, the carbonates and the bicarbonates...
, with a limiting pH of about 5.7. As cloud droplets form in the atmosphere and as raindrops fall through the air minor amounts of are absorbed, and thus most rain is slightly acidic. If high amounts of nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
and sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
oxides are present in the air, they too will dissolve into the cloud and rain drops, producing acid rain
Acid rain
Acid rain is a rain or any other form of precipitation that is unusually acidic, meaning that it possesses elevated levels of hydrogen ions . It can have harmful effects on plants, aquatic animals, and infrastructure. Acid rain is caused by emissions of carbon dioxide, sulfur dioxide and nitrogen...
.
Water in redox reactions
Water contains hydrogen in oxidation stateOxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
+1 and oxygen in oxidation state −2. Because of that, water oxidizes chemicals with reduction potential
Reduction potential
Reduction potential is a measure of the tendency of a chemical species to acquire electrons and thereby be reduced. Reduction potential is measured in volts , or millivolts...
below the potential of /, such as hydrides, alkali
Alkali metal
The alkali metals are a series of chemical elements in the periodic table. In the modern IUPAC nomenclature, the alkali metals comprise the group 1 elements, along with hydrogen. The alkali metals are lithium , sodium , potassium , rubidium , caesium , and francium...
and alkaline earth
Alkaline earth metal
The alkaline earth metals are a group in the periodic table. In the modern IUPAC nomenclature, the alkaline earth metals are called the group 2 elements. Previously, they were called the Group IIA elements . The alkaline earth metals contain beryllium , magnesium , calcium , strontium , barium and...
metals (except for beryllium
Beryllium
Beryllium is the chemical element with the symbol Be and atomic number 4. It is a divalent element which occurs naturally only in combination with other elements in minerals. Notable gemstones which contain beryllium include beryl and chrysoberyl...
), etc. Some other reactive metals, such as aluminum, are oxidized by water as well, but their oxides are not soluble, and the reaction stops because of passivation
Passivation
Passivation is the process of making a material "passive", and thus less reactive with surrounding air, water, or other gases or liquids. The goal is to inhibit corrosion, whether for structural or cosmetic reasons. Passivation of metals is usually achieved by the deposition of a layer of oxide...
. Note, however, that rusting of iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
is a reaction between iron and oxygen, dissolved in water, not between iron and water.
- 2 Na + 2 → 2 NaOH +
Water can be oxidized itself, emitting oxygen gas, but very few oxidants react with water even if their reduction potential is greater than the potential of . Almost all such reactions require a catalyst
- 4 + 2 → 4 AgF + 4 HF +
Geochemistry
Action of water on rock over long periods of time typically leads to weatheringWeathering
Weathering is the breaking down of rocks, soils and minerals as well as artificial materials through contact with the Earth's atmosphere, biota and waters...
and water erosion, physical processes that convert solid rocks and minerals into soil and sediment, but under some conditions chemical reactions with water occur as well, resulting in metasomatism
Metasomatism
Metasomatism is the chemical alteration of a rock by hydrothermal and other fluids.Metasomatism can occur via the action of hydrothermal fluids from an igneous or metamorphic source. In the igneous environment, metasomatism creates skarns, greisen, and may affect hornfels in the contact...
or mineral hydration
Mineral hydration
Mineral hydration is an inorganic chemical reaction where water is added to the crystal structure of a mineral, usually creating a new mineral, usually called a hydrate....
, a type of chemical alteration of a rock which produces clay minerals
Clay minerals
Clay minerals are hydrous aluminium phyllosilicates, sometimes with variable amounts of iron, magnesium, alkali metals, alkaline earths, and other cations. Clays have structures similar to the micas and therefore form flat hexagonal sheets. Clay minerals are common weathering products and low...
in nature and also occurs when Portland cement
Portland cement
Portland cement is the most common type of cement in general use around the world because it is a basic ingredient of concrete, mortar, stucco and most non-specialty grout...
hardens.
Water ice can form clathrate compounds, known as clathrate hydrates, with a variety of small molecules that can be embedded in its spacious crystal lattice. The most notable of these is methane clathrate
Methane clathrate
Methane clathrate, also called methane hydrate, hydromethane, methane ice, "fire ice", natural gas hydrate or just gas hydrate, is a solid clathrate compound in which a large amount of methane is trapped within a crystal structure of water, forming a solid similar to ice...
, 4, naturally found in large quantities on the ocean floor.
Transparency
Water is relatively transparent to visible light, near ultraviolet light, and far-redFar-red
Far-red light is light at the extreme red end of the visible spectrum, between red and infra-red light. Usually regarded as the region between 700 and 800 nm wavelength, it is dimly visible to some eyes. It is reflected or transmitted by plants because of the absorbance spectrum of...
light, but it absorbs most ultraviolet light, infrared light, and microwave
Microwave
Microwaves, a subset of radio waves, have wavelengths ranging from as long as one meter to as short as one millimeter, or equivalently, with frequencies between 300 MHz and 300 GHz. This broad definition includes both UHF and EHF , and various sources use different boundaries...
s. Most photoreceptor
Photoreceptor
A photoreceptor cell is a specialized type of neuron found in the eye's retina that is capable of phototransduction. The great biological importance of photoreceptors is that they convert light into signals that can stimulate biological processes...
s and photosynthetic pigment
Photosynthetic pigment
A photosynthetic pigment is a pigment that is present in chloroplasts or photosynthetic bacteria and captures the light energy necessary for photosynthesis.- Plants :...
s utilize the portion of the light spectrum that is transmitted well through water. Microwave ovens take advantage of water's opacity to microwave radiation to heat the water inside of foods. The very weak onset of absorption in the red end of the visible spectrum lends water its intrinsic blue hue (see Color of water
Color of water
The color of water is a subject of both scientific study and popular misconception. While relatively small quantities of water are observed by humans to be colorless, pure water has a slight blue color that becomes a deeper blue as the thickness of the observed sample increases...
).
Heavy water and isotopologues
Several isotopeIsotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s of both hydrogen and oxygen exist, giving rise to several known isotopologue
Isotopologue
Isotopologues are molecules that differ only in their isotopic composition. Simply, the isotopologue of a chemical species has at least one atom with a different number of neutrons than the parent....
s of water.
Hydrogen occurs naturally in three isotopes. The most common (1H) accounting for more than 99.98% of hydrogen in water, consists of only a single proton in its nucleus. A second, stable isotope, deuterium
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
(chemical symbol D or 2H), has an additional neutron. Deuterium oxide, , is also known as heavy water
Heavy water
Heavy water is water highly enriched in the hydrogen isotope deuterium; e.g., heavy water used in CANDU reactors is 99.75% enriched by hydrogen atom-fraction...
because of its higher density. It is used in nuclear reactor
Nuclear reactor
A nuclear reactor is a device to initiate and control a sustained nuclear chain reaction. Most commonly they are used for generating electricity and for the propulsion of ships. Usually heat from nuclear fission is passed to a working fluid , which runs through turbines that power either ship's...
s as a neutron moderator
Neutron moderator
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, thereby turning them into thermal neutrons capable of sustaining a nuclear chain reaction involving uranium-235....
. The third isotope, tritium
Tritium
Tritium is a radioactive isotope of hydrogen. The nucleus of tritium contains one proton and two neutrons, whereas the nucleus of protium contains one proton and no neutrons...
, has 1 proton and 2 neutrons, and is radioactive, decaying with a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 4500 days. exists in nature only in minute quantities, being produced primarily via cosmic ray-induced nuclear reactions in the atmosphere. Water with one deuterium atom occurs naturally in ordinary water in low concentrations (~0.03%) and in far lower amounts (0.000003%).
The most notable physical differences between and , other than the simple difference in specific mass, involve properties that are affected by hydrogen bonding, such as freezing and boiling, and other kinetic effects. The difference in boiling points allows the isotopologues to be separated.
Consumption of pure isolated may affect biochemical processes – ingestion of large amounts impairs kidney and central nervous system function. Small quantities can be consumed without any ill-effects, and even very large amounts of heavy water must be consumed for any toxicity to become apparent.
Oxygen also has three stable isotopes, with present in 99.76%, in 0.04%, and in 0.2% of water molecules.
Liquid crystal state in the exclusion zone
Near hydrophilicHydrophile
A hydrophile, from the Greek "water" and φιλια "love," is a molecule or other molecular entity that is attracted to, and tends to be dissolved by water. A hydrophilic molecule or portion of a molecule is one that has a tendency to interact with or be dissolved by, water and other polar substances...
surfaces, water exists in a liquid crystal
Liquid crystal
Liquid crystals are a state of matter that have properties between those of a conventional liquid and those of a solid crystal. For instance, an LC may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many different types of LC phases, which can be...
state. This liquid crystal state has the following properties:
- the water molecules are constrained in movement (as shown by nuclear magnetic resonance imagery)
- it is more stable (as shown by infrared radiation imagery)
- it has a negative charge (as shown by a test of its electric potential)
- it absorbs at 270 nm (as shown by light absorption imagery)
- it is more viscous than liquid water (as shown by falling ball viscometry)
- the molecules are aligned (as shown by polarizing microscopy)
Gerald Pollack speculated that this liquid crystal zone remained relatively unexplored recently, despite extensive writing on this topic up through 1949, because of the polywater
Polywater
Polywater was a hypothetical polymerized form of water that was the subject of much scientific controversy during the late 1960s. By 1969 the popular press had taken notice, and by 1970 doubts about its authenticity were being circulated. By 1973 it was found to be illusory...
and water memory
Water memory
Water memory is the claimed ability of water to retain a "memory" of substances previously dissolved in it to arbitrary dilution. No scientific evidence supports this claim. Shaking the water at each stage of a serial dilution is claimed to be necessary for an effect to occur...
debacles.
History
The first decomposition of water into hydrogen and oxygen, by electrolysisElectrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
, was done in 1800 by an English chemist William Nicholson
William Nicholson (chemist)
William Nicholson was a renowned English chemist and writer on "natural philosophy" and chemistry, as well as a translator, journalist, publisher, scientist, and inventor.-Early life:...
. In 1805, Joseph Louis Gay-Lussac
Joseph Louis Gay-Lussac
- External links :* from the American Chemical Society* from the Encyclopædia Britannica, 10th Edition * , Paris...
and Alexander von Humboldt
Alexander von Humboldt
Friedrich Wilhelm Heinrich Alexander Freiherr von Humboldt was a German naturalist and explorer, and the younger brother of the Prussian minister, philosopher and linguist Wilhelm von Humboldt...
showed that water is composed of two parts hydrogen and one part oxygen.
Gilbert Newton Lewis isolated the first sample of pure heavy water
Heavy water
Heavy water is water highly enriched in the hydrogen isotope deuterium; e.g., heavy water used in CANDU reactors is 99.75% enriched by hydrogen atom-fraction...
in 1933.
The properties of water have historically been used to define various temperature scales. Notably, the Kelvin
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
, Celsius
Celsius
Celsius is a scale and unit of measurement for temperature. It is named after the Swedish astronomer Anders Celsius , who developed a similar temperature scale two years before his death...
, Rankine, and Fahrenheit
Fahrenheit
Fahrenheit is the temperature scale proposed in 1724 by, and named after, the German physicist Daniel Gabriel Fahrenheit . Within this scale, the freezing of water into ice is defined at 32 degrees, while the boiling point of water is defined to be 212 degrees...
scales were, or currently are, defined by the freezing and boiling points of water. The less common scales of Delisle
Delisle scale
The Delisle scale is a temperature scale invented in 1732 by the French astronomer Joseph-Nicolas Delisle . Delisle was the author of Mémoires pour servir à l'histoire et aux progrès de l'Astronomie, de la Géographie et de la Physique .He had been invited to Russia by Peter the Great...
, Newton
Newton scale
The Newton scale is a temperature scale devised by Isaac Newton around 1700. Applying his mind to the problem of heat, he elaborated a first qualitative temperature scale, comprising about twenty reference points ranging from "cold air in winter" to "glowing coals in the kitchen fire". This...
, Réaumur and Rømer
Rømer scale
Rømer is a temperature scale named after the Danish astronomer Ole Christensen Rømer, who proposed it in 1701.In this scale, the zero was initially set using freezing brine. The boiling point of water was defined as 60 degrees...
were defined similarly. The triple point
Triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases of that substance coexist in thermodynamic equilibrium...
of water is a more commonly used standard point today.
Systematic naming
The accepted IUPACIUPAC nomenclature of inorganic chemistry
The IUPAC nomenclature of inorganic chemistry is a systematic method of naming inorganic chemical compounds, as recommended by the International Union of Pure and Applied Chemistry . The rules are commonly known as "The Red Book"...
name of water is oxidane or simply water, or its equivalent in different languages, although there are other systematic names which can be used to describe the molecule.
The simplest systematic name of water is hydrogen oxide. This is analogous to related compounds such as hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
, hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
, and deuterium oxide (heavy water). Another systematic name, oxidane, is accepted by IUPAC as a parent name for the systematic naming of oxygen-based substituent groups, although even these commonly have other recommended names. For example, the name hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
is recommended over oxidanyl for the –OH group. The name oxane is explicitly mentioned by the IUPAC as being unsuitable for this purpose, since it is already the name of a cyclic ether also known as tetrahydropyran
Tetrahydropyran
Tetrahydropyran is the organic compound consisting of a saturated six-membered ring containing five carbon atoms and one oxygen atom. The compound is a colourless volatile liquid, but is obscure. Derivatives of tetrahydropyran are, however, more common. Tetrahydropyranyl ethers derived from the...
.
The polarized form of the water molecule, H+OH−, is also called hydron hydroxide by IUPAC nomenclature.
Dihydrogen monoxide (DHMO) is a rarely used name of water. This term has been used in various hoaxes that call for this "lethal chemical" to be banned, such as in the dihydrogen monoxide hoax
Dihydrogen monoxide hoax
In the dihydrogen monoxide hoax, water is called by an unfamiliar name, "dihydrogen monoxide", followed by a listing of real negative effects of this chemical, in an attempt to convince people that it should be carefully regulated, labeled as hazardous, or banned...
. Other systematic names for water include hydroxic acid, hydroxylic acid, and hydrogen hydroxide. Both acid and alkali names exist for water because it is amphoteric
Amphoterism
In chemistry, an amphoteric species is a molecule or ion that can react as an acid as well as a base. The word is derived from the Greek word amphoteroi meaning "both"...
(able to react both as an acid or an alkali). None of these exotic names are used widely.
See also
- Double distilled water
- Flexible SPC water modelFlexible SPC water modelThe Flexible Simple Point Charge water model is a re-parametrization of the three-site SPC water model. The SPC model is rigid, whilst the flexible SPC model is flexible. In the model of Toukan and Rahman, the O-H stretching is made anharmonic and thus the dynamical behavior is well described...
- Hydrodynamics
- Optical properties of water and iceOptical properties of water and iceThe refractive index of water at 20°C is 1.332986. The refractive index of normal ice is 1.31. In general, an index of refraction is a complex number with both a real and imaginary part, where the latter indicates the strength of absorption loss at a particular wavelength...
- Superheated waterSuperheated waterSuperheated water is liquid water under pressure at temperatures between the usual boiling point and the critical temperature . It is also known as subcritical water and pressurized hot water...
- Vienna Standard Mean Ocean Water
- Viscosity of Water
- Water (data page)Water (data page)This page provides supplementary data of the properties of water.Further comprehensive authoritative data can be found at the page on thermophysical properties of fluids.-Structure and properties:-Thermodynamic properties:-Liquid physical properties:...
- Water absorptionWater absorptionDuring the transmission of electromagnetic radiation through a medium containing water molecules, portions of the electromagnetic spectrum are absorbed by water molecules...
of electromagnetic radiationElectromagnetic radiationElectromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space... - Water clusterWater clusterIn chemistry a water cluster is a discrete hydrogen bonded assembly or cluster of molecules of water. These clusters have been found experimentally or predicted in silico in various forms of water; in ice, in crystal lattices and in bulk liquid water, the simplest one being the water dimer 2...
- Water dimerWater dimerThe water dimer consists of two water molecules loosely bound by a hydrogen bond. It is the smallest water cluster. Because it is the simplest model system for studying hydrogen bonding in water, it has been the target of so many theoretical studies that it has been called "a theoretical Guinea...
- Water modelWater modelIn computational chemistry, classical water models are used for the simulation of water clusters, liquid water, and aqueous solutions with explicit solvent. These models use the approximations of molecular mechanics...
External links
- Release on the IAPWS Industrial Formulation 1997 for the Thermodynamic Properties of Water and Steam (fast computation speed)
- Release on the IAPWS Formulation 1995 for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use (simpler formulation)
- Online calculator using the IAPWS Supplementary Release on Properties of Liquid Water at 0.1 MPa, September 2008
- Sigma Xi The Scientific Research Society, Year of Water 2008
- Stockholm International Water Institute (SIWI)
- Calculation of vapor pressure, liquid density, dynamic liquid viscosity, surface tension of water
- Water Density Calculator
- Why does ice float in my drink?, NASANASAThe National Aeronautics and Space Administration is the agency of the United States government that is responsible for the nation's civilian space program and for aeronautics and aerospace research...

