Hydrolysis
Encyclopedia
Hydrolysis is a chemical reaction
during which molecules of water (H2O) are split into hydrogen
cations (H+, conventionally referred to as proton
s) and hydroxide
anions (OH−) in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymer
s, especially those made by condensation polymerization. Such polymer degradation
is usually catalysed by either acid
, e.g., concentrated sulfuric acid
(H2SO4), or alkali
, e.g., sodium hydroxide (NaOH).
(H+) from the split water molecule. The other portion of the target molecule collects the hydroxyl
group (OH−) of the split water molecule. In effect an acid and a base are formed.
The most common hydrolysis occurs when a salt of a weak acid
or weak base
(or both) is dissolved in water. Water spontaneously ionizes into hydroxyl anions and hydrogen cations. The salt, too, dissociates into its constituent anions and cations. For example, sodium acetate
dissociates in water into sodium and acetate
ions. Sodium ions react very little with the hydroxyl ions whereas the acetate ions combine with hydrogen ions to produce acetic acid
. In this case the net result is a relative excess of hydroxyl ions, causing a basic solution
.
However, under normal conditions, only a few reactions between water and organic compound
s occur. In general, strong acid
s or strong bases must be added to catalyze hydrolysis.
Acid–base-catalyzed hydrolyses are very common; one example is the hydrolysis of amide
s or ester
s. Their hydrolysis occurs when the nucleophile
(a nucleus-seeking agent, e.g., water or hydroxyl ion) attacks the carbon of the carbonyl group
of the ester
or amide
. In an aqueous base, hydroxyl ions are better nucleophiles than polar molecules such as water. In acids, the carbonyl group becomes protonated, and this leads to a much easier nucleophilic attack. The products for both hydrolyses are compounds with carboxylic acid
groups.
Perhaps the oldest example of ester hydrolysis is the process called saponification
(formation of soap). It is the hydrolysis of a triglyceride
(fat) with an aqueous base such as sodium hydroxide (NaOH). During the process, glycerol
is formed, and the fatty acid
s react with the base, converting them to salts. These salts are called soaps, commonly used in households.
Moreover, hydrolysis is an important process in plants and animals, the most significant example being energy metabolism and storage. All living cells require a continual supply of energy for two main purposes: for the biosynthesis
of micro and macromolecules, and for the active transport of ions and molecules across cell membranes. The energy derived from the oxidation of nutrients is not used directly but, by means of a complex and long sequence of reactions, it is channeled into a special energy-storage molecule, adenosine triphosphate
(ATP).
The ATP molecule contains pyrophosphate
linkages (bonds formed when two phosphate units are combined together) that release energy when needed. ATP can undergo hydrolysis in two ways: the removal of terminal phosphate to form adenosine diphosphate
(ADP) and inorganic phosphate, or the removal of a terminal diphosphate to yield adenosine monophosphate
(AMP) and pyrophosphate
. The latter usually undergoes further cleavage into its two constituent phosphates. This results in biosynthesis reactions, which usually occur in chains, that can be driven in the direction of synthesis when the phosphate bonds have undergone hydrolysis.
In addition, in living systems, most biochemical reactions (including ATP hydrolysis) take place during the catalysis of enzyme
s. The catalytic action of enzymes allows the hydrolysis of protein
s, fats, oils, and carbohydrate
s. As an example, one may consider protease
s (enzymes that aid digestion
by causing hydrolysis of peptide bond
s in protein
s). They catalyze the hydrolysis of interior peptide bonds in peptide chains, as opposed to exopeptidase
s (another class of enzymes, that catalyze the hydrolysis of terminal peptide bonds, liberating one free amino acid at a time).
However, proteases do not catalyze the hydrolysis of all kinds of proteins. Their action is stereo-selective: Only proteins with a certain tertiary structure will be targeted. As some kind of orienting force is needed to place the amide group in the proper position for catalysis. The necessary contacts between an enzyme and its substrates (proteins) are created because the enzyme folds in such a way as to form a crevice into which the substrate fits; the crevice also contains the catalytic groups. Therefore, proteins that do not fit into the crevice will not undergo hydrolysis. This specificity preserves the integrity of other proteins such as hormone
s, and therefore the biological system continues to function normally.
, it is converted into a carboxylic acid
and an amine
or ammonia
. The carboxylic acid has a hydroxyl group derived from a water molecule and the amine (or ammonia) gains the hydrogen ion.
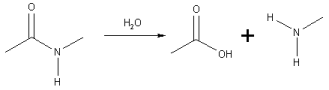
A specific case of the hydrolysis of an amide link is the hydrolysis of peptides
to smaller fragments or amino acid
s.
Many polyamide
polymers such as nylon 6,6 are attacked and hydrolysed in the presence of strong acids. Such attack leads to depolymerization
and nylon products fail by fracturing when exposed to even small amounts of acid. Other polymers made by step-growth polymerization
are susceptible to similar polymer degradation
reactions. The problem is known as stress corrosion cracking
.
 Monosaccharide
Monosaccharide
s can be linked together by glycosidic bond
s, which can be cleaved by hydrolysis. Two, three, several or many monosaccharides thus linked form disaccharide
s, trisaccharides, oligosaccharide
s or polysaccharide
s, respectively. Enzymes that hydrolyse glycosidic bonds are called "glycoside hydrolase
s" or "glycosidases".
The best-known disaccharide is sucrose
(table sugar). Hydrolysis of sucrose yields glucose
and fructose
. Invertase
is a sucrase
used industrially for the hydrolysis of sucrose to so-called invert sugar. Lactase
is essential for digestive hydrolysis of lactose
in milk. Deficiency of lactase in humans causes lactose intolerance
.
The hydrolysis of polysaccharides to soluble sugars is called "saccharification". Malt made from barley
is used as a source of β-amylase to break down starch
into the disaccharide maltose
, which can be used by yeast to produce beer
. Other amylase
enzymes may convert starch to glucose or to oligosaccharides. Cellulose
is converted to glucose or the disaccharide cellobiose
by cellulase
s. Animals such as cows (ruminants) are able to digest cellulose because of symbiotic bacteria that produce cellulases.
irreversible. To give an example:
Assuming that x is the final concentration of products, and that C is the initial concentration of A, and W = [H2O] = 55.5 molar, then x can be calculated with the equation:
let Kd×W = k:
then
For a value of C = 0.001 molar, and k = 1 molar, x/C > 0.999. Less than 0.1% of the original reactant would be present once the reaction is complete.
This theme of physiological irreversibility of hydrolysis is used consistently in metabolic pathways, since many biological processes are driven by the cleavage of anhydrous pyrophosphate
bonds.
s, and in aqueous solution they form aqua ions
, of the general formula M(H2O)nm+.
The aqua ions undergo hydrolysis, to a greater or lesser extent. The first hydrolysis step is given generically as
Thus the aqua ion is behaving as an acid in terms of Brønsted-Lowry acid-base theory. This is easily explained by considering the inductive effect
of the positively charged metal ion, which weakens the O-H bond of an attached water molecule, making the liberation of a proton relatively easy.
The dissociation constant
, pKa, for this reaction is more or less linearly related to the charge-to-size ratio of the metal ion. Ions with low charges, such as Na+ are very weak acids with almost imperceptible hydrolysis. Large divalent ions such as Ca2+, Zn2+, Sn2+ and Pb2+ have a pKa of 6 or more and would not normally be classed as acids, but small divalent ions such as Be2+ undergo extensive hydrolysis. Trivalent ions like Al3+ and Fe3+ are weak acids whose pKa is comparable to that of acetic acid
. Solutions of salts such as BeCl2 or Al(NO3)3 in water are noticeably acidic; the hydrolysis can be suppressed
by adding an acid such as nitric acid
, making the solution more acidic.
Hydrolysis may proceed beyond the first step, often with the formation of polynuclear species.
Some "exotic" species such as Sn3(OH)42+ are well characterized. Hydrolysis tends to increase as pH
rises leading, in many cases, to the precipitation of an hydroxide such as Al(OH)3 or AlO(OH). These substances, the major constituents of bauxite
, are known as laterite
s and are formed by leaching from rocks of most of the ions other than aluminium and iron and subsequent hydrolysis of the remaining aluminium and iron.
Ions with a formal charge of four have undergone extensive hydrolysis and salts of Zr4+, for example, can only be obtained from strongly acidic solutions. With oxidation states five and higher the concentration of the aqua ion in solution is negligible. In effect, the aqua ion is a strong acid. For example, aqueous solutions of Cr(VI) contain CrO42-.
Note that reactions such as
are formally hydrolysis reactions as water molecules are split up yielding hydrogen ions. Such reactions are common among polyoxometalate
s.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
during which molecules of water (H2O) are split into hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
cations (H+, conventionally referred to as proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s) and hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
anions (OH−) in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
s, especially those made by condensation polymerization. Such polymer degradation
Polymer degradation
Polymer degradation is a change in the properties—tensile strength, colour, shape, etc.—of a polymer or polymer-based product under the influence of one or more environmental factors such as heat, light or chemicals such as acids, alkalis and some salts...
is usually catalysed by either acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
, e.g., concentrated sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
(H2SO4), or alkali
Alkali
In chemistry, an alkali is a basic, ionic salt of an alkali metal or alkaline earth metal element. Some authors also define an alkali as a base that dissolves in water. A solution of a soluble base has a pH greater than 7. The adjective alkaline is commonly used in English as a synonym for base,...
, e.g., sodium hydroxide (NaOH).
Types
Hydrolysis is a chemical process in which a water molecule is added to a substance resulting in the split of that substance into two parts. One fragment of the target molecule (or parent molecule) gains a hydrogen ionHydrogen ion
Hydrogen ion is recommended by IUPAC as a general term for all ions of hydrogen and its isotopes.Depending on the charge of the ion, two different classes can be distinguished: positively charged ions and negatively charged ions....
(H+) from the split water molecule. The other portion of the target molecule collects the hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group (OH−) of the split water molecule. In effect an acid and a base are formed.
The most common hydrolysis occurs when a salt of a weak acid
Weak acid
A weak acid is an acid that dissociates incompletely. It does not release all of its hydrogens in a solution, donating only a partial amount of its protons to the solution...
or weak base
Weak base
In chemistry, a weak base is a chemical base that does not ionize fully in an aqueous solution. As Brønsted–Lowry bases are proton acceptors, a weak base may also be defined as a chemical base in which protonation is incomplete. This results in a relatively low pH compared to strong bases...
(or both) is dissolved in water. Water spontaneously ionizes into hydroxyl anions and hydrogen cations. The salt, too, dissociates into its constituent anions and cations. For example, sodium acetate
Sodium acetate
Sodium acetate, CH3COONa, also abbreviated NaOAc, also sodium ethanoate, is the sodium salt of acetic acid. This colourless salt has a wide range of uses.-Industrial:...
dissociates in water into sodium and acetate
Acetate
An acetate is a derivative of acetic acid. This term includes salts and esters, as well as the anion found in solution. Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In...
ions. Sodium ions react very little with the hydroxyl ions whereas the acetate ions combine with hydrogen ions to produce acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
. In this case the net result is a relative excess of hydroxyl ions, causing a basic solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
.
However, under normal conditions, only a few reactions between water and organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s occur. In general, strong acid
Strong acid
A strong acid is an acid that ionizes completely in an aqueous solution by losing one proton, according to the equationFor sulfuric acid which is diprotic, the "strong acid" designation refers only to dissociation of the first protonMore precisely, the acid must be stronger in aqueous solution than...
s or strong bases must be added to catalyze hydrolysis.
Acid–base-catalyzed hydrolyses are very common; one example is the hydrolysis of amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
s or ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s. Their hydrolysis occurs when the nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
(a nucleus-seeking agent, e.g., water or hydroxyl ion) attacks the carbon of the carbonyl group
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
of the ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
or amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
. In an aqueous base, hydroxyl ions are better nucleophiles than polar molecules such as water. In acids, the carbonyl group becomes protonated, and this leads to a much easier nucleophilic attack. The products for both hydrolyses are compounds with carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
groups.
Perhaps the oldest example of ester hydrolysis is the process called saponification
Saponification
Saponification is a process that produces soap, usually from fats and lye. In technical terms, saponification involves base hydrolysis of triglycerides, which are esters of fatty acids, to form the sodium salt of a carboxylate. In addition to soap, such traditional saponification processes...
(formation of soap). It is the hydrolysis of a triglyceride
Triglyceride
A triglyceride is an ester derived from glycerol and three fatty acids. There are many triglycerides, depending on the oil source, some are highly unsaturated, some less so....
(fat) with an aqueous base such as sodium hydroxide (NaOH). During the process, glycerol
Glycerol
Glycerol is a simple polyol compound. It is a colorless, odorless, viscous liquid that is widely used in pharmaceutical formulations. Glycerol has three hydroxyl groups that are responsible for its solubility in water and its hygroscopic nature. The glycerol backbone is central to all lipids...
is formed, and the fatty acid
Fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
s react with the base, converting them to salts. These salts are called soaps, commonly used in households.
Moreover, hydrolysis is an important process in plants and animals, the most significant example being energy metabolism and storage. All living cells require a continual supply of energy for two main purposes: for the biosynthesis
Biosynthesis
Biosynthesis is an enzyme-catalyzed process in cells of living organisms by which substrates are converted to more complex products. The biosynthesis process often consists of several enzymatic steps in which the product of one step is used as substrate in the following step...
of micro and macromolecules, and for the active transport of ions and molecules across cell membranes. The energy derived from the oxidation of nutrients is not used directly but, by means of a complex and long sequence of reactions, it is channeled into a special energy-storage molecule, adenosine triphosphate
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
(ATP).
The ATP molecule contains pyrophosphate
Pyrophosphate
In chemistry, the anion, the salts, and the esters of pyrophosphoric acid are called pyrophosphates. Any salt or ester containing two phosphate groups is called a diphosphate. As a food additive, diphosphates are known as E450.- Chemistry :...
linkages (bonds formed when two phosphate units are combined together) that release energy when needed. ATP can undergo hydrolysis in two ways: the removal of terminal phosphate to form adenosine diphosphate
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
(ADP) and inorganic phosphate, or the removal of a terminal diphosphate to yield adenosine monophosphate
Adenosine monophosphate
Adenosine monophosphate , also known as 5'-adenylic acid, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid and the nucleoside adenosine. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine...
(AMP) and pyrophosphate
Pyrophosphate
In chemistry, the anion, the salts, and the esters of pyrophosphoric acid are called pyrophosphates. Any salt or ester containing two phosphate groups is called a diphosphate. As a food additive, diphosphates are known as E450.- Chemistry :...
. The latter usually undergoes further cleavage into its two constituent phosphates. This results in biosynthesis reactions, which usually occur in chains, that can be driven in the direction of synthesis when the phosphate bonds have undergone hydrolysis.
In addition, in living systems, most biochemical reactions (including ATP hydrolysis) take place during the catalysis of enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s. The catalytic action of enzymes allows the hydrolysis of protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s, fats, oils, and carbohydrate
Carbohydrate
A carbohydrate is an organic compound with the empirical formula ; that is, consists only of carbon, hydrogen, and oxygen, with a hydrogen:oxygen atom ratio of 2:1 . However, there are exceptions to this. One common example would be deoxyribose, a component of DNA, which has the empirical...
s. As an example, one may consider protease
Protease
A protease is any enzyme that conducts proteolysis, that is, begins protein catabolism by hydrolysis of the peptide bonds that link amino acids together in the polypeptide chain forming the protein....
s (enzymes that aid digestion
Digestion
Digestion is the mechanical and chemical breakdown of food into smaller components that are more easily absorbed into a blood stream, for instance. Digestion is a form of catabolism: a breakdown of large food molecules to smaller ones....
by causing hydrolysis of peptide bond
Peptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
s in protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s). They catalyze the hydrolysis of interior peptide bonds in peptide chains, as opposed to exopeptidase
Exopeptidase
An exopeptidase is an enzyme produced in the pancreas that catalyses the removal of an amino acid from the end of a polypeptide chain. Exopeptidase cleaves the end of a polypeptide chain....
s (another class of enzymes, that catalyze the hydrolysis of terminal peptide bonds, liberating one free amino acid at a time).
However, proteases do not catalyze the hydrolysis of all kinds of proteins. Their action is stereo-selective: Only proteins with a certain tertiary structure will be targeted. As some kind of orienting force is needed to place the amide group in the proper position for catalysis. The necessary contacts between an enzyme and its substrates (proteins) are created because the enzyme folds in such a way as to form a crevice into which the substrate fits; the crevice also contains the catalytic groups. Therefore, proteins that do not fit into the crevice will not undergo hydrolysis. This specificity preserves the integrity of other proteins such as hormone
Hormone
A hormone is a chemical released by a cell or a gland in one part of the body that sends out messages that affect cells in other parts of the organism. Only a small amount of hormone is required to alter cell metabolism. In essence, it is a chemical messenger that transports a signal from one...
s, and therefore the biological system continues to function normally.
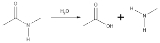
Hydrolysis of amide links
In the hydrolysis of an amideAmide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
, it is converted into a carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
and an amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
or ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
. The carboxylic acid has a hydroxyl group derived from a water molecule and the amine (or ammonia) gains the hydrogen ion.

A specific case of the hydrolysis of an amide link is the hydrolysis of peptides
Peptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
to smaller fragments or amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s.
Many polyamide
Polyamide
A polyamide is a polymer containing monomers of amides joined by peptide bonds. They can occur both naturally and artificially, examples being proteins, such as wool and silk, and can be made artificially through step-growth polymerization or solid-phase synthesis, examples being nylons, aramids,...
polymers such as nylon 6,6 are attacked and hydrolysed in the presence of strong acids. Such attack leads to depolymerization
Depolymerization
Depolymerization is the process of converting a polymer into a monomer or a mixture of monomers.Thioglycolysis, thiolysis and phloroglucinolysis are reactions used to study condensed tannins by means of their depolymerisation. Thioglycolysis is also used to study lignin....
and nylon products fail by fracturing when exposed to even small amounts of acid. Other polymers made by step-growth polymerization
Step-growth polymerization
Step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Many naturally occurring and some synthetic polymers are produced by step-growth...
are susceptible to similar polymer degradation
Polymer degradation
Polymer degradation is a change in the properties—tensile strength, colour, shape, etc.—of a polymer or polymer-based product under the influence of one or more environmental factors such as heat, light or chemicals such as acids, alkalis and some salts...
reactions. The problem is known as stress corrosion cracking
Stress corrosion cracking
Stress corrosion cracking is the unexpected sudden failure of normally ductile metals subjected to a tensile stress in a corrosive environment, especially at elevated temperature in the case of metals. SCC is highly chemically specific in that certain alloys are likely to undergo SCC only when...
.
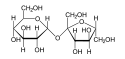
Hydrolysis of polysaccharides

Monosaccharide
Monosaccharides are the most basic units of biologically important carbohydrates. They are the simplest form of sugar and are usually colorless, water-soluble, crystalline solids. Some monosaccharides have a sweet taste. Examples of monosaccharides include glucose , fructose , galactose, xylose...
s can be linked together by glycosidic bond
Glycosidic bond
In chemistry, a glycosidic bond is a type of covalent bond that joins a carbohydrate molecule to another group, which may or may not be another carbohydrate....
s, which can be cleaved by hydrolysis. Two, three, several or many monosaccharides thus linked form disaccharide
Disaccharide
A disaccharide or biose is the carbohydrate formed when two monosaccharides undergo a condensation reaction which involves the elimination of a small molecule, such as water, from the functional groups only. Like monosaccharides, disaccharides form an aqueous solution when dissolved in water...
s, trisaccharides, oligosaccharide
Oligosaccharide
An oligosaccharide is a saccharide polymer containing a small number of component sugars, also known as simple sugars...
s or polysaccharide
Polysaccharide
Polysaccharides are long carbohydrate molecules, of repeated monomer units joined together by glycosidic bonds. They range in structure from linear to highly branched. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure,...
s, respectively. Enzymes that hydrolyse glycosidic bonds are called "glycoside hydrolase
Glycoside hydrolase
Glycoside hydrolases catalyze the hydrolysis of the glycosidic linkage to release smaller sugars...
s" or "glycosidases".
The best-known disaccharide is sucrose
Sucrose
Sucrose is the organic compound commonly known as table sugar and sometimes called saccharose. A white, odorless, crystalline powder with a sweet taste, it is best known for its role in human nutrition. The molecule is a disaccharide composed of glucose and fructose with the molecular formula...
(table sugar). Hydrolysis of sucrose yields glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
and fructose
Fructose
Fructose, or fruit sugar, is a simple monosaccharide found in many plants. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed directly into the bloodstream during digestion. Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847...
. Invertase
Invertase
Invertase is an enzyme that catalyzes the hydrolysis of sucrose . The resulting mixture of fructose and glucose is called inverted sugar syrup. Related to invertases are sucrases. Invertases and sucrases hydrolyze sucrose to give the same mixture of glucose and fructose...
is a sucrase
Sucrase
Sucrase is the name given to a number of enzymes that catalyze the hydrolysis of sucrose to fructose and glucose. The enzyme invertase, which occurs more commonly in plants, also hydrolyzes sucrose but by a different mechanism.-Physiology:...
used industrially for the hydrolysis of sucrose to so-called invert sugar. Lactase
Lactase
Lactase , a part of the β-galactosidase family of enzymes, is a glycoside hydrolase involved in the hydrolysis of the disaccharide lactose into constituent galactose and glucose monomers...
is essential for digestive hydrolysis of lactose
Lactose
Lactose is a disaccharide sugar that is found most notably in milk and is formed from galactose and glucose. Lactose makes up around 2~8% of milk , although the amount varies among species and individuals. It is extracted from sweet or sour whey. The name comes from or , the Latin word for milk,...
in milk. Deficiency of lactase in humans causes lactose intolerance
Lactose intolerance
Lactose intolerance, also called lactase deficiency or hypolactasia, is the inability to digest and metabolize lactose, a sugar found in milk...
.
The hydrolysis of polysaccharides to soluble sugars is called "saccharification". Malt made from barley
Barley
Barley is a major cereal grain, a member of the grass family. It serves as a major animal fodder, as a base malt for beer and certain distilled beverages, and as a component of various health foods...
is used as a source of β-amylase to break down starch
Starch
Starch or amylum is a carbohydrate consisting of a large number of glucose units joined together by glycosidic bonds. This polysaccharide is produced by all green plants as an energy store...
into the disaccharide maltose
Maltose
Maltose , or malt sugar, is a disaccharide formed from two units of glucose joined with an αbond, formed from a condensation reaction. The isomer "isomaltose" has two glucose molecules linked through an α bond. Maltose is the second member of an important biochemical series of glucose chains....
, which can be used by yeast to produce beer
Brewing
Brewing is the production of beer through steeping a starch source in water and then fermenting with yeast. Brewing has taken place since around the 6th millennium BCE, and archeological evidence suggests that this technique was used in ancient Egypt...
. Other amylase
Amylase
Amylase is an enzyme that catalyses the breakdown of starch into sugars. Amylase is present in human saliva, where it begins the chemical process of digestion. Food that contains much starch but little sugar, such as rice and potato, taste slightly sweet as they are chewed because amylase turns...
enzymes may convert starch to glucose or to oligosaccharides. Cellulose
Cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to over ten thousand β linked D-glucose units....
is converted to glucose or the disaccharide cellobiose
Cellobiose
Cellobiose is a disaccharide with the formula [HOCH2CHO3]2O. Cellobiose consists of two glucose molecules linked by a β bond. It can be hydrolyzed to glucose enzymatically or with acid. Cellobiose has eight free alcohol groups, one acetal linkage and one hemiacetal linkages, which give rise to...
by cellulase
Cellulase
400px|thumb|right|alt = Colored dice with checkered background|Ribbon representation of the Streptomyces lividans beta-1,4-endoglucanase catalytic domain - an example from the family 12 glycoside hydrolases...
s. Animals such as cows (ruminants) are able to digest cellulose because of symbiotic bacteria that produce cellulases.
Irreversibility of hydrolysis under physiological conditions
Under physiological conditions (e.g., in dilute aqueous solution), a hydrolytic cleavage reaction, in which the concentration of a metabolic precursor is low (on the order of 10−3 to 10−6 molar), is essentially thermodynamicallyThermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
irreversible. To give an example:
- A + H2O → X + Y
Assuming that x is the final concentration of products, and that C is the initial concentration of A, and W = [H2O] = 55.5 molar, then x can be calculated with the equation:
let Kd×W = k:
then

For a value of C = 0.001 molar, and k = 1 molar, x/C > 0.999. Less than 0.1% of the original reactant would be present once the reaction is complete.
This theme of physiological irreversibility of hydrolysis is used consistently in metabolic pathways, since many biological processes are driven by the cleavage of anhydrous pyrophosphate
Pyrophosphate
In chemistry, the anion, the salts, and the esters of pyrophosphoric acid are called pyrophosphates. Any salt or ester containing two phosphate groups is called a diphosphate. As a food additive, diphosphates are known as E450.- Chemistry :...
bonds.
Hydrolysis of metal aqua ions
Metal ions are Lewis acidLewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
s, and in aqueous solution they form aqua ions
Metal ions in aqueous solution
A metal ion in aqueous solution is a cation, dissolved in water, of chemical formula [Mn]z+. The solvation number, n, determined by a variety of experimental methods is 4 for Li+ and Be2+ and 6 for elements in rows 3 and 4 of the periodic table. Lanthanide and actinide aqua ions have solvation...
, of the general formula M(H2O)nm+.
The aqua ions undergo hydrolysis, to a greater or lesser extent. The first hydrolysis step is given generically as
- M(H2O)nm+ + H2O M(H2O)n-1(OH)(m-1)+ + H3O+
Thus the aqua ion is behaving as an acid in terms of Brønsted-Lowry acid-base theory. This is easily explained by considering the inductive effect
Inductive effect
In chemistry and physics, the inductive effect is an experimentally observable effect of the transmission of charge through a chain of atoms in a molecule by electrostatic induction...
of the positively charged metal ion, which weakens the O-H bond of an attached water molecule, making the liberation of a proton relatively easy.
The dissociation constant
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
, pKa, for this reaction is more or less linearly related to the charge-to-size ratio of the metal ion. Ions with low charges, such as Na+ are very weak acids with almost imperceptible hydrolysis. Large divalent ions such as Ca2+, Zn2+, Sn2+ and Pb2+ have a pKa of 6 or more and would not normally be classed as acids, but small divalent ions such as Be2+ undergo extensive hydrolysis. Trivalent ions like Al3+ and Fe3+ are weak acids whose pKa is comparable to that of acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
. Solutions of salts such as BeCl2 or Al(NO3)3 in water are noticeably acidic; the hydrolysis can be suppressed
Le Châtelier's principle
In chemistry, Le Chatelier's principle, also called the Chatelier's principle, can be used to predict the effect of a change in conditions on a chemical equilibrium. The principle is named after Henry Louis Le Chatelier and sometimes Karl Ferdinand Braun who discovered it independently...
by adding an acid such as nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
, making the solution more acidic.
Hydrolysis may proceed beyond the first step, often with the formation of polynuclear species.
Some "exotic" species such as Sn3(OH)42+ are well characterized. Hydrolysis tends to increase as pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
rises leading, in many cases, to the precipitation of an hydroxide such as Al(OH)3 or AlO(OH). These substances, the major constituents of bauxite
Bauxite
Bauxite is an aluminium ore and is the main source of aluminium. This form of rock consists mostly of the minerals gibbsite Al3, boehmite γ-AlO, and diaspore α-AlO, in a mixture with the two iron oxides goethite and hematite, the clay mineral kaolinite, and small amounts of anatase TiO2...
, are known as laterite
Laterite
Laterites are soil types rich in iron and aluminium, formed in hot and wet tropical areas. Nearly all laterites are rusty-red because of iron oxides. They develop by intensive and long-lasting weathering of the underlying parent rock...
s and are formed by leaching from rocks of most of the ions other than aluminium and iron and subsequent hydrolysis of the remaining aluminium and iron.
Ions with a formal charge of four have undergone extensive hydrolysis and salts of Zr4+, for example, can only be obtained from strongly acidic solutions. With oxidation states five and higher the concentration of the aqua ion in solution is negligible. In effect, the aqua ion is a strong acid. For example, aqueous solutions of Cr(VI) contain CrO42-.
- Cr(H2O)66+ → CrO42- + 2 H2O + 8 H+
Note that reactions such as
- Cr2O72- + H2O 2 CrO42- + 2 H+
are formally hydrolysis reactions as water molecules are split up yielding hydrogen ions. Such reactions are common among polyoxometalate
Polyoxometalate
In chemistry, a polyoxometalate is a polyatomic ion, usually an anion, that consists of three or more transition metal oxyanions linked together by shared oxygen atoms to form a large, closed 3-dimensional framework....
s.
See also
- Adenosine triphosphateAdenosine triphosphateAdenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
- Aluminium hydroxideAluminium hydroxideAluminium hydroxide, Al3, ATH, sometimes erroneously called Hydrate of alumina, is found in nature as the mineral gibbsite and its three, much more rare forms, polymorphs: bayerite, doyleite and nordstrandite. Closely related are aluminium oxide hydroxide, AlO, and aluminium oxide, Al2O3,...
- CatabolismCatabolismCatabolism is the set of metabolic pathways that break down molecules into smaller units and release energy. In catabolism, large molecules such as polysaccharides, lipids, nucleic acids and proteins are broken down into smaller units such as monosaccharides, fatty acids, nucleotides, and amino...
- Condensation reactionCondensation reactionA condensation reaction is a chemical reaction in which two molecules or moieties combine to form one single molecule, together with the loss of a small molecule. When this small molecule is water, it is known as a dehydration reaction; other possible small molecules lost are hydrogen chloride,...
- Dehydration synthesis
- Hydrolysis constantHydrolysis constantA hydrolysis constant is an equilibrium constant for a hydrolysis reaction.For example, if a metal salt such as AlCl3 dissolves in an aqueous solution, the metal cation behaves as a Lewis acid and hydrolyzes the water molecules in the solvent....
- Inhibitor proteinInhibitor proteinThe inhibitor protein is situated in the mitochondrial matrix and protects the cell against rapid ATP hydrolysis during momentary ischaemia. In oxygen absence, the pH of the matrix drops...
- Polymer degradationPolymer degradationPolymer degradation is a change in the properties—tensile strength, colour, shape, etc.—of a polymer or polymer-based product under the influence of one or more environmental factors such as heat, light or chemicals such as acids, alkalis and some salts...
- ResomationResomationAlkaline hydrolysis is a process for the disposal of human remains, which its creator states is more ecologically favorable than cremation. The process is being marketed worldwide as an alternative to the traditional options of burial or cremation...
- SolvolysisSolvolysisSolvolysis is a special type of nucleophilic substitution or elimination where the nucleophile is a solvent molecule. For certain nucleophiles, there are specific terms for the type of solvolysis reaction...
- Thermal hydrolysisThermal hydrolysisThermal hydrolysis is the process where waste or sludge is boiled under high pressure and high temperature, between 160-180 degrees. Cells rich in energy are released and the solution gives a doubling of the amount of biogas compared to the traditional solutions. The biogas can be used to generate...
- Tissue DigestionTissue DigestionTissue digestion is a method of disposing bodies. The scientific term is "alkaline hydrolysis". It is used at several universities for the remains of animal cadavers as well as for human remains. In mortuary usage, the process is called "water reduction" "resomation" or "aquamation".-Methods:The...



