
Liquid crystal
Encyclopedia
State of matter
States of matter are the distinct forms that different phases of matter take on. Solid, liquid and gas are the most common states of matter on Earth. However, much of the baryonic matter of the universe is in the form of hot plasma, both as rarefied interstellar medium and as dense...
that have properties between those of a conventional liquid and those of a solid crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
. For instance, an LC may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many different types of LC phases, which can be distinguished by their different optical
Optics
Optics is the branch of physics which involves the behavior and properties of light, including its interactions with matter and the construction of instruments that use or detect it. Optics usually describes the behavior of visible, ultraviolet, and infrared light...
properties (such as birefringence
Birefringence
Birefringence, or double refraction, is the decomposition of a ray of light into two rays when it passes through certain anisotropic materials, such as crystals of calcite or boron nitride. The effect was first described by the Danish scientist Rasmus Bartholin in 1669, who saw it in calcite...
). When viewed under a microscope
Microscope
A microscope is an instrument used to see objects that are too small for the naked eye. The science of investigating small objects using such an instrument is called microscopy...
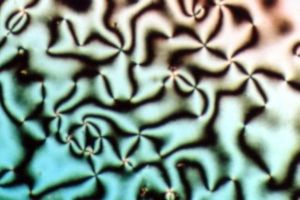
using a polarized light source, different liquid crystal phases will appear to have distinct texture
Texture (crystalline)
In materials science, texture is the distribution of crystallographic orientations of a polycrystalline sample. A sample in which these orientations are fully random is said to have no texture. If the crystallographic orientations are not random, but have some preferred orientation, then the...
s. The contrasting areas in the textures correspond to domains where the LC molecules are oriented in different directions. Within a domain, however, the molecules are well ordered. LC materials may not always be in an LC phase (just as water may turn into ice or steam).
Liquid crystals can be divided into thermotropic
Thermotropic
A liquid crystal is thermotropic if the order of its components is determined or changed by temperature.If temperature is too high, the rise in energy and therefore in motion of the components will induce a phase change: the LC will become an isotropic liquid.If, on the contrary, temperature is...
, lyotropic
Lyotropic
A material is called lyotropic if it forms liquid crystal phases because of the addition of a solvent. Historically the term was used to describe materials composed of amphiphilic molecules. Such molecules comprise a water-loving 'hydrophilic' head-group attached to a water-hating 'hydrophobic'...
and metallotropic phases. Thermotropic and lyotropic LCs consist of organic molecules. Thermotropic LCs exhibit a phase transition
Phase transition
A phase transition is the transformation of a thermodynamic system from one phase or state of matter to another.A phase of a thermodynamic system and the states of matter have uniform physical properties....
into the LC phase as temperature is changed. Lyotropic LCs exhibit phase transitions as a function of both temperature and concentration
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
of the LC molecules in a solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
(typically water). Metallotropic LCs are composed of both organic and inorganic molecules; their LC transition depends not only on temperature and concentration, but also on the inorganic-organic composition ratio.
Examples of liquid crystals can be found both in the natural world and in technological applications. Most modern electronic displays
Electronic visual display
An electronic visual display is display technology which incorporates flat panel displays, performs as a video display, output device for presentation of images transmitted electronically, for visual reception, without producing a permanent record....
are liquid crystal based. Lyotropic liquid-crystalline phases are abundant in living systems. For example, many proteins and cell membranes are LCs. Other well-known LC examples are solutions of soap and various related detergent
Detergent
A detergent is a surfactant or a mixture of surfactants with "cleaning properties in dilute solutions." In common usage, "detergent" refers to alkylbenzenesulfonates, a family of compounds that are similar to soap but are less affected by hard water...
s, as well as the tobacco mosaic virus
Tobacco mosaic virus
Tobacco mosaic virus is a positive-sense single stranded RNA virus that infects plants, especially tobacco and other members of the family Solanaceae. The infection causes characteristic patterns on the leaves . TMV was the first virus to be discovered...
.
History
In 1888, Austrian botanical physiologist Friedrich ReinitzerFriedrich Reinitzer
Friedrich Richard Reinitzer was an Austrian botanist and chemist. In late 1880s, experimenting with cholesteryl benzoate, he discovered properties of liquid crystals ....
, working at the Charles University in Prague
Charles University in Prague
Charles University in Prague is the oldest and largest university in the Czech Republic. Founded in 1348, it was the first university in Central Europe and is also considered the earliest German university...
, examined the physico-chemical properties of various derivatives
Derivative (chemistry)
In chemistry, a derivative is a compound that is derived from a similar compound by some chemical or physical process. In the past it was also used to mean a compound that can be imagined to arise from another compound, if one atom is replaced with another atom or group of atoms, but modern...
of cholesterol
Cholesterol
Cholesterol is a complex isoprenoid. Specifically, it is a waxy steroid of fat that is produced in the liver or intestines. It is used to produce hormones and cell membranes and is transported in the blood plasma of all mammals. It is an essential structural component of mammalian cell membranes...
, which are now known as cholesteric liquid crystals. Previously, other researchers had observed distinct color effects when cooling cholesterol derivatives just above the freezing point
Freezing Point
Freezing Point is a news journal in the People's Republic of China which has been the subject of controversy over its criticism of Communist Party officials and the sympathetic ear it lent to a Chinese historian who had criticized official history textbooks...
, but had not associated it with a new phenomenon. Reinitzer perceived that color changes in a derivative cholesteryl benzoate
Cholesteryl benzoate
Cholesteryl benzoate, also called 5-cholesten-3-yl benzoate, is an organic chemical, an ester of cholesterol and benzoic acid. It is a liquid crystal material forming cholesteric liquid crystals with helical structure....
were not the most peculiar feature. He found that cholesteryl benzoate does not melt
Melting
Melting, or fusion, is a physical process that results in the phase change of a substance from a solid to a liquid. The internal energy of a substance is increased, typically by the application of heat or pressure, resulting in a rise of its temperature to the melting point, at which the rigid...
in the same manner as other compounds, but has two melting point
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
s. At 145.5 °C (293.9 °F) it melts into a cloudy liquid, and at 178.5 °C (353.3 °F) it melts again and the cloudy liquid becomes clear. The phenomenon is reversible. Seeking help from a physicist, on March 14, 1888, he wrote to Otto Lehmann
Otto Lehmann
Otto Lehmann was a German physicist and "father" of liquid crystal technology.-Life:...
, at that time a Privatdozent
Privatdozent
Privatdozent or Private lecturer is a title conferred in some European university systems, especially in German-speaking countries, for someone who pursues an academic career and holds all formal qualifications to become a tenured university professor...
in Aachen
Aachen
Aachen has historically been a spa town in North Rhine-Westphalia, Germany. Aachen was a favoured residence of Charlemagne, and the place of coronation of the Kings of Germany. Geographically, Aachen is the westernmost town of Germany, located along its borders with Belgium and the Netherlands, ...
. They exchanged letters and samples. Lehmann examined the intermediate cloudy fluid, and reported seeing crystallite
Crystallite
Crystallites are small, often microscopic crystals that, held together through highly defective boundaries, constitute a polycrystalline solid. Metallurgists often refer to crystallites as grains.- Details :...
s. Reinitzer's Viennese colleague von Zepharovich also indicated that the intermediate "fluid" was crystalline. The exchange of letters with Lehmann ended on April 24, with many questions unanswered. Reinitzer presented his results, with credits to Lehmann and von Zepharovich, at a meeting of the Vienna Chemical Society on May 3, 1888.
By that time, Reinitzer had discovered and described three important features of cholesteric liquid crystals (the name coined by Otto Lehmann
Otto Lehmann
Otto Lehmann was a German physicist and "father" of liquid crystal technology.-Life:...
in 1904): the existence of two melting points, the reflection of circularly polarized light
Circular polarization
In electrodynamics, circular polarization of an electromagnetic wave is a polarization in which the electric field of the passing wave does not change strength but only changes direction in a rotary type manner....
, and the ability to rotate the polarization direction of light.
After his accidental discovery, Reinitzer did not pursue studying liquid crystals further. The research was continued by Lehmann, who realized that he had encountered a new phenomenon and was in a position to investigate it: In his postdoctoral years he had acquired expertise in crystallography and microscopy. Lehmann started a systematic study, first of cholesteryl benzoate, and then of related compounds which exhibited the double-melting phenomenon. He was able to make observations in polarized light, and his microscope was equipped with a hot stage (sample holder equipped with a heater) enabling high temperature observations. The intermediate cloudy phase clearly sustained flow, but other features, particularly the signature under a microscope, convinced Lehmann that he was dealing with a solid. By the end of August 1889 he had published his results in the Zeitschrift für Physikalische Chemie
Zeitschrift für Physikalische Chemie
Zeitschrift für Physikalische Chemie is a physical chemistry journal published in Germany. Its name literally means "Journal of Physical Chemistry", but its official English-language subtitle is "International journal of research in physical chemistry and chemical physics". Its emphasis is on...
.
Lehmann's work was continued and significantly expanded by the German chemist Daniel Vorländer
Daniel Vorländer
Daniel Vorländer was a German chemist who synthesized most of the liquid crystals known until his retirement in 1935.Vorländer was born in Eupen in Rhenish Prussia...
, who from the beginning of 20th century until his retirement in 1935, had synthesized most of the liquid crystals known. However, liquid crystals were not popular among scientists and the material remained a pure scientific curiosity for about 80 years.
In 1969, Hans Kelker succeeded in synthesizing a substance that had a nematic phase at room temperature, MBBA
MBBA
N--4-butylaniline is an organic compound often used in liquid crystals.-External links:*...
, which is one of the most popular subjects of liquid crystal research. The next step to commercialization of liquid crystal displays was the synthesis of further chemically stable substances (cyanobiphenyls) with low melting temperatures by George Gray
George William Gray
George William Gray CBE, FRS is a Professor of Organic Chemistry at the University of Hull who was instrumental in developing the long-lasting materials which made liquid crystal displays possible...
. That work with Ken Harrison and the UK MOD (RRE Malvern
Royal Radar Establishment
The name Royal Radar Establishment was given to the existing Radar Research Establishment following a visit by Queen Elizabeth II in 1957. Both names were abbreviated to RRE. The establishment had been formed, under its first name, in 1953 by merging the Telecommunications Research Establishment ...
), in 1973, led to design of new materials resulting in rapid adoption of small area LCDs within electronic products.
In 1991, when liquid crystal displays were already well established, Pierre-Gilles de Gennes
Pierre-Gilles de Gennes
Pierre-Gilles de Gennes was a French physicist and the Nobel Prize laureate in physics in 1991.-Biography:...
received the Nobel Prize in physics "for discovering that methods developed for studying order phenomena in simple systems can be generalized to more complex forms of matter, in particular to liquid crystals and polymers".
Liquid crystal phases
The various LC phases (called mesophaseMesophase
In physics, a mesophase is a state of matter intermediate between liquid and solid. Gelatin is a common example of a partially-ordered structure in a mesophase...
s) can be characterized by the type of ordering. One can distinguish positional order (whether molecules are arranged in any sort of ordered lattice) and orientational order (whether molecules are mostly pointing in the same direction), and moreover order can be either short-range (only between molecules close to each other) or long-range (extending to larger, sometimes macroscopic, dimensions). Most thermotropic LCs will have an isotropic phase at high temperature. That is that heating will eventually drive them into a conventional liquid phase characterized by random and isotropic molecular ordering (little to no long-range order), and fluid
Fluid
In physics, a fluid is a substance that continually deforms under an applied shear stress. Fluids are a subset of the phases of matter and include liquids, gases, plasmas and, to some extent, plastic solids....
-like flow behavior. Under other conditions (for instance, lower temperature), an LC might inhabit one or more phases with significant anisotropic orientational structure and short-range orientational order while still having an ability to flow.
The ordering of liquid crystalline phases is extensive on the molecular scale. This order extends up to the entire domain size, which may be on the order of micrometers, but usually does not extend to the macroscopic scale as often occurs in classical crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
line solids. However some techniques, such as the use of boundaries or an applied electric field
Electric field
In physics, an electric field surrounds electrically charged particles and time-varying magnetic fields. The electric field depicts the force exerted on other electrically charged objects by the electrically charged particle the field is surrounding...
, can be used to enforce a single ordered domain in a macroscopic liquid crystal sample. The ordering in a liquid crystal might extend along only one dimension
Dimension
In physics and mathematics, the dimension of a space or object is informally defined as the minimum number of coordinates needed to specify any point within it. Thus a line has a dimension of one because only one coordinate is needed to specify a point on it...
, with the material being essentially disordered in the other two directions.
Thermotropic liquid crystals
Thermotropic phases are those that occur in a certain temperature range. If the temperature rise is too high, thermal motion will destroy the delicate cooperative ordering of the LC phase, pushing the material into a conventional isotropic liquid phase. At too low temperature, most LC materials will form a conventional crystal. Many thermotropic LCs exhibit a variety of phases as temperature is changed. For instance, a particular type of LC molecule (called mesogenMesogen
Mesogen is the fundamental unit of a liquid crystal that induces structural order in the crystals.Typically, a liquid-crystalline molecule consists of a rigid moiety and one or more flexible parts. The rigid part aligns molecules in one direction, whereas the flexible parts induce fluidity in the...
) may exhibit various smectic and nematic (and finally isotropic) phases as temperature is increased. An example of a compound displaying thermotropic LC behavior is para-azoxyanisole
Para-Azoxyanisole
para-Azoxyanisole is an organic, aromatic compound. In a solid state, it appears as a white powder, but when heated it forms a liquid crystal. As one of the first known and most readily prepared liquid crystals, PAA has been played an important role in the development of liquid crystal...
.
Nematic phase


Greek language
Greek is an independent branch of the Indo-European family of languages. Native to the southern Balkans, it has the longest documented history of any Indo-European language, spanning 34 centuries of written records. Its writing system has been the Greek alphabet for the majority of its history;...
(nema), which means "thread". This term originates from the thread-like topological defect
Topological defect
In mathematics and physics, a topological soliton or a topological defect is a solution of a system of partial differential equations or of a quantum field theory homotopically distinct from the vacuum solution; it can be proven to exist because the boundary conditions entail the existence of...
s observed in nematics, which are formally called 'disclination
Disclination
A disclination is a line defect in which rotational symmetry is violated. In analogy with dislocations in crystals, the term, disinclination, for liquid crystals first used by F. C. Frank and since then has been modified to its current usage, disclination.It is a defect in the orientation of...
s'. Nematics also exhibit so-called hedgehog topological
Hedgehog space
In mathematics, a hedgehog space is a topological space, consisting of a set of spines joined at a point.For any cardinal number K, the K-hedgehog space is formed by taking the disjoint union of K real unit intervals identified at the origin...
defects. In a nematic phase, the calamitic or rod-shaped organic molecules have no positional order, but they self-align to have long-range directional order with their long axes roughly parallel. Thus, the molecules are free to flow and their center of mass positions are randomly distributed as in a liquid, but still maintain their long-range directional order. Most nematics are uniaxial: they have one axis that is longer and preferred, with the other two being equivalent (can be approximated as cylinders or rods). However, some liquid crystals are biaxial nematics, meaning that in addition to orienting their long axis, they also orient along a secondary axis. Nematics have fluidity similar to that of ordinary (isotropic) liquids but they can be easily aligned by an external magnetic or electric field. Aligned nematics have the optical properties of uniaxial crystals and this makes them extremely useful in liquid crystal display
Liquid crystal display
A liquid crystal display is a flat panel display, electronic visual display, or video display that uses the light modulating properties of liquid crystals . LCs do not emit light directly....
s (LCD).
p-azoxy amisole they poses parlel order
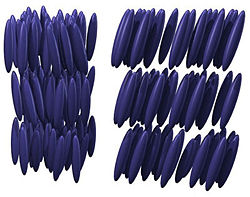
Smectic phases
Chiral phases

Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
nematic phase exhibits chirality
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
(handedness). This phase is often called the cholesteric
Cholesteric liquid crystal
A cholesteric liquid crystal is a type of liquid crystal with a helical structure and which is therefore chiral. Cholesteric liquid crystals are also known as chiral nematic liquid crystals. They organize in layers with no positional ordering within layers, but a director axis which varies with...
phase because it was first observed for cholesterol
Cholesterol
Cholesterol is a complex isoprenoid. Specifically, it is a waxy steroid of fat that is produced in the liver or intestines. It is used to produce hormones and cell membranes and is transported in the blood plasma of all mammals. It is an essential structural component of mammalian cell membranes...
derivatives. Only chiral molecules
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
(i.e., those that lack inversion symmetry) can give rise to such a phase. This phase exhibits a twisting of the molecules perpendicular to the director, with the molecular axis parallel to the director. The finite twist angle between adjacent molecules is due to their asymmetric packing, which results in longer-range chiral order. In the smectic C* phase (an asterisk denotes a chiral phase), the molecules have positional ordering in a layered structure (as in the other smectic phases), with the molecules tilted by a finite angle with respect to the layer normal. The chirality induces a finite azimuthal twist from one layer to the next, producing a spiral twisting of the molecular axis along the layer normal.
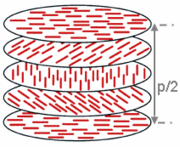
Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
of visible light. This causes these systems to exhibit unique optical properties, such as Bragg reflection and low-threshold laser
Laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of photons. The term "laser" originated as an acronym for Light Amplification by Stimulated Emission of Radiation...
emission, and these properties are exploited in a number of optical applications. For the case of Bragg reflection only the lowest-order reflection is allowed if the light is incident along the helical axis, whereas for oblique incidence higher-order reflections become permitted. Cholesteric liquid crystals also exhibit the unique property that they reflect circularly polarized light when it is incident along the helical axis and elliptically polarized if it comes in obliquely.
Blue phases
Blue phases are liquid crystal phases that appear in the temperature range between a chiralChirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
nematic phase and an isotropic
Isotropy
Isotropy is uniformity in all orientations; it is derived from the Greek iso and tropos . Precise definitions depend on the subject area. Exceptions, or inequalities, are frequently indicated by the prefix an, hence anisotropy. Anisotropy is also used to describe situations where properties vary...
liquid phase. Blue phases have a regular three-dimensional cubic structure of defects with lattice
Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
periods of several hundred nanometers, and thus they exhibit selective Bragg reflections
Bragg's law
In physics, Bragg's law gives the angles for coherent and incoherent scattering from a crystal lattice. When X-rays are incident on an atom, they make the electronic cloud move as does any electromagnetic wave...
in the wavelength range of visible light corresponding to the cubic lattice. It was theoretically predicted in 1981 that these phases can possesses icosahedral symmetry similar to quasicrystal
Quasicrystal
A quasiperiodic crystal, or, in short, quasicrystal, is a structure that is ordered but not periodic. A quasicrystalline pattern can continuously fill all available space, but it lacks translational symmetry...
s.
Although blue phases are of interest for fast light modulators or tunable photonic crystal
Photonic crystal
Photonic crystals are periodic optical nanostructures that are designed to affect the motion of photons in a similar way that periodicity of a semiconductor crystal affects the motion of electrons...
s, they exist in a very narrow temperature range, usually less than a few kelvin
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
. Recently the stabilization of blue phases over a temperature range of more than 60 K including room temperature (260–326 K) has been demonstrated. Blue phases stabilized at room temperature allow electro-optical switching with response times of the order of 10−4 s.
In May 2008, the first Blue Phase Mode LCD
Blue Phase Mode LCD
A Blue Phase Mode LCD is a liquid crystal display technology that uses highly twisted cholesteric phases in a blue phase. It was first proposed in 2007 to obtain a better display of moving images with, for example, frame rates of 100–120 Hz to improve the temporal response of liquid crystal...
panel had been developed.
Discotic phases
Disk-shaped LC molecules can orient themselves in a layer-like fashion known as the discotic nematic phase. If the disks pack into stacks, the phase is called a discotic columnarColumnar phase
The columnar phase is a class of mesophases in which molecules assemble into cylindrical structures to act as mesogens. Originally, these kinds of liquid crystals were called discotic liquid crystals because the columnar structures are composed of flat-shaped discotic molecules stacked...
. The columns themselves may be organized into rectangular or hexagonal arrays. Chiral discotic phases, similar to the chiral nematic phase, are also known.
Lyotropic liquid crystals
A lyotropic liquid crystalLyotropic liquid crystal
A liquid crystalline material is called lyotropic if phases having long-ranged orientational order are induced by the addition of a solvent. Historically the term was used to describe materials composed of amphiphilic molecules. Such molecules comprise a water-loving 'hydrophilic' head-group ...
consists of two or more components that exhibit liquid-crystalline properties in certain concentration ranges. In the lyotropic
Lyotropic
A material is called lyotropic if it forms liquid crystal phases because of the addition of a solvent. Historically the term was used to describe materials composed of amphiphilic molecules. Such molecules comprise a water-loving 'hydrophilic' head-group attached to a water-hating 'hydrophobic'...
phases, solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
molecules fill the space around the compounds to provide fluidity
Viscosity
Viscosity is a measure of the resistance of a fluid which is being deformed by either shear or tensile stress. In everyday terms , viscosity is "thickness" or "internal friction". Thus, water is "thin", having a lower viscosity, while honey is "thick", having a higher viscosity...
to the system. In contrast to thermotropic liquid crystals, these lyotropics have another degree of freedom of concentration that enables them to induce a variety of different phases.
A compound, which has two immiscible hydrophilic and hydrophobic parts within the same molecule, is called an amphiphilic molecule. Many amphiphilic molecules show lyotropic liquid-crystalline phase sequences depending on the volume balances between the hydrophilic part and hydrophobic part. These structures are formed through the micro-phase segregation of two incompatible components on a nanometer scale. Soap is an everyday example of a lyotropic liquid crystal.
The content of water or other solvent molecules changes the self-assembled structures. At very low amphiphile concentration, the molecules will be dispersed randomly without any ordering. At slightly higher (but still low) concentration, amphiphilic molecules will spontaneously assemble into micelle
Micelle
A micelle is an aggregate of surfactant molecules dispersed in a liquid colloid. A typical micelle in aqueous solution forms an aggregate with the hydrophilic "head" regions in contact with surrounding solvent, sequestering the hydrophobic single tail regions in the micelle centre. This phase is...
s or vesicles
Vesicle (biology)
A vesicle is a bubble of liquid within another liquid, a supramolecular assembly made up of many different molecules. More technically, a vesicle is a small membrane-enclosed sack that can store or transport substances. Vesicles can form naturally because of the properties of lipid membranes , or...
. This is done so as to 'hide' the hydrophobic tail of the amphiphile inside the micelle core, exposing a hydrophilic (water-soluble) surface to aqueous solution. These spherical objects do not order themselves in solution, however. At higher concentration, the assemblies will become ordered. A typical phase is a hexagonal columnar phase, where the amphiphiles form long cylinders (again with a hydrophilic surface) that arrange themselves into a roughly hexagonal lattice. This is called the middle soap phase. At still higher concentration, a lamellar phase (neat soap phase) may form, wherein extended sheets of amphiphiles are separated by thin layers of water. For some systems, a cubic (also called viscous isotropic) phase may exist between the hexagonal and lamellar phases, wherein spheres are formed that create a dense cubic lattice. These spheres may also be connected to one another, forming a bicontinuous cubic phase.
The objects created by amphiphiles are usually spherical (as in the case of micelles), but may also be disc-like (bicelles), rod-like, or biaxial (all three micelle axes are distinct). These anisotropic self-assembled nano-structures can then order themselves in much the same way as thermotropic liquid crystals do, forming large-scale versions of all the thermotropic phases (such as a nematic phase of rod-shaped micelles).
For some systems, at high concentrations, inverse phases are observed. That is, one may generate an inverse hexagonal columnar phase (columns of water encapsulated by amphiphiles) or an inverse micellar phase (a bulk liquid crystal sample with spherical water cavities).
A generic progression of phases, going from low to high amphiphile concentration, is:
- Discontinuous cubic phase (micellar cubicMicellar cubicA micellar cubic phase is a lyotropic liquid crystal phase formed when the concentration of micelles dispersed in a solvent is sufficiently high that they are forced to pack into a structure having long-ranged positional order. For example, spherical micelles a subic packing of a body-centred...
phase) - Hexagonal phaseHexagonal phaseA hexagonal phase of lyotropic liquid crystal is formed by some amphiphilic molecules when they are mixed with water or another polar solvent. In this phase the amphiphile molecules are aggregated into cylindrical structures of indefinite length and these cylindrical aggregates are disposed on a...
(hexagonal columnar phase) (middle phase) - Lamellar phase
- Bicontinuous cubic phase
- Reverse hexagonal columnar phase
- Inverse cubic phase (Inverse micellar phase)
Even within the same phases, their self-assembled structures are tunable by the concentration: for example, in lamellar phases, the layer distances increase with the solvent volume. Since lyotropic liquid crystals rely on a subtle balance of intermolecular interactions, it is more difficult to analyze their structures and properties than those of thermotropic liquid crystals.
Similar phases and characteristics can be observed in immiscible diblock copolymers.
Metallotropic liquid crystals
Liquid crystal phases can also be based on low-melting inorganic phases like ZnCl2Zinc chloride
Zinc chloride is the name of chemical compound with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. ZnCl2 itself is hygroscopic and even deliquescent. Samples should therefore be protected from...
that have a structure formed of linked tetrahedra and easily form glasses. The addition of long chain soap-like molecules leads to a series of new phases that show a variety of liquid crystalline behavior both as a function of the inorganic-organic composition ratio and of temperature. This class of materials has been named metallotropic.
Biological liquid crystals
Lyotropic liquid-crystalline phases are abundant in living systems, the study of which is referred to as lipid polymorphismLipid polymorphism
Polymorphism in biophysics is the aspect of the behaviour of lipids that influences their long-range order, i.e. how they aggregate. This can be in the form of spheres of lipid molecules , pairs of layers that face one another , a tubular arrangement , or various cubic phases Polymorphism in...
. Accordingly, lyotropic liquid crystals attract particular attention in the field of biomimetic chemistry. In particular, biological membrane
Biological membrane
A biological membrane or biomembrane is an enclosing or separatingmembrane that acts as a selective barrier, within or around a cell. It consists of a lipid bilayer with embedded proteins that may constitute close to 50% of membrane content...
s and cell membranes are a form of liquid crystal. Their constituent molecules (e.g., phospholipid
Phospholipid
Phospholipids are a class of lipids that are a major component of all cell membranes as they can form lipid bilayers. Most phospholipids contain a diglyceride, a phosphate group, and a simple organic molecule such as choline; one exception to this rule is sphingomyelin, which is derived from...
s) are perpendicular to the membrane surface, yet the membrane is flexible. These lipids vary in shape (see page on lipid polymorphism
Lipid polymorphism
Polymorphism in biophysics is the aspect of the behaviour of lipids that influences their long-range order, i.e. how they aggregate. This can be in the form of spheres of lipid molecules , pairs of layers that face one another , a tubular arrangement , or various cubic phases Polymorphism in...
). The constituent molecules can inter-mingle easily, but tend not to leave the membrane due to the high energy requirement of this process. Lipid molecules can flip from one side of the membrane to the other, this process being catalyzed by flippase
Flippase
Flippases are a family of transmembrane lipid transporter enzymes located in the membrane responsible for aiding the movement of phospholipid molecules between the two leaflets that compose a cell's membrane...
s and floppases (depending on the direction of movement). These liquid crystal membrane phases can also host important proteins such as receptors freely "floating" inside, or partly outside, the membrane, e.g. CCT.
Many other biological structures exhibit LC behavior. For instance, the concentrated protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
solution that is extruded by a spider to generate silk
Spider silk
Spider silk is a protein fiber spun by spiders. Spiders use their silk to make webs or other structures, which function as nets to catch other animals, or as nests or cocoons for protection for their offspring...
is, in fact, a liquid crystal phase. The precise ordering of molecules in silk is critical to its renowned strength. DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
and many polypeptides
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
can also form LC phases and this too forms an important part of current academic research.
Pattern formation in liquid crystals
Anisotropy of liquid crystals is a property not observed in other fluids. This anisotropy makes flows of liquid crystals behave more differentially than those of ordinary fluids. For example, injection of a flux of a liquid crystal between two close parallel plates (viscous fingeringViscous fingering
Viscous fingering is the formation of patterns in a morphologically unstable interface between two fluids in a porous medium or in a Hele-Shaw cell. It occurs when a less viscous fluid is injected displacing a more viscous one...
), causes orientation of the molecules to couple with the flow, with the resulting emergence of dendritic patterns. This anisotropy is also manifested in the interfacial energy (surface tension
Surface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
) between different liquid crystal phases. This anisotropy determines the equilibrium shape at the coexistence temperature, and is so strong that usually facets appear. When temperature is changed one of the phases grows, forming different morphologies depending on the temperature change. Since growth is controlled by heat diffusion, anisotropy in thermal conductivity favors growth in specific directions, which has also an effect on the final shape.
Theoretical treatment of liquid crystals
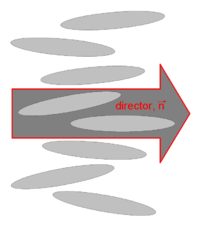
Microscopic theoretical treatment of fluid phases can become quite complicated, owing to the high material density, meaning that strong interactions, hard-core repulsions, and many-body correlations cannot be ignored. In the case of liquid crystals, anisotropy in all of these interactions further complicates analysis. There are a number of fairly simple theories, however, that can at least predict the general behavior of the phase transitions in liquid crystal systems.Director
As we already saw above, the nematic liquid crystals are composed of rod-like molecules with the long axes of neighboring molecules aligned approximately to one another. To allow this anisotropic structure, a dimensionless unit vector n called the director, is introduced to represent the direction of preferred orientation of molecules in the neighborhood of any point. Because there is no physical polarity along the director axis, n and -n are fully equivalent.Order parameter

where
 is the angle between the LC molecular axis and the local director (which is the 'preferred direction' in a volume element of a liquid crystal sample, also representing its local optical axis
is the angle between the LC molecular axis and the local director (which is the 'preferred direction' in a volume element of a liquid crystal sample, also representing its local optical axisOptical axis
An optical axis is a line along which there is some degree of rotational symmetry in an optical system such as a camera lens or microscope.The optical axis is an imaginary line that defines the path along which light propagates through the system...
). The brackets denote both a temporal and spatial average. This definition is convenient, since for a completely random and isotropic sample, S=0, whereas for a perfectly aligned sample S=1. For a typical liquid crystal sample, S is on the order of 0.3 to 0.8, and generally decreases as the temperature is raised. In particular, a sharp drop of the order parameter to 0 is observed when the system undergoes a phase transition from an LC phase into the isotropic phase. The order parameter can be measured experimentally in a number of ways. For instance, diamagnetism
Diamagnetism
Diamagnetism is the property of an object which causes it to create a magnetic field in opposition to an externally applied magnetic field, thus causing a repulsive effect. Specifically, an external magnetic field alters the orbital velocity of electrons around their nuclei, thus changing the...
, birefringence
Birefringence
Birefringence, or double refraction, is the decomposition of a ray of light into two rays when it passes through certain anisotropic materials, such as crystals of calcite or boron nitride. The effect was first described by the Danish scientist Rasmus Bartholin in 1669, who saw it in calcite...
, Raman scattering
Raman scattering
Raman scattering or the Raman effect is the inelastic scattering of a photon. It was discovered by Sir Chandrasekhara Venkata Raman and Kariamanickam Srinivasa Krishnan in liquids, and by Grigory Landsberg and Leonid Mandelstam in crystals....
, NMR
Nuclear magnetic resonance
Nuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation...
and EPR
Electron paramagnetic resonance
Electron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion...
can also be used to determine S.
The order of a liquid crystal could also be characterized by using other even Legendre polynomials (all the odd polynomials average to zero since the director can point in either of two antiparallel directions). These higher-order averages are more difficult to measure, but can yield additional information about molecular ordering.
A positional order parameter is also used to describe the ordering of a liquid crystal. It is characterized by the variation of the density of the center of mass of the liquid crystal molecules along a given vector. In the case of positional variation along the z-axis the density
 is often given by:
is often given by:
The complex positional order parameter is defined as
 and
and  the average density. Typically only the first two terms are kept and higher order terms are ignored since most phases can be described adequately using sinusoidal functions. For a perfect nematic
the average density. Typically only the first two terms are kept and higher order terms are ignored since most phases can be described adequately using sinusoidal functions. For a perfect nematic  and for a smectic phase
and for a smectic phase  will take on complex values. The complex nature of this order parameter allows for many parallels between nematic to smectic phase transitions and conductor to superconductor transitions.
will take on complex values. The complex nature of this order parameter allows for many parallels between nematic to smectic phase transitions and conductor to superconductor transitions.Onsager hard-rod model
A simple model which predicts lyotropic phase transitions is the hard-rod model proposed by Lars OnsagerLars Onsager
Lars Onsager was a Norwegian-born American physical chemist and theoretical physicist, winner of the 1968 Nobel Prize in Chemistry.He held the Gibbs Professorship of Theoretical Chemistry at Yale University....
. This theory considers the volume excluded from the center-of-mass of one idealized cylinder as it approaches another. Specifically, if the cylinders are oriented parallel to one another, there is very little volume that is excluded from the center-of-mass of the approaching cylinder (it can come quite close to the other cylinder). If, however, the cylinders are at some angle to one another, then there is a large volume surrounding the cylinder which the approaching cylinder's center-of-mass cannot enter (due to the hard-rod repulsion between the two idealized objects). Thus, this angular arrangement sees a decrease in the net positional entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
of the approaching cylinder (there are fewer states available to it).
The fundamental insight here is that, whilst parallel arrangements of anisotropic objects lead to a decrease in orientational entropy, there is an increase in positional entropy. Thus in some case greater positional order will be entropically favorable. This theory thus predicts that a solution of rod-shaped objects will undergo a phase transition, at sufficient concentration, into a nematic phase. Although this model is conceptually helpful, its mathematical formulation makes several assumptions that limit its applicability to real systems.
Maier–Saupe mean field theory
This statistical theory, proposed by Alfred SaupeAlfred Saupe
-Biography:Alfred Saupe was a German Physicist born in Badenweiler, who laid groundbreaking work in the area of liquid crystal studies....
and Wilhelm Maier, includes contributions from an attractive intermolecular potential from an induced dipole moment between adjacent liquid crystal molecules. The anisotropic attraction stabilizes parallel alignment of neighboring molecules, and the theory then considers a mean-field average of the interaction. Solved self-consistently, this theory predicts thermotropic nematic-isotropic phase transitions, consistent with experiment.
McMillan's model
McMillan's model, proposed by William McMillan, is an extension of the Maier–Saupe mean field theory used to describe the phase transition of a liquid crystal from a nematic to a smectic A phase. It predicts that the phase transition can be either continuous or discontinuous depending on the strength of the short-range interaction between the molecules. As a result, it allows for a triple critical point where the nematic, isotropic, and smectic A phase meet. Although it predicts the existence of a triple critical point, it does not successfully predict its value. The model utilizes two order parameters that describe the orientational and positional order of the liquid crystal. The first is simply the average of the second Legendre polynomial and the second order parameter is given by:
The values zi, θi, and d are the position of the molecule, the angle between the molecular axis and director, and the layer spacing. The postulated potential energy of a single molecule is given by:

Here constant α quantifies the strength of the interaction between adjacent molecules. The potential is then used to derive the thermodynamic properties of the system assuming thermal equilibrium. It results in two self-consistency equations that must be solved numerically, the solutions of which are the three stable phases of the liquid crystal.
Elastic continuum theory
In this formalism, a liquid crystal material is treated as a continuum; molecular details are entirely ignored. Rather, this theory considers perturbations to a presumed oriented sample. The distortions of the liquid crystal are commonly described by the Frank free energy density. One can identify three types of distortions that could occur in an oriented sample: (1) twists of the material, where neighboring molecules are forced to be angled with respect to one another, rather than aligned; (2) splay of the material, where bending occurs perpendicular to the director; and (3) bend of the material, where the distortion is parallel to the director and molecular axis. All three of these types of distortions incur an energy penalty. They are distortions that are induced by the boundary conditions at domain walls or the enclosing container. The response of the material can then be decomposed into terms based on the elastic constants corresponding to the three types of distortions. Elastic continuum theory is a particularly powerful tool for modeling liquid crystal devices.Effect of chirality
As already described, chiralChirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
LC molecules usually give rise to chiral mesophases. This means that the molecule must possess some form of asymmetry, usually a stereogenic center. An additional requirement is that the system not be racemic
Racemic
In chemistry, a racemic mixture, or racemate , is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule. The first known racemic mixture was "racemic acid", which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid.- Nomenclature :A...
: a mixture of right- and left-handed molecules will cancel the chiral effect. Due to the cooperative nature of liquid crystal ordering, however, a small amount of chiral dopant in an otherwise achiral mesophase is often enough to select out one domain handedness, making the system overall chiral.
Chiral phases usually have a helical twisting of the molecules. If the pitch of this twist is on the order of the wavelength of visible light, then interesting optical interference effects can be observed. The chiral twisting that occurs in chiral LC phases also makes the system respond differently from right- and left-handed circularly polarized light. These materials can thus be used as polarization filters
Polarizer
A polarizer is an optical filter that passes light of a specific polarization and blocks waves of other polarizations. It can convert a beam of light of undefined or mixed polarization into a beam with well-defined polarization. The common types of polarizers are linear polarizers and circular...
.
It is possible for chiral LC molecules to produce essentially achiral mesophases. For instance, in certain ranges of concentration and molecular weight, DNA will form an achiral line hexatic phase. An interesting recent observation is of the formation of chiral mesophases from achiral LC molecules. Specifically, bent-core molecules (sometimes called banana liquid crystals) have been shown to form liquid crystal phases that are chiral. In any particular sample, various domains will have opposite handedness, but within any given domain, strong chiral ordering will be present. The appearance mechanism of this macroscopic chirality is not yet entirely clear. It appears that the molecules stack in layers and orient themselves in a tilted fashion inside the layers. These liquid crystals phases may be ferroelectric or anti-ferroelectric, both of which are of interest for applications.
Chirality can also be incorporated into a phase by adding a chiral dopant
Dopant
A dopant, also called a doping agent, is a trace impurity element that is inserted into a substance in order to alter the electrical properties or the optical properties of the substance. In the case of crystalline substances, the atoms of the dopant very commonly take the place of elements that...
, which may not form LCs itself. Twisted-nematic
Twisted nematic field effect
The twisted nematic effect was a main technology breakthrough that made liquid crystal displays practical. Unlike earlier displays, TN-cells did not require a current to flow for operation and used low operating voltages suitable for use with batteries...
or super-twisted nematic
Super-twisted nematic display
A super-twisted nematic display is a type of monochrome passive matrix liquid crystal display . STN displays provide more contrast than twisted nematic displays by twisting the molecules from 180 to 270 degrees...
mixtures often contain a small amount of such dopants.
Applications of liquid crystals
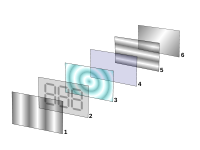
Optics
Optics is the branch of physics which involves the behavior and properties of light, including its interactions with matter and the construction of instruments that use or detect it. Optics usually describes the behavior of visible, ultraviolet, and infrared light...
properties of certain liquid crystalline substances in the presence or absence of an electric field
Electric field
In physics, an electric field surrounds electrically charged particles and time-varying magnetic fields. The electric field depicts the force exerted on other electrically charged objects by the electrically charged particle the field is surrounding...
. In a typical device, a liquid crystal layer (typically 10 μm thick) sits between two polarizer
Polarizer
A polarizer is an optical filter that passes light of a specific polarization and blocks waves of other polarizations. It can convert a beam of light of undefined or mixed polarization into a beam with well-defined polarization. The common types of polarizers are linear polarizers and circular...
s that are crossed (oriented at 90° to one another). The liquid crystal alignment is chosen so that its relaxed phase is a twisted one (see Twisted nematic field effect
Twisted nematic field effect
The twisted nematic effect was a main technology breakthrough that made liquid crystal displays practical. Unlike earlier displays, TN-cells did not require a current to flow for operation and used low operating voltages suitable for use with batteries...
). This twisted phase reorients light that has passed through the first polarizer, allowing its transmission through the second polarizer (and reflected back to the observer if a reflector is provided). The device thus appears transparent. When an electric field is applied to the LC layer, the long molecular axes tend to align parallel to the electric field thus gradually untwisting in the center of the liquid crystal layer. In this state, the LC molecules do not reorient light, so the light polarized at the first polarizer is absorbed at the second polarizer, and the device loses transparency with increasing voltage. In this way, the electric field can be used to make a pixel switch between transparent or opaque on command. Color LCD systems use the same technique, with color filters used to generate red, green, and blue pixels. Similar principles can be used to make other liquid crystal based optical devices.
Liquid crystal tunable filter
Liquid crystal tunable filter
Liquid crystal tunable filters are solid-state optical filters that use electronically controlled liquid crystal elements to transmit a selectable wavelength of light and exclude others...
s are used as electrooptical devices, e.g. in hyperspectral imaging
Hyperspectral imaging
Hyperspectral imaging collects and processes information from across the electromagnetic spectrum. Much as the human eye sees visible light in three bands , spectral imaging divides the spectrum into many more bands...
.
Thermotropic
Thermochromism
Thermochromism is the ability of substance to change color due to a change in temperature. A mood ring is an excellent example of this, but it has many other uses such as baby bottles and kettles. Thermochromism is one of several types of chromism.The two basic approaches are based on liquid...
chiral LCs whose pitch varies strongly with temperature can be used as crude liquid crystal thermometer
Liquid crystal thermometer
A liquid crystal thermometer or plastic strip thermometer is a type of thermometer that contains heat-sensitive liquid crystals in a plastic strip that change color to indicate different temperatures....
s, since the color of the material will change as the pitch is changed. Liquid crystal color transitions are used on many aquarium and pool thermometers as well as on thermometers for infants or baths. Other liquid crystal materials change color when stretched or stressed. Thus, liquid crystal sheets are often used in industry to look for hot spots, map heat flow, measure stress distribution patterns, and so on. Liquid crystal in fluid form is used to detect electrically generated hot spots for failure analysis
Failure analysis
Failure analysis is the process of collecting and analyzing data to determine the cause of a failure. It is an important discipline in many branches of manufacturing industry, such as the electronics industry, where it is a vital tool used in the development of new products and for the improvement...
in the semiconductor
Semiconductor
A semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
industry.
Liquid crystal laser
Liquid crystal laser
A liquid crystal laser is a laser that uses a liquid crystal as the resonator cavity, allowing selection of emission wavelength and polarization from the active laser medium. The lasing medium is usually a dye doped into the liquid crystal...
s use a liquid crystal in the lasing medium
Active laser medium
The active laser medium is the source of optical gain within a laser. The gain results from the stimulated emission of electronic or molecular transitions to a lower energy state from a higher energy state...
as a distributed feedback mechanism instead of external mirrors. Emission at a photonic bandgap
Photonic crystal
Photonic crystals are periodic optical nanostructures that are designed to affect the motion of photons in a similar way that periodicity of a semiconductor crystal affects the motion of electrons...
created by the periodic dielectric structure of the liquid crystal gives a low-threshold high-output device with stable monochromatic emission.
Many common fluids, such as soapy water
Soap
In chemistry, soap is a salt of a fatty acid.IUPAC. "" Compendium of Chemical Terminology, 2nd ed. . Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford . XML on-line corrected version: created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. ISBN...
, are in fact liquid crystals. Soap forms a variety of LC phases depending on its concentration in water.
See also
- Biaxial nematic
- Columnar phaseColumnar phaseThe columnar phase is a class of mesophases in which molecules assemble into cylindrical structures to act as mesogens. Originally, these kinds of liquid crystals were called discotic liquid crystals because the columnar structures are composed of flat-shaped discotic molecules stacked...
- ChromonicChromonicIn a chromonic, relatively flat molecules form linear aggregates. For aqueous solutions, the molecules have a flat core, such as a carbon ring system, with highly water-soluble side groups, such as a sulfonate or carboxyl. The molecules aggregate in linear stacks with the flat cores lying one on...
- LCD classificationLCD classificationThere are various classifications of the electro-optical modes of liquid crystal displays .- LCD operation in a nutshell :The operation of TN, VA and IPS-LCDs can be summarized as follows:...
- Liquid crystal displayLiquid crystal displayA liquid crystal display is a flat panel display, electronic visual display, or video display that uses the light modulating properties of liquid crystals . LCs do not emit light directly....
- Liquid crystal polymerLiquid crystal polymerLiquid-crystal polymers are a class of aromatic polyester polymers. They are extremely unreactive and inert, and highly resistant to fire.-Background:...
- Liquid crystal tunable filterLiquid crystal tunable filterLiquid crystal tunable filters are solid-state optical filters that use electronically controlled liquid crystal elements to transmit a selectable wavelength of light and exclude others...
- Lyotropic liquid crystalLyotropic liquid crystalA liquid crystalline material is called lyotropic if phases having long-ranged orientational order are induced by the addition of a solvent. Historically the term was used to describe materials composed of amphiphilic molecules. Such molecules comprise a water-loving 'hydrophilic' head-group ...
- Pattern formationPattern formationThe science of pattern formation deals with the visible, orderly outcomes of self-organisation and the common principles behind similar patterns....
- Plastic crystallinityPlastic crystallinityA Plastic crystal is a crystal composed of weakly interacting molecules that possess some orientational or conformational degree of freedom. The name plastic crystal refers to the mechanical softness of such phases: they resemble waxes and are easily deformed...
- Smart glass
- Thermochromics
- Thermotropic crystal
- Twisted nematic field effectTwisted nematic field effectThe twisted nematic effect was a main technology breakthrough that made liquid crystal displays practical. Unlike earlier displays, TN-cells did not require a current to flow for operation and used low operating voltages suitable for use with batteries...
- nematiconNematiconIn optics, a nematicon is a spatial optical soliton in a nematic liquid crystal. The name was invented in 2003 by G. Assanto . Nematicons are generated by the special type of optical nonlinearity that is present in nematic LCs: the optical field induced director reorientation...
- Liquid crystal thermometerLiquid crystal thermometerA liquid crystal thermometer or plastic strip thermometer is a type of thermometer that contains heat-sensitive liquid crystals in a plastic strip that change color to indicate different temperatures....
- Mood ringMood ringA mood ring is a ring which contains a thermochromic element, such as liquid crystal. The ring changes color in response to the body temperature of its wearer...
External links
- Water, Energy, and Life: Fresh Views From the Water's Edge Dr. Pollack, U. of Washington, 2006 one hour lecture
- Definitions of basic terms relating to low-molar-mass and polymer liquid crystals (IUPAC Recommendations 2001)
- An intelligible introduction to liquid crystals from Case Western Reserve University
- Liquid Crystal Physics tutorial from the Liquid Crystals Group, University of Colorado
- Introduction to liquid crystals from the Liquid Crystal Technology Group, Oxford University
- Liquid Crystals & Photonics Group – Ghent University (Belgium), good tutorial
- Simulation of light propagation in liquid crystals, free program
- Liquid Crystals Interactive Online
- Liquid Crystal Institute Kent State University
- Liquid Crystals a journal by Taylor&Francis
- Molecular Crystals and Liquid Crystals a journal by Taylor & Francis
- Hot-spot detection techniques for ICs
- What are liquid crystals? from Chalmers University of Technology, Sweden
- Progress in liquid crystal chemistry Thematic series in the Open Access Beilstein Journal of Organic Chemistry
- DoITPoMS Teaching and Learning Package- "Liquid Crystals"

