Alkali metal
Encyclopedia
| Group → | 1 | |||||
|---|---|---|---|---|---|---|
| ↓ Period | ||||||
| 2 Period 2 element A period 2 element is one of the chemical elements in the second row of the periodic table. The periodic table is laid out in rows to illustrate recurring trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to... |
||||||
| 3 Period 3 element A period 3 element is one of the chemical elements in the third row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical... |
||||||
| 4 Period 4 element A period 4 element is one of the chemical elements in the fourth row of the periodic table of the elements. The periodic table is laid out in rows to illustrate recurring trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical behaviour... |
||||||
| 5 Period 5 element A period 5 element is one of the chemical elements in the fifth row of the periodic table of the elements. The periodic table is laid out in rows to illustrate recurring trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical behaviour... |
||||||
| 6 Period 6 element A period 6 element is one of the chemical elements in the sixth row of the periodic table of the elements, including the lanthanides... |
||||||
| 7 Period 7 element A period 7 element is one of the chemical elements in the seventh row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical... |
||||||
----
|
||||||
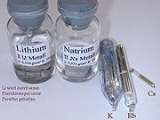
The alkali metals are a series of chemical element
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
s in the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
. In the modern IUPAC nomenclature, the alkali metals comprise the group 1 elements, along with hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
. The alkali metals are lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
(Li), sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
(Na), potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
(K), rubidium
Rubidium
Rubidium is a chemical element with the symbol Rb and atomic number 37. Rubidium is a soft, silvery-white metallic element of the alkali metal group. Its atomic mass is 85.4678. Elemental rubidium is highly reactive, with properties similar to those of other elements in group 1, such as very rapid...
(Rb), caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
(Cs), and francium
Francium
Francium is a chemical element with symbol Fr and atomic number 87. It was formerly known as eka-caesium and actinium K.Actually the least unstable isotope, francium-223 It has the lowest electronegativity of all known elements, and is the second rarest naturally occurring element...
(Fr). Hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
(H), although nominally also a member of group 1, rarely exhibits behavior comparable to the alkali metals. This group lies in the s-block
S-block
The s-block of the periodic table of elements consists of the first two groups: the alkali metals and alkaline earth metals, plus hydrogen and helium.Except in hydrogen and helium, these electrons are very easily lost to form positive ions...
of the periodic table; all alkali metals' outermost electrons
Electron configuration
In atomic physics and quantum chemistry, electron configuration is the arrangement of electrons of an atom, a molecule, or other physical structure...
lie in an s-orbital
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
. The alkali metals provide one of the best examples of group trends in properties in the periodic table, with elements exhibiting well characterized homologous
Homology (chemistry)
In chemistry, homology refers to the appearance of homologues. A homologue is a compound belonging to a series of compounds differing from each other by a repeating unit, such as a methylene group, a peptide residue, etcetera....
behavior. For instance, when moving down the table, all alkali metals show decreasing electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
, increasing reactivity, and decreasing melting and boiling points. In general, their densities increase, with the notable exception that potassium is less dense than sodium.
All discovered alkali metals are naturally occurring, although francium is the second-rarest naturally occurring element, after astatine
Astatine
Astatine is a radioactive chemical element with the symbol At and atomic number 85. It occurs on the Earth only as the result of decay of heavier elements, and decays away rapidly, so much less is known about this element than its upper neighbors in the periodic table...
. All are highly reactive metals under standard conditions. Experiments have been conducted to attempt the synthesis of ununennium
Ununennium
Ununennium also known as eka-francium or element 119, is the temporary name of a hypothetical chemical element in the periodic table that has the temporary symbol Uue and has the atomic number 119. To date, attempted syntheses of this element have been unsuccessful. Since it is below the alkali...
(Uue), likely the next member of the group, but have all met with failure.
Most alkali metals have many different applications. Two of the most well-known applications of the pure elements are rubidium and caesium atomic clock
Atomic clock
An atomic clock is a clock that uses an electronic transition frequency in the microwave, optical, or ultraviolet region of the electromagnetic spectrum of atoms as a frequency standard for its timekeeping element...
s, of which caesium atomic clocks are the most accurate representation of time known as of . A common application of the compounds of sodium is the sodium vapor lamp
Sodium vapor lamp
A sodium vapor lamp is a gas discharge lamp that uses sodium in an excited state to produce light. There are two varieties of such lamps: low pressure and high pressure...
, which emit very efficient light. Table salt, or sodium chloride, has been known of for a long time. The alkali metals can also be used as fertilizers.
Hydrogen
The element hydrogenHydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, with one electron per neutral atom, is usually placed at the top of Group 1 of the periodic table for convenience, but hydrogen is not usually considered as an alkali metal. Under typical conditions, pure hydrogen exists as a diatomic
Diatomic
Diatomic molecules are molecules composed only of two atoms, of either the same or different chemical elements. The prefix di- means two in Greek. Common diatomic molecules are hydrogen , nitrogen , oxygen , and carbon monoxide . Seven elements exist in the diatomic state in the liquid and solid...
gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
consisting of two atoms per molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
(H2).
Hydrogen, like the other alkali metals, has one valence electron; however, the similarities end there. In fact, its placement above lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
is primarily due to its electron configuration
Electron configuration
In atomic physics and quantum chemistry, electron configuration is the arrangement of electrons of an atom, a molecule, or other physical structure...
and not its chemical properties. It is sometimes placed above carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
due to similar electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
or fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
due to similar chemical properties.
The first ionization energy
Ionization energy
The ionization energy of a chemical species, i.e. an atom or molecule, is the energy required to remove an electron from the species to a practically infinite distance. Large atoms or molecules have a low ionization energy, while small molecules tend to have higher ionization energies.The property...
of hydrogen is much higher than that of the alkali metals. As only one additional electron is required to fill in the outermost shell of the hydrogen atom, hydrogen often behaves like a halogen, forming the negative hydride
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
ion. Binary compound
Binary compound
A binary compound is a chemical compound that contains exactly two different elements. Examples of binary ionic compounds include calcium chloride , sodium fluoride , and magnesium oxide , whilst examples of binary covalent compounds include water , carbon monoxide , and sulfur hexafluoride...
s of hydrogen with the alkali metals and some transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
s have been produced in the laboratory, but these are only laboratory curiosities without any practical use. Under extremely high pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
s and low temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
s, however, such as those found at the cores of the planets Jupiter
Jupiter
Jupiter is the fifth planet from the Sun and the largest planet within the Solar System. It is a gas giant with mass one-thousandth that of the Sun but is two and a half times the mass of all the other planets in our Solar System combined. Jupiter is classified as a gas giant along with Saturn,...
and Saturn
Saturn
Saturn is the sixth planet from the Sun and the second largest planet in the Solar System, after Jupiter. Saturn is named after the Roman god Saturn, equated to the Greek Cronus , the Babylonian Ninurta and the Hindu Shani. Saturn's astronomical symbol represents the Roman god's sickle.Saturn,...
, hydrogen does become metallic and behaves like an alkali metal; in this phase, it is known as metallic hydrogen
Metallic hydrogen
Metallic hydrogen is a state of hydrogen which results when it is sufficiently compressed and undergoes a phase transition; it is an example of degenerate matter. Solid metallic hydrogen is predicted to consist of a crystal lattice of hydrogen nuclei , with a spacing which is significantly smaller...
.
Chemical

| Z Atomic number In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element... | Element Chemical element A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements... | No. of electrons/shell Electron shell An electron shell may be thought of as an orbit followed by electrons around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" , followed by the "2 shell" , then the "3 shell" , and so on further and further from the nucleus. The shell letters K,L,M,..... | Electron configuration Electron configuration In atomic physics and quantum chemistry, electron configuration is the arrangement of electrons of an atom, a molecule, or other physical structure... |
|---|---|---|---|
| 3 | lithium Lithium Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly... |
2, 1 | [He]2s1 |
| 11 | sodium Sodium Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride... |
2, 8, 1 | [Ne]3s1 |
| 19 | potassium Potassium Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are... |
2, 8, 8, 1 | [Ar]4s1 |
| 37 | rubidium Rubidium Rubidium is a chemical element with the symbol Rb and atomic number 37. Rubidium is a soft, silvery-white metallic element of the alkali metal group. Its atomic mass is 85.4678. Elemental rubidium is highly reactive, with properties similar to those of other elements in group 1, such as very rapid... |
2, 8, 18, 8, 1 | [Kr]5s1 |
| 55 | caesium Caesium Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature... |
2, 8, 18, 18, 8, 1 | [Xe]6s1 |
| 87 | francium Francium Francium is a chemical element with symbol Fr and atomic number 87. It was formerly known as eka-caesium and actinium K.Actually the least unstable isotope, francium-223 It has the lowest electronegativity of all known elements, and is the second rarest naturally occurring element... |
2, 8, 18, 32, 18, 8, 1 | [Rn]7s1 |
Most of the chemistry has been observed only for the first five members of the group. The chemistry of francium is not well established; thus, the presentation of its properties here is limited. All the alkali metals are all highly reactive and are never found in elemental forms in nature. Because of this, they are usually stored in mineral oil
Mineral oil
A mineral oil is any of various colorless, odorless, light mixtures of alkanes in the C15 to C40 range from a non-vegetable source, particularly a distillate of petroleum....
or kerosene
Kerosene
Kerosene, sometimes spelled kerosine in scientific and industrial usage, also known as paraffin or paraffin oil in the United Kingdom, Hong Kong, Ireland and South Africa, is a combustible hydrocarbon liquid. The name is derived from Greek keros...
(paraffin oil).
The alkali metals are all silver-colored except for metallic caesium, which can have a golden tint. All are soft and have low density, melting point
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
s, and boiling point
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
s. In chemical terms
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, all of the alkali metals react aggressively with the halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
s to form ionic salts and also react with water to form strongly alkali
Alkali
In chemistry, an alkali is a basic, ionic salt of an alkali metal or alkaline earth metal element. Some authors also define an alkali as a base that dissolves in water. A solution of a soluble base has a pH greater than 7. The adjective alkaline is commonly used in English as a synonym for base,...
ne hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
s and thus should be handled with great care. The heavier alkali metals react more vigorously than the lighter ones. For example, when dropped into water, caesium produces a larger explosion than potassium. The alkali metals have the lowest first ionization energies
Ionization energy
The ionization energy of a chemical species, i.e. an atom or molecule, is the energy required to remove an electron from the species to a practically infinite distance. Large atoms or molecules have a low ionization energy, while small molecules tend to have higher ionization energies.The property...
in their respective periods of the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
because of their low effective nuclear charge
Effective nuclear charge
The effective nuclear charge is the net positive charge experienced by an electron in a multi-electron atom. The term "effective" is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge by the repelling effect...
and the ability to attain noble gas
Noble gas
The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
configuration by losing just one electron. The second ionization energy of all of the alkali metals is very high, thus they almost always lose a single electron, forming cations.
Alkali metals form a very wide range of amalgams.
They tend to form ionically bonded salts with most electronegative elements on the periodic table, for example caesium fluoride
Caesium fluoride
Caesium fluoride , is an inorganic compound usually encountered as a hygroscopic white solid. It is more soluble and more readily dissociated than sodium fluoride or potassium fluoride. It is available in anhydrous form, and if water has been absorbed it is easy to dry by heating at 100 °C for...
and sodium chloride
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
.
The ammonium
Ammonium
The ammonium cation is a positively charged polyatomic cation with the chemical formula NH. It is formed by the protonation of ammonia...
ion (NH) has very similar properties to the heavier alkali metals and is often considered a close relative.
Physical and atomic
The table below is a summary of the key physical properties of the alkali metals. The density of Francium is an estimation partially based on periodic trends rather than observations.| Group 1 element | Standard atomic weight Atomic weight Atomic weight is a dimensionless physical quantity, the ratio of the average mass of atoms of an element to 1/12 of the mass of an atom of carbon-12... (u) | Melting point Melting point The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure... (K Kelvin The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all... ) | Melting point Melting point The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure... (°C) | Boiling point Boiling point The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid.... (K Kelvin The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all... ) | Boiling point Boiling point The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid.... (°C) | Density Density The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight... (g Gram The gram is a metric system unit of mass.... /cm3) | Electronegativity Electronegativity Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus... (Pauling) |
|---|---|---|---|---|---|---|---|
| Lithium Lithium Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly... |
6.941 | 454 | 180.5 | 1615 | 1342 | 0.534 | 0.98 |
| Sodium Sodium Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride... |
22.98976928 | 370 | 97.8 | 1156 | 883 | 0.968 | 0.93 |
| Potassium Potassium Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are... |
39.0983 | 336 | 63.38 | 1032 | 759 | 0.89 | 0.82 |
| Rubidium Rubidium Rubidium is a chemical element with the symbol Rb and atomic number 37. Rubidium is a soft, silvery-white metallic element of the alkali metal group. Its atomic mass is 85.4678. Elemental rubidium is highly reactive, with properties similar to those of other elements in group 1, such as very rapid... |
85.4678 | 312 | 39.31 | 961 | 688 | 1.532 | 0.82 |
| Caesium Caesium Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature... |
132.9054519 | 301 | 28.44 | 944 | 671 | 1.93 | 0.79 |
| Francium Francium Francium is a chemical element with symbol Fr and atomic number 87. It was formerly known as eka-caesium and actinium K.Actually the least unstable isotope, francium-223 It has the lowest electronegativity of all known elements, and is the second rarest naturally occurring element... |
(223) | 300.15 | 27 | 950.15 | 677 | 1.87 | 0.70 |
Lithium
PetalitePetalite
Petalite, also known as castorite, is a lithium aluminium tectosilicate mineral LiAlSi4O10, crystallizing in the monoclinic system. Petalite is a member of the feldspathoid group. It occurs as colorless, grey, yellow, yellow grey, to white tabular crystals and columnar masses. Occurs in...
(LiAlSi4O10) was discovered in 1800 by the Brazil
Brazil
Brazil , officially the Federative Republic of Brazil , is the largest country in South America. It is the world's fifth largest country, both by geographical area and by population with over 192 million people...
ian chemist José Bonifácio de Andrada e Silva in a mine on the island of Utö, Sweden
Utö, Sweden
Utö is a small island in the East of Stockholm archipelago, famous for its nature. Utö has the oldest iron-ore mines in Sweden.A famous landmark of Utö is its windmill, which is over 200 years old, and from which there is a good view of the bay Mysingen....
. However, it was not until 1817 that Johan August Arfwedson
Johan August Arfwedson
Johan August Arfwedson was a Swedish chemist who discovered the chemical element lithium in 1817 by isolating it as a salt.- Life and work :...
, then working in the laboratory of the chemist Jöns Jakob Berzelius
Jöns Jakob Berzelius
Jöns Jacob Berzelius was a Swedish chemist. He worked out the modern technique of chemical formula notation, and is together with John Dalton, Antoine Lavoisier, and Robert Boyle considered a father of modern chemistry...
, detected the presence of a new element while analyzing petalite ore. This new element formed compounds similar to those of sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
and potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
, though its carbonate
Lithium carbonate
Lithium carbonate is a chemical compound of lithium, carbon, and oxygen with the formula Li2CO3. This colorless salt is widely used in the processing of metal oxides and has received attention for its use in psychiatry. It is found in nature as the rare mineral zabuyelite.-Properties:Like almost...
and hydroxide
Lithium hydroxide
Lithium hydroxide is an inorganic compound with the formula LiOH. It is a white hygroscopic crystalline material. It is soluble in water and slightly soluble in ethanol...
were less soluble in water
Solubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
and more alkaline
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
than the other alkali metals. Berzelius gave the unknown material the name "lithion/lithina", from the Greek
Greek language
Greek is an independent branch of the Indo-European family of languages. Native to the southern Balkans, it has the longest documented history of any Indo-European language, spanning 34 centuries of written records. Its writing system has been the Greek alphabet for the majority of its history;...
word λιθoς (transliterated as lithos, meaning "stone"), to reflect its discovery in a solid mineral, as opposed to potassium, which had been discovered in plant ashes, and sodium, which was known partly for its high abundance in animal blood. He named the metal inside the material "lithium".
Sodium

Humphry Davy
Sir Humphry Davy, 1st Baronet FRS MRIA was a British chemist and inventor. He is probably best remembered today for his discoveries of several alkali and alkaline earth metals, as well as contributions to the discoveries of the elemental nature of chlorine and iodine...
through the electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
of caustic soda, a very similar method to the one used to isolate potassium earlier that year.
Potassium
Potassium metal was first isolated in 1807 in England by Sir Humphry DavyHumphry Davy
Sir Humphry Davy, 1st Baronet FRS MRIA was a British chemist and inventor. He is probably best remembered today for his discoveries of several alkali and alkaline earth metals, as well as contributions to the discoveries of the elemental nature of chlorine and iodine...
, who derived it from caustic potash
Potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
(KOH) by the use of electrolysis of the molten salt with the newly invented voltaic pile
Voltaic pile
A voltaic pile is a set of individual Galvanic cells placed in series. The voltaic pile, invented by Alessandro Volta in 1800, was the first electric battery...
. Potassium was the first metal that was isolated by electrolysis. Later that same year, Davy reported extraction of the metal sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
from the similar substance caustic soda (NaOH, or lye) by a similar technique, demonstrating the elements, and thus the salts, to be different.
Rubidium
Rubidium was discovered in 1861 in Heidelberg, Germany by Robert BunsenRobert Bunsen
Robert Wilhelm Eberhard Bunsen was a German chemist. He investigated emission spectra of heated elements, and discovered caesium and rubidium with Gustav Kirchhoff. Bunsen developed several gas-analytical methods, was a pioneer in photochemistry, and did early work in the field of organoarsenic...
and Gustav Kirchhoff
Gustav Kirchhoff
Gustav Robert Kirchhoff was a German physicist who contributed to the fundamental understanding of electrical circuits, spectroscopy, and the emission of black-body radiation by heated objects...
, the first people to suggest finding new elements by spectrum analysis
Spectrum analysis
Spectrum, also known as emission spectrochemical analysis, is the original scientific method of charting and analyzing the chemical properties of matter and gases by looking at the bands in their optical spectrum...
, in the mineral
Mineral
A mineral is a naturally occurring solid chemical substance formed through biogeochemical processes, having characteristic chemical composition, highly ordered atomic structure, and specific physical properties. By comparison, a rock is an aggregate of minerals and/or mineraloids and does not...
lepidolite
Lepidolite
Lepidolite Lepidolite Lepidolite (KLi2Al(Al,Si)3O10(F,OH)2 is a lilac-gray or rose-colored phyllosilicate mineral of the mica group that is a secondary source of lithium. It is associated with other lithium-bearing minerals like spodumene in pegmatite bodies. It is one of the major sources of the...
through the use of a spectroscope. Because of the bright red lines in its emission spectrum
Emission spectrum
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted by the element's atoms or the compound's molecules when they are returned to a lower energy state....
, they chose a name derived from the Latin
Latin
Latin is an Italic language originally spoken in Latium and Ancient Rome. It, along with most European languages, is a descendant of the ancient Proto-Indo-European language. Although it is considered a dead language, a number of scholars and members of the Christian clergy speak it fluently, and...
word rubidus, meaning dark red. Rubidium's discovery succeeded that of caesium, also discovered by Bunsen and Kirchhoff through spectroscopy.
Caesium
In 1860, Robert BunsenRobert Bunsen
Robert Wilhelm Eberhard Bunsen was a German chemist. He investigated emission spectra of heated elements, and discovered caesium and rubidium with Gustav Kirchhoff. Bunsen developed several gas-analytical methods, was a pioneer in photochemistry, and did early work in the field of organoarsenic...
and Gustav Kirchhoff
Gustav Kirchhoff
Gustav Robert Kirchhoff was a German physicist who contributed to the fundamental understanding of electrical circuits, spectroscopy, and the emission of black-body radiation by heated objects...
discovered caesium in the mineral water
Mineral water
Mineral water is water containing minerals or other dissolved substances that alter its taste or give it therapeutic value, generally obtained from a naturally occurring mineral spring or source. Dissolved substances in the water may include various salts and sulfur compounds...
from Dürkheim
Bad Dürkheim
Bad Dürkheim is a spa town in the Rhine-Neckar urban agglomeration, and is the seat of the Bad Dürkheim district in Rhineland-Palatinate, Germany.- Location :...
, Germany
Germany
Germany , officially the Federal Republic of Germany , is a federal parliamentary republic in Europe. The country consists of 16 states while the capital and largest city is Berlin. Germany covers an area of 357,021 km2 and has a largely temperate seasonal climate...
. Due to the bright-blue lines in its emission spectrum
Emission spectrum
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted by the element's atoms or the compound's molecules when they are returned to a lower energy state....
, they chose a name derived from the Latin word caesius, meaning sky-blue. Caesium was the first element to be discovered spectroscopically
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
, only one year after the invention of the spectroscope by Bunsen and Kirchhoff.
Francium
There were at least three erroneous and incomplete discoveries before Marguerite PereyMarguerite Perey
Marguerite Catherine Perey was a French physicist. In 1939, Perey discovered the element francium by purifying samples of lanthanum that contained actinium. She was a student of Marie Curie...
of the Curie Institute
Curie Institute (Paris)
thumb|Centre of protontherapyInstitut Curie is one of the leading medical, biological and biophysical research centres in the world.It is a private non-profit foundation operating a research center on biophysics, cell biology and oncology and a hospital specialized in treatment of cancer...
in Paris, France discovered Francium in 1939 by purifying a sample of actinium
Actinium
Actinium is a radioactive chemical element with the symbol Ac and atomic number 89, which was discovered in 1899. It was the first non-primordial radioactive element to be isolated. Polonium, radium and radon were observed before actinium, but they were not isolated until 1902...
-227, which had been reported to have a decay energy of 220 keV. However, Perey noticed decay particles with an energy level below 80 keV. Perey thought this decay activity might have been caused by a previously unidentified decay product, one that was separated during purification, but emerged again out of the pure actinium-227. Various tests eliminated the possibility of the unknown element being thorium
Thorium
Thorium is a natural radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 and named after Thor, the Norse god of thunder....
, radium
Radium
Radium is a chemical element with atomic number 88, represented by the symbol Ra. Radium is an almost pure-white alkaline earth metal, but it readily oxidizes on exposure to air, becoming black in color. All isotopes of radium are highly radioactive, with the most stable isotope being radium-226,...
, lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
, bismuth
Bismuth
Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead...
, or thallium
Thallium
Thallium is a chemical element with the symbol Tl and atomic number 81. This soft gray poor metal resembles tin but discolors when exposed to air. The two chemists William Crookes and Claude-Auguste Lamy discovered thallium independently in 1861 by the newly developed method of flame spectroscopy...
. The new product exhibited chemical properties of an alkali metal (such as coprecipitating with caesium salts), which led Perey to believe that it was element 87, caused by the alpha decay
Alpha decay
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less...
of actinium-227. Perey then attempted to determine the proportion of beta decay
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
to alpha decay in actinium-227. Her first test put the alpha branching at 0.6%, a figure that she later revised to 1%.
Eka-francium
The next element below franciumFrancium
Francium is a chemical element with symbol Fr and atomic number 87. It was formerly known as eka-caesium and actinium K.Actually the least unstable isotope, francium-223 It has the lowest electronegativity of all known elements, and is the second rarest naturally occurring element...
(Eka-francium) is likely to be ununennium
Ununennium
Ununennium also known as eka-francium or element 119, is the temporary name of a hypothetical chemical element in the periodic table that has the temporary symbol Uue and has the atomic number 119. To date, attempted syntheses of this element have been unsuccessful. Since it is below the alkali...
(Uue), element 119, although this is not certain. The synthesis of ununennium was first attempted in 1985 by bombarding a target of einsteinium
Einsteinium
Einsteinium is a synthetic element with the symbol Es and atomic number 99. It is the seventh transuranic element, and an actinide.Einsteinium was discovered in the debris of the first hydrogen bomb explosion in 1952, and named after Albert Einstein...
-254 with calcium
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
-48 ions at the superHILAC accelerator at Berkeley, California. No atoms were identified, leading to a limiting yield of 300 nb.
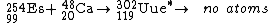
It is highly unlikely that this reaction will be able to create any atoms of ununennium in the near future, given the extremely difficult task of making sufficient amounts of 254Es
Isotopes of einsteinium
Einsteinium is an artificial element, and thus a standard atomic mass cannot be given. Like all artificial elements, it has no stable isotopes. The first isotope to be discovered was 253Es in 1952...
to make a large enough target to increase the sensitivity of the experiment to the required level; einsteinium has not been found in nature and has only been produced in laboratories. However, given that ununennium is only the first period 8 element
Period 8 element
A period 8 element is any one of 50 hypothetical chemical elements belonging to an eighth period of the periodic table of the elements. They may be referred to using IUPAC systematic element names. None of these elements have been created, and it is possible that none have isotopes with stable...
on the extended periodic table, it may well be discovered in the near future through other reactions. Currently, none of the period 8 elements have been discovered yet. It is also possible that, due to drip instabilities, only the lower period 8 elements are physically possible.
Production
The production of pure alkali metals is difficult due to their extreme reactivity with commonly used substances, such as water. Lithium salts have to be extracted from the water of mineral springMineral spring
Mineral springs are naturally occurring springs that produce water containing minerals, or other dissolved substances, that alter its taste or give it a purported therapeutic value...
s, brine
Brine
Brine is water, saturated or nearly saturated with salt .Brine is used to preserve vegetables, fruit, fish, and meat, in a process known as brining . Brine is also commonly used to age Halloumi and Feta cheeses, or for pickling foodstuffs, as a means of preserving them...
pools, and brine deposits. The metal is produced electrolytically
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
from a mixture of fused lithium chloride
Lithium chloride
Lithium chloride is a chemical compound with the formula LiCl. The salt is a typical ionic compound, although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlorides, such as extraordinary solubility in polar solvents and its hygroscopic...
and potassium chloride
Potassium chloride
The chemical compound potassium chloride is a metal halide salt composed of potassium and chlorine. In its pure state, it is odorless and has a white or colorless vitreous crystal appearance, with a crystal structure that cleaves easily in three directions. Potassium chloride crystals are...
.
Potassium is occasionally produced through separating the potassium from the chlorine in potassium chloride
Potassium chloride
The chemical compound potassium chloride is a metal halide salt composed of potassium and chlorine. In its pure state, it is odorless and has a white or colorless vitreous crystal appearance, with a crystal structure that cleaves easily in three directions. Potassium chloride crystals are...
, but is more often produced through electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
of potassium hydroxide
Potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
, found extensively in places such as Canada
Canada
Canada is a North American country consisting of ten provinces and three territories. Located in the northern part of the continent, it extends from the Atlantic Ocean in the east to the Pacific Ocean in the west, and northward into the Arctic Ocean...
, Russia
Russia
Russia or , officially known as both Russia and the Russian Federation , is a country in northern Eurasia. It is a federal semi-presidential republic, comprising 83 federal subjects...
, Belarus
Belarus
Belarus , officially the Republic of Belarus, is a landlocked country in Eastern Europe, bordered clockwise by Russia to the northeast, Ukraine to the south, Poland to the west, and Lithuania and Latvia to the northwest. Its capital is Minsk; other major cities include Brest, Grodno , Gomel ,...
, Germany
Germany
Germany , officially the Federal Republic of Germany , is a federal parliamentary republic in Europe. The country consists of 16 states while the capital and largest city is Berlin. Germany covers an area of 357,021 km2 and has a largely temperate seasonal climate...
, Israel
Israel
The State of Israel is a parliamentary republic located in the Middle East, along the eastern shore of the Mediterranean Sea...
, United States
United States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
, and Jordan
Jordan
Jordan , officially the Hashemite Kingdom of Jordan , Al-Mamlaka al-Urduniyya al-Hashemiyya) is a kingdom on the East Bank of the River Jordan. The country borders Saudi Arabia to the east and south-east, Iraq to the north-east, Syria to the north and the West Bank and Israel to the west, sharing...
, in a method similar to how sodium was produced in in the late 1800s and early 1900s. Sodium is now produced through electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
of sodium chloride
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
by lowering the melting point of the substance to below 700 °C through the use of a Downs Cell
Downs cell
The Downs process is an electrochemical method for the commercial preparation of metallic sodium, in which molten NaCl is electrolyzed in a special apparatus called the Downs cell.-How it works:...
. Extremely pure sodium can be produced through the thermal decomposition of sodium azide
Sodium azide
Sodium azide is the inorganic compound with the formula NaN3. This colourless azide salt is the gas-forming component in many car airbag systems. It is used for the preparation of other azide compounds. It is an ionic substance and is highly soluble in water. It is extremely...
.
usgov.jpg)
Tanco Mine
Tanco Mine is an underground caesium and tantalum mine, owned and operated by Cabot Corporation on the north west shore of Bernic Lake, Manitoba, Canada. The mine has the largest known deposit of pollucite and is also the world's largest producer of caesium....
, Manitoba, Canada, produce rubidium as by-product from pollucite
Pollucite
Pollucite is a zeolite mineral with the formula 2Al2Si4O12·2H2O with iron, calcium, rubidium and potassium as common substituting elements. It is important as a significant ore of caesium and sometimes rubidium. It forms a solid solution series with analcime. It crystallizes in the isometric -...
. Today, a common method for separating rubidium from potassium and caesium is the fractional crystallization
Fractional crystallization (chemistry)
In chemistry, fractional crystallization is a method of refining substances based on differences in solubility. If a mixture of two or more substances in solution is allowed to crystallize, for example by allowing the temperature of the solution to decrease, the precipitate will contain more of...
of a rubidium and caesium alum (Cs,Rb)Al(SO4)2·12H2O, which yields pure rubidium alum after approximately 30 different reactions. The limited applications and the lack of a mineral rich in rubidium limits the production of rubidium compounds to 2 to 4 tonne
Tonne
The tonne, known as the metric ton in the US , often put pleonastically as "metric tonne" to avoid confusion with ton, is a metric system unit of mass equal to 1000 kilograms. The tonne is not an International System of Units unit, but is accepted for use with the SI...
s per year. Caesium, however, is not produced from the above reaction. Instead, the mining of pollucite
Pollucite
Pollucite is a zeolite mineral with the formula 2Al2Si4O12·2H2O with iron, calcium, rubidium and potassium as common substituting elements. It is important as a significant ore of caesium and sometimes rubidium. It forms a solid solution series with analcime. It crystallizes in the isometric -...
ore is the main method of obtaining pure caesium, extracted from the ore mainly by three methods: acid digestion, alkaline decomposition, and direct reduction.

Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
and thorium
Thorium
Thorium is a natural radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 and named after Thor, the Norse god of thunder....
mineral
Mineral
A mineral is a naturally occurring solid chemical substance formed through biogeochemical processes, having characteristic chemical composition, highly ordered atomic structure, and specific physical properties. By comparison, a rock is an aggregate of minerals and/or mineraloids and does not...
s; it has been calculated that at most there are 30 g of francium in the earth's crust
Crust (geology)
In geology, the crust is the outermost solid shell of a rocky planet or natural satellite, which is chemically distinct from the underlying mantle...
at any given time. This makes it the second rarest element
Abundance of the chemical elements
The abundance of a chemical element measures how relatively common the element is, or how much of the element is present in a given environment by comparison to all other elements...
in the crust after astatine
Astatine
Astatine is a radioactive chemical element with the symbol At and atomic number 85. It occurs on the Earth only as the result of decay of heavier elements, and decays away rapidly, so much less is known about this element than its upper neighbors in the periodic table...
. As a result of its extreme rarity in nature, most francium is synthesized in the nuclear reaction 197Au + 18O → 210Fr + 5 n, yielding francium-209, francium-210, and francium-211.
As of , the synthesis of Ununennium
Ununennium
Ununennium also known as eka-francium or element 119, is the temporary name of a hypothetical chemical element in the periodic table that has the temporary symbol Uue and has the atomic number 119. To date, attempted syntheses of this element have been unsuccessful. Since it is below the alkali...
has been met with failure.
Occurrence
The alkali metals, due to their high reactivity, do not occur naturally in pure form in nature. Lithium, due to its relatively low reactivity, can be found in seawater in large amounts; it is estimated that seawater is approximately 0.14 to 0.25 parts per million (ppm) or 25 micromolar.Sodium and potassium are very abundant in earth; sodium makes up approximately 2.6% of the Earth
Earth
Earth is the third planet from the Sun, and the densest and fifth-largest of the eight planets in the Solar System. It is also the largest of the Solar System's four terrestrial planets...
's crust measured by weight, making it the sixth most abundant element
Abundance of the chemical elements
The abundance of a chemical element measures how relatively common the element is, or how much of the element is present in a given environment by comparison to all other elements...
overall and the most abundant alkali metal. Potassium makes up approximately 1.5% of the Earth's crust and is the seventh most abundant element. Sodium is found in many different minerals, of which the most common is ordinary salt
Salt
In chemistry, salts are ionic compounds that result from the neutralization reaction of an acid and a base. They are composed of cations and anions so that the product is electrically neutral...
(sodium chloride), which occurs in vast quantities dissolved in seawater. Other solid deposits include halite
Halite
Halite , commonly known as rock salt, is the mineral form of sodium chloride . Halite forms isometric crystals. The mineral is typically colorless or white, but may also be light blue, dark blue, purple, pink, red, orange, yellow or gray depending on the amount and type of impurities...
, amphibole
Amphibole
Amphibole is the name of an important group of generally dark-colored rock-forming inosilicate minerals, composed of double chain tetrahedra, linked at the vertices and generally containing ions of iron and/or magnesium in their structures.-Mineralogy:...
, cryolite
Cryolite
Cryolite is an uncommon mineral identified with the once large deposit at Ivigtût on the west coast of Greenland, depleted by 1987....
, soda niter, and zeolite
Zeolite
Zeolites are microporous, aluminosilicate minerals commonly used as commercial adsorbents. The term zeolite was originally coined in 1756 by Swedish mineralogist Axel Fredrik Cronstedt, who observed that upon rapidly heating the material stilbite, it produced large amounts of steam from water that...
.
Caesium is more abundant than some commonly known elements, such as antimony
Antimony
Antimony is a toxic chemical element with the symbol Sb and an atomic number of 51. A lustrous grey metalloid, it is found in nature mainly as the sulfide mineral stibnite...
, cadmium
Cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low...
, tin
Tin
Tin is a chemical element with the symbol Sn and atomic number 50. It is a main group metal in group 14 of the periodic table. Tin shows chemical similarity to both neighboring group 14 elements, germanium and lead and has two possible oxidation states, +2 and the slightly more stable +4...
, and tungsten
Tungsten
Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as...
, but is much less abundant than rubidium. Rubidium is approximately as abundant as zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
and more abundant than copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
. It occurs naturally in the minerals leucite
Leucite
Leucite is a rock-forming mineral composed of potassium and aluminium tectosilicate K[AlSi2O6]. Crystals have the form of cubic icositetrahedra but, as first observed by Sir David Brewster in 1821, they are not optically isotropic, and are therefore pseudo-cubic. Goniometric measurements made by...
, pollucite
Pollucite
Pollucite is a zeolite mineral with the formula 2Al2Si4O12·2H2O with iron, calcium, rubidium and potassium as common substituting elements. It is important as a significant ore of caesium and sometimes rubidium. It forms a solid solution series with analcime. It crystallizes in the isometric -...
, carnallite
Carnallite
Carnallite is an evaporite mineral, a hydrated potassium magnesium chloride with formula: KMgCl3·6. It is variably colored yellow to white, reddish, and sometimes colorless or blue. It is usually massive to fibrous with rare pseudohexagonal orthorhombic crystals...
, zinnwaldite
Zinnwaldite
Zinnwaldite, KLiFeAlO102, is a potassium lithium iron aluminium silicate hydroxide fluoride silicate mineral in the mica group. IMA status: a series, not a mineral.-Name and discovery:...
, and lepidolite
Lepidolite
Lepidolite Lepidolite Lepidolite (KLi2Al(Al,Si)3O10(F,OH)2 is a lilac-gray or rose-colored phyllosilicate mineral of the mica group that is a secondary source of lithium. It is associated with other lithium-bearing minerals like spodumene in pegmatite bodies. It is one of the major sources of the...
.
Francium-223
Isotopes of francium
Francium has no stable isotopes. A standard atomic mass cannot be given. Its most stable isotope is 223Fr with a half-life of 22 minutes, which is also the only naturally occurring isotope, occurring in trace quantities as an intermediate decay product of 235U.Of elements whose most stable...
, the only naturally occurring isotope of francium
Francium
Francium is a chemical element with symbol Fr and atomic number 87. It was formerly known as eka-caesium and actinium K.Actually the least unstable isotope, francium-223 It has the lowest electronegativity of all known elements, and is the second rarest naturally occurring element...
, is the result of the alpha decay of actinium-227 and can be found in trace amounts in uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
and thorium
Thorium
Thorium is a natural radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 and named after Thor, the Norse god of thunder....
mineral
Mineral
A mineral is a naturally occurring solid chemical substance formed through biogeochemical processes, having characteristic chemical composition, highly ordered atomic structure, and specific physical properties. By comparison, a rock is an aggregate of minerals and/or mineraloids and does not...
s In a given sample of uranium, there is estimated to be only one francium atom for every 1×1018 uranium atoms. It has been calculated that there is at most 30 g of francium in the earth's crust
Crust (geology)
In geology, the crust is the outermost solid shell of a rocky planet or natural satellite, which is chemically distinct from the underlying mantle...
at any time, due to its extremely short half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 22 minutes, making it the second-rarest element
Abundance of the chemical elements
The abundance of a chemical element measures how relatively common the element is, or how much of the element is present in a given environment by comparison to all other elements...
in the crust after astatine
Astatine
Astatine is a radioactive chemical element with the symbol At and atomic number 85. It occurs on the Earth only as the result of decay of heavier elements, and decays away rapidly, so much less is known about this element than its upper neighbors in the periodic table...
.
Ununennium does not occur naturally in nature, and until it is synthesized in laboratories, it is not likely that any exists on earth.
Applications
All of the discovered alkali metals excluding francium have many applications. Lithium is often used in batteries, and lithium oxideLithium oxide
Lithium oxide or lithia is an inorganic chemical compound. Lithium oxide is formed along with small amounts of lithium peroxide when lithium metal is burned in the air and combines with oxygen:Pure can be produced by the thermal decomposition of lithium peroxide, at 450°C-Structure:In the solid...
can help process silica. Lithium can also be used to make lubricating greases, air treatment, and aluminium production.
Pure sodium has many applications, including use in sodium-vapor lamps, which produce very efficient light compared to other types of lighting, and can help smooth the surface of other metals. Sodium compounds have many applications as well, the most well-known compound being table salt. Sodium is also used in soap as salts of fatty acid
Fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
s.
Potassium is often used as a fertilizer, as it is an important element for plant
Plant
Plants are living organisms belonging to the kingdom Plantae. Precise definitions of the kingdom vary, but as the term is used here, plants include familiar organisms such as trees, flowers, herbs, bushes, grasses, vines, ferns, mosses, and green algae. The group is also called green plants or...
nutrition. Other potassium ions are often used to hold anions. Potassium hydroxide is a very strong base, and is used to control pH of various substances
Rubidium and caesium are often used in atomic clock
Atomic clock
An atomic clock is a clock that uses an electronic transition frequency in the microwave, optical, or ultraviolet region of the electromagnetic spectrum of atoms as a frequency standard for its timekeeping element...
s. Caesium atomic clocks are extraordinarily accurate; if a clock had been made at the time of the dinosaurs, it would be off by less than two seconds. For that reason, cesium atoms are used as the definition of the second. Rubidium ions are often used in purple fireworks, and caesium is often used in drilling fluids in the petroleum industry.
Francium has no commercial applications, but because of francium's relatively simple atomic structure, among other things, it has been used in spectroscopy
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
experiments, leading to more information regarding energy level
Energy level
A quantum mechanical system or particle that is bound -- that is, confined spatially—can only take on certain discrete values of energy. This contrasts with classical particles, which can have any energy. These discrete values are called energy levels...
s and the coupling constant
Coupling constant
In physics, a coupling constant, usually denoted g, is a number that determines the strength of an interaction. Usually the Lagrangian or the Hamiltonian of a system can be separated into a kinetic part and an interaction part...
s between subatomic particle
Subatomic particle
In physics or chemistry, subatomic particles are the smaller particles composing nucleons and atoms. There are two types of subatomic particles: elementary particles, which are not made of other particles, and composite particles...
s. Studies on the light emitted by laser-trapped francium-210 ions have provided accurate data on transitions between atomic energy levels, similar to those predicted by quantum theory
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
.
Biological occurrences
The lighter alkali metals all have a role in the human body. Lithium carbonateLithium carbonate
Lithium carbonate is a chemical compound of lithium, carbon, and oxygen with the formula Li2CO3. This colorless salt is widely used in the processing of metal oxides and has received attention for its use in psychiatry. It is found in nature as the rare mineral zabuyelite.-Properties:Like almost...
is a mood stabilizer and is used to treat bipolar disorder
Bipolar disorder
Bipolar disorder or bipolar affective disorder, historically known as manic–depressive disorder, is a psychiatric diagnosis that describes a category of mood disorders defined by the presence of one or more episodes of abnormally elevated energy levels, cognition, and mood with or without one or...
(manic-depression), although there are side-effects. Excessive ingestion of lithium poison
Poison
In the context of biology, poisons are substances that can cause disturbances to organisms, usually by chemical reaction or other activity on the molecular scale, when a sufficient quantity is absorbed by an organism....
s the central nervous system
Central nervous system
The central nervous system is the part of the nervous system that integrates the information that it receives from, and coordinates the activity of, all parts of the bodies of bilaterian animals—that is, all multicellular animals except sponges and radially symmetric animals such as jellyfish...
, which is even more dangerous than the disorder itself, as the lethal dose is only just above the therapeutic dose.
Sodium is an essential nutrient that regulates blood volume, blood pressure, osmotic equilibrium and pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
; the minimum physiological requirement for sodium is 500 milligrams per day. Sodium chloride
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
is the principal source of sodium in the diet, and is used as seasoning and preservative, such as for pickling
Pickling
Pickling, also known as brining or corning is the process of preserving food by anaerobic fermentation in brine to produce lactic acid, or marinating and storing it in an acid solution, usually vinegar . The resulting food is called a pickle. This procedure gives the food a salty or sour taste...
and jerky
Jerky (food)
Jerky is lean meat that has been trimmed of fat, cut into strips, and then been dried to prevent spoilage. Normally, this drying includes the addition of salt, to prevent bacteria from developing on the meat before sufficient moisture has been removed. The word "jerky" is a bastardization of the...
; most of it comes from processed foods. The DRI
Dietary Reference Intake
The Dietary Reference Intake is a system of nutrition recommendations from the Institute of Medicine of the U.S. National Academy of Sciences. The DRI system is used by both the United States and Canada and is intended for the general public and health professionals...
for sodium is 1.5 grams per day, but most people in the United States consume more than 2.3 grams per day, the minimum amount that promotes hypertension; this in turn causes 7.6 million premature deaths worldwide.
Potassium is the major cation (positive ion) inside animal cells
Cell (biology)
The cell is the basic structural and functional unit of all known living organisms. It is the smallest unit of life that is classified as a living thing, and is often called the building block of life. The Alberts text discusses how the "cellular building blocks" move to shape developing embryos....
, while sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
is the major cation outside animal cells. The concentration
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
differences of these charged particles causes a difference in electric potential
Electric potential
In classical electromagnetism, the electric potential at a point within a defined space is equal to the electric potential energy at that location divided by the charge there...
between the inside and outside of cells, known as the membrane potential
Membrane potential
Membrane potential is the difference in electrical potential between the interior and exterior of a biological cell. All animal cells are surrounded by a plasma membrane composed of a lipid bilayer with a variety of types of proteins embedded in it...
. The balance between potassium and sodium is maintained by ion pumps in the cell membrane
Cell membrane
The cell membrane or plasma membrane is a biological membrane that separates the interior of all cells from the outside environment. The cell membrane is selectively permeable to ions and organic molecules and controls the movement of substances in and out of cells. It basically protects the cell...
. All potassium ion channels are tetramers with several conserved secondary structural elements. The most recently resolved potassium ion channel is KirBac3.1, which gives a total of five potassium ion channels (KcsA, KirBac1.1, KirBac3.1, KvAP, MthK) with a determined structure. All five are from prokaryotic species. The cell membrane potential created by potassium and sodium ions allows the cell to generate an action potential
Action potential
In physiology, an action potential is a short-lasting event in which the electrical membrane potential of a cell rapidly rises and falls, following a consistent trajectory. Action potentials occur in several types of animal cells, called excitable cells, which include neurons, muscle cells, and...
—a "spike" of electrical discharge. The ability of cells to produce electrical discharge is critical for body functions such as neurotransmission
Neurotransmission
Neurotransmission , also called synaptic transmission, is the process by which signaling molecules called neurotransmitters are released by a neuron , and bind to and activate the receptors of another neuron...
, muscle contraction, and heart function.
Rubidium has no known biological role, but may help stimulate metabolism
Metabolism
Metabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
, and, similarly to caesium, replace potassium in the body causing potassium deficiency
Potassium deficiency
Potassium deficiency has two different contexts:*For the medical condition in humans, see hypokalemia*Potassium deficiency , the disease in plants...
.
Caesium compounds are rarely encountered by most people, but most caesium compounds are mildly toxic because of chemical similarity of caesium to potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
. Exposure to large amounts of caesium compounds can cause hyperirritability
Irritability
Irritability is an excessive response to stimuli. The term is used for both the physiological reaction to stimuli and for the pathological, abnormal or excessive sensitivity to stimuli; It is usually used to refer to anger or frustration....
and spasm
Spasm
In medicine a spasm is a sudden, involuntary contraction of a muscle, a group of muscles, or a hollow organ, or a similarly sudden contraction of an orifice. It is sometimes accompanied by a sudden burst of pain, but is usually harmless and ceases after a few minutes...
s, but as such amounts would not ordinarily be encountered in natural sources, caesium is not a major chemical environmental pollutant. The median lethal dose (LD50) value for caesium chloride
Caesium chloride
Caesium chloride is the inorganic compound with the formula CsCl. This colorless solid is an important source of caesium ions in a variety of applications. Its crystal structure forms a major structural type where each caesium ion is coordinated by 8 chlorine ions...
in mice is 2.3 g per kilogram, which is comparable to the LD50 values of potassium chloride
Potassium chloride
The chemical compound potassium chloride is a metal halide salt composed of potassium and chlorine. In its pure state, it is odorless and has a white or colorless vitreous crystal appearance, with a crystal structure that cleaves easily in three directions. Potassium chloride crystals are...
and sodium chloride
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
. Caesium chloride
Caesium chloride
Caesium chloride is the inorganic compound with the formula CsCl. This colorless solid is an important source of caesium ions in a variety of applications. Its crystal structure forms a major structural type where each caesium ion is coordinated by 8 chlorine ions...
has been promoted as an alternative cancer therapy, but has been linked to the deaths of over 50 patients, on whom it was used as part of a scientifically unvalidated cancer treatment.
Francium has no biological role and is most likely to be toxic due to its high radioactivity, although the greatest quantity of francium ever assembled to date is a sphere of radius 1 mm (about 300,000 neutral atoms).
External links
-
- Science aid: Alkali metals A simple look at alkali metals
- Atomic and Physical Properties of the Group 1 Elements An in-depth look at alkali metals
Links to related articles

