Glossary of chemistry terms
Encyclopedia
This page is a glossary of chemistry terms. Chemistry
has an extensive vocabulary and a significant amount of jargon. This is a list of chemical terms, including laboratory tools, glassware, and equipment. Chemistry itself is a physical science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions.
Note: All periodic table references refer to the IUPAC
Style of the Periodic Table



Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
has an extensive vocabulary and a significant amount of jargon. This is a list of chemical terms, including laboratory tools, glassware, and equipment. Chemistry itself is a physical science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions.
International Union of Pure and Applied Chemistry
The International Union of Pure and Applied Chemistry is an international federation of National Adhering Organizations that represents chemists in individual countries. It is a member of the International Council for Science . The international headquarters of IUPAC is located in Zürich,...
Style of the Periodic Table
A

- absolute zeroAbsolute zeroAbsolute zero is the theoretical temperature at which entropy reaches its minimum value. The laws of thermodynamics state that absolute zero cannot be reached using only thermodynamic means....
- a theoreticalTheoryThe English word theory was derived from a technical term in Ancient Greek philosophy. The word theoria, , meant "a looking at, viewing, beholding", and referring to contemplation or speculation, as opposed to action...
condition concerning a system at zero KelvinKelvinThe kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
where a systemPhysical systemIn physics, the word system has a technical meaning, namely, it is the portion of the physical universe chosen for analysis. Everything outside the system is known as the environment, which in analysis is ignored except for its effects on the system. The cut between system and the world is a free...
does not emit or absorb energy (all atoms are at rest) - accuracy - how close a value is to the actual or true value; also see precision
- acidAcidAn acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
- a compound that, when dissolved in water, gives a pH of less than 7.0 or a compound that donates a hydrogen ion - acid anhydride - a compound with two acyl groups bound to a single oxygen atom
- acid dissociation constantAcid dissociation constantAn acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
- an equilibrium constant for the dissociation of a weak acid - actinides - the fifteen chemical elements that are between actiniumActiniumActinium is a radioactive chemical element with the symbol Ac and atomic number 89, which was discovered in 1899. It was the first non-primordial radioactive element to be isolated. Polonium, radium and radon were observed before actinium, but they were not isolated until 1902...
(89) and lawrenciumLawrenciumLawrencium is a radioactive synthetic chemical element with the symbol Lr and atomic number 103. In the periodic table of the elements, it is a period 7 d-block element and the last element of actinide series...
(103) - activated complexActivated complexIn chemistry an activated complex is defined by the International Union of Pure and Applied Chemistry as "that assembly of atoms which corresponds to an arbitrary infinitesimally small region at or near the col of a potential energy surface"...
- a structure that forms because of a collision between molecules while new bonds are formed - activation energyActivation energyIn chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
- activity series
- actual yield
- addition reactionAddition reactionAn addition reaction, in organic chemistry, is in its simplest terms an organic reaction where two or more molecules combine to form a larger one....
- within organic chemistry, when two or more molecules combine to make a larger one - aerationAerationAeration is the process by which air is circulated through, mixed with or dissolved in a liquid or substance.-Aeration of liquids:-Methods:Aeration of liquids is achieved by:...
- the mixing of air into a liquid or solid - alkali metals - the metals of GroupPeriodic table groupIn chemistry, a group is a vertical column in the periodic table of the chemical elements. There are 18 groups in the standard periodic table, including the d-block elements, but excluding the f-block elements....
1 on the periodic table - alkaline earth metals - the metals of Group 2 on the periodic table
- allomer - a substance that has different composition than another, but has the same crystalline structure
- allotropyAllotropyAllotropy or allotropism is the property of some chemical elements to exist in two or more different forms, known as allotropes of these elements...
- elements that can have different structures (and therefore different forms), such as CarbonCarbonCarbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
(diamonds, graphiteGraphiteThe mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
, and fullereneFullereneA fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
) - anion - negatively charge ionIonAn ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s - anodeAnodeAn anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
- aromaticityAromaticityIn organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
- chemical property of conjugated rings that results in unusual stability. See also benzeneBenzeneBenzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
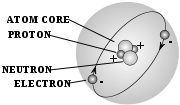
. - atomAtomThe atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
- a chemical element in its smallest form, and is made up of neutrons and protons within the nucleus and electrons circling the nucleus - atomic mass unitAtomic mass unitThe unified atomic mass unit or dalton is a unit that is used for indicating mass on an atomic or molecular scale. It is defined as one twelfth of the rest mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state, and has a value of...
- atomic numberAtomic numberIn chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
- the number representing an element which corresponds with the number of protons within the nucleus - atomic orbitalAtomic orbitalAn atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
- the region where the electron of the atom may be found - average atomic mass
- Avogadro's lawAvogadro's lawAvogadro's law is a gas law named after Amedeo Avogadro who, in 1811, hypothesized that two given samples of an ideal gas, at the same temperature, pressure and volume, contain the same number of molecules...
- Avogadro's numberAvogadro's numberIn chemistry and physics, the Avogadro constant is defined as the ratio of the number of constituent particles N in a sample to the amount of substance n through the relationship NA = N/n. Thus, it is the proportionality factor that relates the molar mass of an entity, i.e...
B
- barometerBarometerA barometer is a scientific instrument used in meteorology to measure atmospheric pressure. Pressure tendency can forecast short term changes in the weather...
- - baseBase (chemistry)For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
- a substance that accepts a proton and has a high pHPHIn chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
; a common example is sodium hydroxide (NaOH) - biochemistryBiochemistryBiochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
- the chemistry of organisms - boilingBoilingBoiling is the rapid vaporization of a liquid, which occurs when a liquid is heated to its boiling point, the temperature at which the vapor pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding environmental pressure. While below the boiling point a liquid...
- the phase transitionPhase transitionA phase transition is the transformation of a thermodynamic system from one phase or state of matter to another.A phase of a thermodynamic system and the states of matter have uniform physical properties....
of liquid vaporizing - boiling pointBoiling pointThe boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
- - boiling-point elevationBoiling-point elevationBoiling-point elevation describes the phenomenon that the boiling point of a liquid will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such...
- - bondChemical bondA chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
- the attraction and repulsion between atoms and molecules that is a cornerstone of chemistry - Boyle's lawBoyle's lawBoyle's law is one of many gas laws and a special case of the ideal gas law. Boyle's law describes the inversely proportional relationship between the absolute pressure and volume of a gas, if the temperature is kept constant within a closed system...
- - Brønsted-Lowrey acid -
- Brønsted–Lowry acid–base reaction -
- Brønsted-Lowrey base -
- buffered solution -
- buretteBuretteA burette is a vertical cylindrical piece of laboratory glassware with a volumetric graduation on its full length and a precision tap, or stopcock, on the bottom. It is used to dispense known amounts of a liquid reagent in experiments for which such precision is necessary, such as a titration...
(also buret) - glassware used to dispense specific amounts of liquid when precision is necessary (e.g. titration and resource dependent reactions)
C

- catalyst - a chemical compound used to change the rate (either to speed up or slow down) of a reaction, but is regenerated at the end of the reaction.
- cation - positively charged ion
- centrifugeCentrifugeA centrifuge is a piece of equipment, generally driven by an electric motor , that puts an object in rotation around a fixed axis, applying a force perpendicular to the axis...
- equipment used to separate substances based on density by rotating the tubes around a centred axis - cell potentialResting potentialThe relatively static membrane potential of quiescent cells is called the resting membrane potential , as opposed to the specific dynamic electrochemical phenomena called action potential and graded membrane potential....
- the force in a galvanic cellGalvanic cellA Galvanic cell, or Voltaic cell, named after Luigi Galvani, or Alessandro Volta respectively, is an electrochemical cell that derives electrical energy from spontaneous redox reaction taking place within the cell...
that pulls electron through reducing agent to oxidizing agent - chemical LawChemical lawChemical laws are those laws of nature relevant to chemistry. The most fundamental concept in chemistry is the law of conservation of mass, which states that there is no detectable change in the quantity of matter during an ordinary chemical reaction...
- certain rules that pertain to the laws of nature and chemistry - examples - chemical reactionChemical reactionA chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- the change of one or more substances into another or multiple substances - colloidColloidA colloid is a substance microscopically dispersed evenly throughout another substance.A colloidal system consists of two separate phases: a dispersed phase and a continuous phase . A colloidal system may be solid, liquid, or gaseous.Many familiar substances are colloids, as shown in the chart below...
- mixture of evenly dispersed substances, such as many milkMilkMilk is a white liquid produced by the mammary glands of mammals. It is the primary source of nutrition for young mammals before they are able to digest other types of food. Early-lactation milk contains colostrum, which carries the mother's antibodies to the baby and can reduce the risk of many...
s - combustionCombustionCombustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
- an exothermic reaction between an oxidant and fuel with heat and often light - compoundChemical compoundA chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
- a substanceChemical substanceIn chemistry, a chemical substance is a form of matter that has constant chemical composition and characteristic properties. It cannot be separated into components by physical separation methods, i.e. without breaking chemical bonds. They can be solids, liquids or gases.Chemical substances are...
that is made up of two or more chemically bonded elements - condensationCondensationCondensation is the change of the physical state of matter from gaseous phase into liquid phase, and is the reverse of vaporization. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition....
- the phase change from gas to liquid - conductorElectrical conductorIn physics and electrical engineering, a conductor is a material which contains movable electric charges. In metallic conductors such as copper or aluminum, the movable charged particles are electrons...
- material that allows electric flow more freely - covalent bondCovalent bondA covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
- chemical bond that involves sharing electrons - crystalCrystalA crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
- a solid that is packed with ions, molecules or atoms in an orderly fashion - cuvetteCuvetteA cuvette is a small tube of circular or square cross section, sealed at one end, made of plastic, glass, or fused quartz and designed to hold samples for spectroscopic experiments. The best cuvettes are as clear as possible, without impurities that might affect a spectroscopic reading...
- glassware used in spectroscopic experiments. It is usually made of plastic, glass or quartz and should be as clean and clear as possible
D
- deionization - the removal of ions, and in water's case mineral ions such as sodium, iron and calcium
- deliquescence - substances that absorb water from the atmosphere to form liquid solutions
- depositionDeposition (chemistry)In chemistry, deposition is the settling of particles or sediment from a solution, suspension and mixture or vapor onto a pre-existing surface...
- settling of particles within a solution or mixture - dipoleDipoleIn physics, there are several kinds of dipoles:*An electric dipole is a separation of positive and negative charges. The simplest example of this is a pair of electric charges of equal magnitude but opposite sign, separated by some distance. A permanent electric dipole is called an electret.*A...
- electric or magnetic separation of charge - dipole momentBond dipole momentThe bond dipole moment uses the idea of electric dipole moment to measure the polarity of a chemical bond within a molecule. The bond dipole μ is given by:\mu = \delta \, d....
- the polarity of a polar covalent bond - dissolution or solvationSolvationSolvation, also sometimes called dissolution, is the process of attraction and association of molecules of a solvent with molecules or ions of a solute...
- the spread of ions in a solvent - double bond - sharing of two pairs of electrons
E

- earth metal - see alkaline earth metal
- electrolyteElectrolyteIn chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
- a solution that conducts a certain amount of current and can be split categorically as weak and strong electrlytes - electrochemical cellElectrochemical cellAn electrochemical cell is a device capable of either deriving electrical energy from chemical reactions, or facilitating chemical reactions through the introduction of electrical energy. A common example of an electrochemical cell is a standard 1.5-volt "battery"...
- using a chemical reaction's current, electromotive force is made - electromagnetic radiationElectromagnetic radiationElectromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space...
- a type of wave that can go through vacuums as well as material and classified as a self-propagating wave - electromagnetismElectromagnetismElectromagnetism is one of the four fundamental interactions in nature. The other three are the strong interaction, the weak interaction and gravitation...
- fields that have electric charge and electric properties that change the way that particles move and interact - electromotive forceElectromotive forceIn physics, electromotive force, emf , or electromotance refers to voltage generated by a battery or by the magnetic force according to Faraday's Law, which states that a time varying magnetic field will induce an electric current.It is important to note that the electromotive "force" is not a...
- a device that gains energy as electric charges pass through it - electronElectronThe electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
- a subatomic particle with a net charge that is negative - electron shells - an orbital around the atom's nucleus that has a fixed number electrons (usually two or eight)
- electric chargeElectric chargeElectric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
- a measured property (coulombs) that determine electromagnetic interaction - element - an atom that is defined by its atomic numberAtomic numberIn chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
- energyEnergyIn physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
- A system's ability to do workMechanical workIn physics, work is a scalar quantity that can be described as the product of a force times the distance through which it acts, and it is called the work of the force. Only the component of a force in the direction of the movement of its point of application does work... - enthalpyEnthalpyEnthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
- measure of the total energy of a thermodynamic system (usually symbolized as H) - entropyEntropyEntropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
- the amount of energy not available for work in a closed thermodynamic system (usually symbolized as S) - enzymeEnzymeEnzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
- a protein that speeds up (catalyses) a reaction - eppendorf tube - generalized and trademarked term used for a type of tube; see microcentrifuge
F
- freezingFreezingFreezing or solidification is a phase change in which a liquid turns into a solid when its temperature is lowered below its freezing point. The reverse process is melting....
- phase transition from liquid to solid - Faraday constant - a unit of electrical charge widely used in electrochemistryElectrochemistryElectrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
and equal to ~ 96,500 coulombs.
- It represents 1 molMole (unit)The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
of electrons, or the Avogadro number of electrons: 6.022 × 1023 electrons. F = 96 485.339 9(24) C/molMole (unit)The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
- Faraday's law of electrolysis - a two part law that Michael FaradayMichael FaradayMichael Faraday, FRS was an English chemist and physicist who contributed to the fields of electromagnetism and electrochemistry....
published about electrolysis- the mass of a substance altered at an electrode during electrolysis is directly proportional to the quantity of electricity transferred at that electrode
- the mass of an elemental material altered at an electrode is directly proportional to the element's equivalent weight.
- frequencyFrequencyFrequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
- number of cycles per unit of time. Unit: 1 hertzHertzThe hertz is the SI unit of frequency defined as the number of cycles per second of a periodic phenomenon. One of its most common uses is the description of the sine wave, particularly those used in radio and audio applications....
= 1 cycle per 1 second
- Faraday's law of electrolysis - a two part law that Michael Faraday
G
- galvanic cellGalvanic cellA Galvanic cell, or Voltaic cell, named after Luigi Galvani, or Alessandro Volta respectively, is an electrochemical cell that derives electrical energy from spontaneous redox reaction taking place within the cell...
- battery made up of electrochemical with two different metals connected by salt bridgeSalt bridgeA salt bridge, in chemistry, is a laboratory device used to connect the oxidation and reduction half-cells of a galvanic cell , a type of electrochemical cell... - gasGasGas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
- particles that fill their container though have no definite shape or volume - geochemistryGeochemistryThe field of geochemistry involves study of the chemical composition of the Earth and other planets, chemical processes and reactions that govern the composition of rocks, water, and soils, and the cycles of matter and energy that transport the Earth's chemical components in time and space, and...
- the chemistry of and chemical composition of the Earth - Gibbs energy - value that indicates the spontaneity of a reaction (usually symbolized as G)
I
- indicatorPH indicatorA pH indicator is a halochromic chemical compound that is added in small amounts to a solution so that the pH of the solution can be determined visually. Hence a pH indicator is a chemical detector for hydronium ions or hydrogen ions in the Arrhenius model. Normally, the indicator causes the...
- a special compound added to solution that changes color depending on the acidity of the solution; different indicators have different colors and effective pH ranges - inorganic compoundInorganic compoundInorganic compounds have traditionally been considered to be of inanimate, non-biological origin. In contrast, organic compounds have an explicit biological origin. However, over the past century, the classification of inorganic vs organic compounds has become less important to scientists,...
- compounds that do not contain carbon, though there are exceptions (see main article) - inorganic chemistryInorganic chemistryInorganic chemistry is the branch of chemistry concerned with the properties and behavior of inorganic compounds. This field covers all chemical compounds except the myriad organic compounds , which are the subjects of organic chemistry...
- a part of chemistry concerned with inorganic compounds - International Union of Pure and Applied Chemistry (IUPACInternational Union of Pure and Applied ChemistryThe International Union of Pure and Applied Chemistry is an international federation of National Adhering Organizations that represents chemists in individual countries. It is a member of the International Council for Science . The international headquarters of IUPAC is located in Zürich,...
) - - insulator - material that resists the flow of electric current
- ionIonAn ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
- a molecule that has gained or lost one or more electrons - ionic bondIonic bondAn ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. Pure ionic bonding cannot exist: all ionic compounds have some...
- electrostatic attraction between oppositely charged ions - ionizationIonizationIonization is the process of converting an atom or molecule into an ion by adding or removing charged particles such as electrons or other ions. This is often confused with dissociation. A substance may dissociate without necessarily producing ions. As an example, the molecules of table sugar...
-The breaking up of a compound into separate ions.
K
- KineticsChemical kineticsChemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
- A sub-field of chemistry specializing in reaction rates - Kinetic energyKinetic energyThe kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
- The energy of an object due to its motion.
L
- lanthanides - Elements 57 through 71
- latticeCrystal structureIn mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
- Unique arrangement of atoms or molecules in a crystalline liquid or solid. - Laws of thermodynamicsLaws of thermodynamicsThe four laws of thermodynamics summarize its most important facts. They define fundamental physical quantities, such as temperature, energy, and entropy, in order to describe thermodynamic systems. They also describe the transfer of energy as heat and work in thermodynamic processes...
- liquidLiquidLiquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
- A state of matter which takes the shape of its container - lightLightLight or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
- Portion of the electromagnetic spectrum which is visible to the naked eye. Also called "visible light." - London dispersion forces - A weak intermolecular force
M

- MetalMetalA metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
- Chemical element that is a good conductor of both electricity and heat and forms cations and ionic bonds with non-metals. - meltingMeltingMelting, or fusion, is a physical process that results in the phase change of a substance from a solid to a liquid. The internal energy of a substance is increased, typically by the application of heat or pressure, resulting in a rise of its temperature to the melting point, at which the rigid...
- The phase change from a solid to a liquid - metalloidMetalloidMetalloid is a term used in chemistry when classifying the chemical elements. On the basis of their general physical and chemical properties, each element can usually be classified as a metal or a nonmetal. However, some elements with intermediate or mixed properties can be harder to characterize...
- A substance possessing both the properties of metals and non-metals - methylene blueMethylene blueMethylene blue is a heterocyclic aromatic chemical compound with the molecular formula C16H18N3SCl. It has many uses in a range of different fields, such as biology and chemistry. At room temperature it appears as a solid, odorless, dark green powder, that yields a blue solution when dissolved in...
- a heterocyclic aromaticAromaticityIn organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
chemical compoundChemical compoundA chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
with the molecular formula CCarbonCarbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
16HHydrogenHydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
18NNitrogenNitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
3SSulfurSulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
ClChlorineChlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine... - microcentrifuge - a small plastic container that is used to store small amounts of liquid
- moleMole (unit)The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
- abbreviated mol - a measurement of an amount of substance; a single mole contains approximately 6.022×1023 units or entities- a mole of water contains 6.022×1023 H2O molecules
- moleculeMoleculeA molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
- a chemically bonded number of atoms that are electrically neutral - molecular orbitalMolecular orbitalIn chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
- region where an electron can be found in a molecule (as opposed to an atom)
N
- neutronNeutronThe neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
- a neutral unit or subatomic particleSubatomic particleIn physics or chemistry, subatomic particles are the smaller particles composing nucleons and atoms. There are two types of subatomic particles: elementary particles, which are not made of other particles, and composite particles...
that has no net charge - neutrinoNeutrinoA neutrino is an electrically neutral, weakly interacting elementary subatomic particle with a half-integer spin, chirality and a disputed but small non-zero mass. It is able to pass through ordinary matter almost unaffected...
- a particle that can travel at speeds close to the speed of light and are created as a result of radioactive decayRadioactive decayRadioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom... - nucleusAtomic nucleusThe nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
- the centre of an atom made up of neutrons and protons, with a net positive charge - noble gasNoble gasThe noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
es - group 18 elements, those whose outer electron shell is filled - non-metal - an element which is not metallic
- nuclearAtomic nucleusThe nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
- of or pertaining to the atomic nucleus - nuclear magnetic resonance spectroscopy - technique that exploits the magnetic properties of certain nuclei, useful for identifying unknown compounds
- number densityNumber densityIn physics, astronomy, and chemistry, number density is an intensive quantity used to describe the degree of concentration of countable objects in the three-dimensional physical space...
– a measure of concentration of countable objects (atoms, molecules, etc.) in space; number per volume
O
- orbital - may refer to either an atomic orbitalAtomic orbitalAn atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
or a molecular orbitalMolecular orbitalIn chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first... - organic compound - compounds that contain carbon
- organic chemistryOrganic chemistryOrganic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
- a part of chemistry concerned with organic compounds
P
- pHPHIn chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
- the measure of acidity (or basicity) of a solution - plasma - state of matter similar to gas in which a certain portion of the particles are ionized
- poor metal - Metallic elements in the p-block, characterized by lower melting and boiling points than other metals
- potential energyPotential energyIn physics, potential energy is the energy stored in a body or in a system due to its position in a force field or due to its configuration. The SI unit of measure for energy and work is the Joule...
- energy stored in a body or in a system due to its position in a force field or due to its configuration - precipitate - formation of a solid in a solution or inside another solid during a chemical reaction or by diffusion in a solid
- precision - How close the results of multiple experimental trials are. See also accuracy.
- photonPhotonIn physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
- a carrier of electromagnetic radiationElectromagnetic radiationElectromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space...
of all wavelength (such as gamma rays and radio waves) - protonProtonThe proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
- a positive unit or subatomic particle that has a positive charge - protonationProtonationIn chemistry, protonation is the addition of a proton to an atom, molecule, or ion. Some classic examples include*the protonation of water by sulfuric acid:*the protonation of isobutene in the formation of a carbocation:2C=CH2 + HBF4 → 3C+ + BF4−*the protonation of ammonia in the...
- the addition of a proton (H+) to an atom, molecule, or ion
Q
- Quantum mechanicsQuantum mechanicsQuantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
- the study of how atoms, molecules, subatomic particles, etc. behave and are structured - quarks - elementary particle and a fundamental constituent of matter
R
- radiationRadiationIn physics, radiation is a process in which energetic particles or energetic waves travel through a medium or space. There are two distinct types of radiation; ionizing and non-ionizing...
- energy in the form of waves or subatomic particles when there is a change from high energy to low energy states - radioactive decayRadioactive decayRadioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
- the process of an unstable atomic nucleus losing energy by emitting radiation
S
- s-block elementsS-blockThe s-block of the periodic table of elements consists of the first two groups: the alkali metals and alkaline earth metals, plus hydrogen and helium.Except in hydrogen and helium, these electrons are very easily lost to form positive ions...
- Group 1 and 2 elements (alkali and alkaline metals), which includes Hydrogen and Helium - salts - ionic compounds composed of anions and cations
- salt bridgeSalt bridgeA salt bridge, in chemistry, is a laboratory device used to connect the oxidation and reduction half-cells of a galvanic cell , a type of electrochemical cell...
- devices used to connection reduction with oxidation half-cells in an electrochemical cell - saline solutionSaline (medicine)In medicine, saline is a general term referring to a sterile solution of sodium chloride in water but is only sterile when it is to be placed intravenously, otherwise, a saline solution is a salt water solution...
- general term for NaCl in water - Schrödinger equationSchrödinger equationThe Schrödinger equation was formulated in 1926 by Austrian physicist Erwin Schrödinger. Used in physics , it is an equation that describes how the quantum state of a physical system changes in time....
- quantum state equation which represents the behaviour of an election around an atom - semiconductorSemiconductorA semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
- an electrically conductive solid that is between a conductor and an insulator - single bond - sharing of one pair of electrons
- solSol (colloid)A sol is a colloidal suspension of very small solid particles in a continuous liquid medium. They are quite stable and show the Tyndall effect. Examples include blood, pigmented ink, and paint....
- a suspension of solid particles in liquid. Artificial examples include sol-gels. - solidSolidSolid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
- one of the states of matter, where the molecules are packed close together, there is a resistance of movement/deformation and volume change; see Young's modulusYoung's modulusYoung's modulus is a measure of the stiffness of an elastic material and is a quantity used to characterize materials. It is defined as the ratio of the uniaxial stress over the uniaxial strain in the range of stress in which Hooke's Law holds. In solid mechanics, the slope of the stress-strain... - soluteSolutionIn chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
- the part of the solution that is mixed into the solvent (NaCl in saline water) - solutionSolutionIn chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
- homogeneous mixture made up of multiple substances. It is made up of solutes and solvents. - solventSolventA solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
- the part of the solution that dissolves the solute (H2O in saline water) - spectroscopySpectroscopySpectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
- study of radiation and matter, such as X-ray absorption and emission spectroscopy - speed of lightSpeed of lightThe speed of light in vacuum, usually denoted by c, is a physical constant important in many areas of physics. Its value is 299,792,458 metres per second, a figure that is exact since the length of the metre is defined from this constant and the international standard for time...
- the speed of anything that has zero rest mass (Energyrest = mc² where m is the mass and c is the speed of light) - Standard conditions for temperature and pressureStandard conditions for temperature and pressureStandard condition for temperature and pressure are standard sets of conditions for experimental measurements established to allow comparisons to be made between different sets of data...
or SATP - a standardisation used in order compare experimental results (25 °C and 100.000 kPa) - state of matterState of matterStates of matter are the distinct forms that different phases of matter take on. Solid, liquid and gas are the most common states of matter on Earth. However, much of the baryonic matter of the universe is in the form of hot plasma, both as rarefied interstellar medium and as dense...
- matterMatterMatter is a general term for the substance of which all physical objects consist. Typically, matter includes atoms and other particles which have mass. A common way of defining matter is as anything that has mass and occupies volume...
having a homogeneous, macroscopic phase; gasGasGas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
, plasmaPlasma (physics)In physics and chemistry, plasma is a state of matter similar to gas in which a certain portion of the particles are ionized. Heating a gas may ionize its molecules or atoms , thus turning it into a plasma, which contains charged particles: positive ions and negative electrons or ions...
, liquidLiquidLiquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
, and solidSolidSolid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
are the most well known (in increasing concentration) - sublimation - a phase transition from solid to gas
- subatomic particles - particles that are smaller than an atom; examples are protons, neutrons and electrons
- substanceChemical substanceIn chemistry, a chemical substance is a form of matter that has constant chemical composition and characteristic properties. It cannot be separated into components by physical separation methods, i.e. without breaking chemical bonds. They can be solids, liquids or gases.Chemical substances are...
- material with definite chemical composition
T

- talcTalcTalc is a mineral composed of hydrated magnesium silicate with the chemical formula H2Mg34 or Mg3Si4O102. In loose form, it is the widely-used substance known as talcum powder. It occurs as foliated to fibrous masses, its crystals being so rare as to be almost unknown...
- a mineral representing the one on the Mohs Scale and composed of hydrated magnesium silicate with the chemical formula H2Mg3(SiO3)4 or Mg3Si4O10(OH)2 - temperatureTemperatureTemperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
- the average energy of microscopic motions of particles - theoretical yieldYield (chemistry)In chemistry, yield, also referred to as chemical yield and reaction yield, is the amount of product obtained in a chemical reaction. The absolute yield can be given as the weight in grams or in moles...
- see yield - theory - a model describing the nature of a phenomenon
- thermal conductivityThermal conductivityIn physics, thermal conductivity, k, is the property of a material's ability to conduct heat. It appears primarily in Fourier's Law for heat conduction....
- a property of a material to conduct heat (often noted as )
) - thermochemistryThermochemistryThermochemistry is the study of the energy and heat associated with chemical reactions and/or physical transformations. A reaction may release or absorb energy, and a phase change may do the same, such as in melting and boiling. Thermochemistry focuses on these energy changes, particularly on the...
- the study of absorption/release of heat within a chemical reaction - thermodynamicsThermodynamicsThermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
- the study of the effects of changing temperature, volume or pressure (or work, heat, and energy) on a macroscopic scale - thermodynamic stabilityChemical stabilityChemical stability when used in the technical sense in chemistry, means thermodynamic stability of a chemical system.Thermodynamic stability occurs when a system is in its lowest energy state, or chemical equilibrium with its environment. This may be a dynamic equilibrium, where individual atoms...
- when a system is in its lowest energy state with its environment (equilibrium) - thermometerThermometerDeveloped during the 16th and 17th centuries, a thermometer is a device that measures temperature or temperature gradient using a variety of different principles. A thermometer has two important elements: the temperature sensor Developed during the 16th and 17th centuries, a thermometer (from the...
- device that measures the average energy of a system - titrationTitrationTitration, also known as titrimetry, is a common laboratory method of quantitative chemical analysis that is used to determine the unknown concentration of an identified analyte. Because volume measurements play a key role in titration, it is also known as volumetric analysis. A reagent, called the...
- the process of titrating one solution with another, also called volumetric analysis - torrTorrThe torr is a non-SI unit of pressure with the ratio of 760 to 1 standard atmosphere, chosen to be roughly equal to the fluid pressure exerted by a millimetre of mercury, i.e., a pressure of 1 torr is approximately equal to 1 mmHg...
- a unit to measure pressure (1 Torr is equivalent to 133.322 PaPascal (unit)The pascal is the SI derived unit of pressure, internal pressure, stress, Young's modulus and tensile strength, named after the French mathematician, physicist, inventor, writer, and philosopher Blaise Pascal. It is a measure of force per unit area, defined as one newton per square metre...
or 1.3158×10−3 atmAtmosphere (unit)The standard atmosphere is an international reference pressure defined as 101325 Pa and formerly used as unit of pressure. For practical purposes it has been replaced by the bar which is 105 Pa...
) - transition metalTransition metalThe term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
- elements that have incomplete d sub-shells, but also may be referred to as the d-block elements - transuranic element - element with atomic number greater than 92; none of the transuranic elements are stable
- triple bond - the sharing of three pairs of electrons within a covalent bond (example N2)
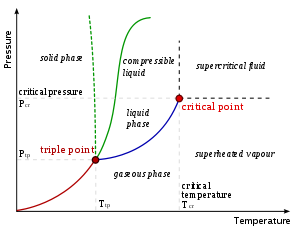
- triple pointTriple pointIn thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases of that substance coexist in thermodynamic equilibrium...
- the place where temperature and pressure of three phasesPhase (matter)In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
are the same (Water has a special phase diagram) - Tyndall effectTyndall effectThe Tyndall effect, also known as Tyndall scattering, is light scattering by particles in a colloid or particles in a fine suspension. It is named after the 19th century physicist John Tyndall. It is similar to Rayleigh scattering, in that the intensity of the scattered light depends on the fourth...
- the effect of light scattering by colloidal (mixture where one substance is dispersed evenly through another) or suspended particles
U
- UN numberUN numberUN numbers or UN IDs are four-digit numbers that identify hazardous substances, and articles in the framework of international transport....
- a four digit code used to note hazardous and flammable substances - uncertaintyUncertaintyUncertainty is a term used in subtly different ways in a number of fields, including physics, philosophy, statistics, economics, finance, insurance, psychology, sociology, engineering, and information science...
- a characteristic that any measurement that involves estimation of any amount cannot be exactly reproducible - Uncertainty principleUncertainty principleIn quantum mechanics, the Heisenberg uncertainty principle states a fundamental limit on the accuracy with which certain pairs of physical properties of a particle, such as position and momentum, can be simultaneously known...
- knowing the location of a particle makes the momentum uncertain, while knowing the momentum of a particle makes the location uncertain - unit cellCrystal structureIn mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
- the smallest repeating unit of a lattice - unit factorUnits conversion by factor-labelMany, if not most, parameters and measurements in the physical sciences and engineering are expressed as a numerical quantity and a corresponding dimensional unit; for example: 1000 kg/m³, 100 kPa/bar, 50 miles per hour, 1000 Btu/lb. Converting from one dimensional unit to another is often...
- statements used in converting between units - universal or ideal gas constantGas constantThe gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
- proportionality constant in the ideal gas law (0.08206 L·atm/(K·mol))
V
- valence electronValence electronIn chemistry, valence electrons are the electrons of an atom that can participate in the formation of chemical bonds with other atoms. Valence electrons are the "own" electrons, present in the free neutral atom, that combine with valence electrons of other atoms to form chemical bonds. In a single...
- the outermost electrons of an atom, which are located in electron shells - Valence bond theoryValence bond theoryIn chemistry, valence bond theory is one of two basic theories, along with molecular orbital theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds...
- a theory explaining the chemical bonding within molecules by discussing valenciesValence (chemistry)In chemistry, valence, also known as valency or valence number, is a measure of the number of bonds formed by an atom of a given element. "Valence" can be defined as the number of valence bonds...
, the number of chemical bonds formed by an atom - van der Waals forceVan der Waals forceIn physical chemistry, the van der Waals force , named after Dutch scientist Johannes Diderik van der Waals, is the sum of the attractive or repulsive forces between molecules other than those due to covalent bonds or to the electrostatic interaction of ions with one another or with neutral...
- one of the forces (attraction/repulsion) between molecules - van 't Hoff factorVan 't Hoff factorThe van 't Hoff factor i is a measure of the effect of a solute upon colligative properties, such as vapor pressure, osmotic pressure and freezing point depression. The van 't Hoff factor is the ratio between the actual concentration of particles produced when the substance is dissolved, and the...
- ratio of moles of particles in solution to moles of solute dissolved - vaporVaporA vapor or vapour is a substance in the gas phase at a temperature lower than its critical point....
- when a substance is below the critical temperature while in the gas phase - vapour pressureVapor pressureVapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
- pressure of vapour over a liquid at equilibrium - vaporizationVaporizationVaporization of an element or compound is a phase transition from the liquid or solid phase to gas phase. There are three types of vaporization: evaporation, boiling and sublimation....
- phase change from liquid to gas - viscosityViscosityViscosity is a measure of the resistance of a fluid which is being deformed by either shear or tensile stress. In everyday terms , viscosity is "thickness" or "internal friction". Thus, water is "thin", having a lower viscosity, while honey is "thick", having a higher viscosity...
- the resistance of a liquid to flow (oil) - voltVoltThe volt is the SI derived unit for electric potential, electric potential difference, and electromotive force. The volt is named in honor of the Italian physicist Alessandro Volta , who invented the voltaic pile, possibly the first chemical battery.- Definition :A single volt is defined as the...
- one joule of work per coulomb - the unit of electrical potential transferred - voltmeterVoltmeterA voltmeter is an instrument used for measuring electrical potential difference between two points in an electric circuit. Analog voltmeters move a pointer across a scale in proportion to the voltage of the circuit; digital voltmeters give a numerical display of voltage by use of an analog to...
- instrument that measures the cell potential - volumetric analysis - see titration
W
- water - H2O - a chemical substance, a major part of cells and Earth, and covalently bonded
- wave function - a function describing the electron's position in a three dimensional space
- workMechanical workIn physics, work is a scalar quantity that can be described as the product of a force times the distance through which it acts, and it is called the work of the force. Only the component of a force in the direction of the movement of its point of application does work...
- the amount of force over distance and is in terms of jouleJouleThe joule ; symbol J) is a derived unit of energy or work in the International System of Units. It is equal to the energy expended in applying a force of one newton through a distance of one metre , or in passing an electric current of one ampere through a resistance of one ohm for one second...
s (energy)
X
- X-rayX-rayX-radiation is a form of electromagnetic radiation. X-rays have a wavelength in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz and energies in the range 120 eV to 120 keV. They are shorter in wavelength than UV rays and longer than gamma...
- form of ionizing, electromagnetic radiation, between gamma and UVUltravioletUltraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
rays - X-ray diffractionX-ray scattering techniquesX-ray scattering techniques are a family of non-destructive analytical techniques which reveal information about the crystallographic structure, chemical composition, and physical properties of materials and thin films...
- a method for establishing structures of crystalline solids using singe wavelength X-rays and looking at diffraction pattern - X-ray photoelectron spectroscopyX-ray photoelectron spectroscopyX-ray photoelectron spectroscopy is a quantitative spectroscopic technique that measures the elemental composition, empirical formula, chemical state and electronic state of the elements that exist within a material...
- a spectroscopic technique to measure composition of a material
Z
- zone meltingZone meltingZone melting is a group of similar methods of purifying crystals, in which a narrow region of a crystal is molten, and this molten zone is moved along the crystal...
- a way to remove impurities from an element by melting it and slowly travel down an ingot (cast)

