
State of matter
Encyclopedia
States of matter are the distinct forms that different phases
of matter
take on. Solid
, liquid
and gas
are the most common states of matter on Earth. However, much of the baryonic matter of the universe is in the form of hot plasma
, both as rarefied interstellar medium
and as dense star
s.
Historically, the distinction is made based on qualitative differences in bulk properties. Solid is the state in which matter maintains a fixed volume and shape; liquid is the state in which matter maintains a fixed volume but adapts to the shape of its container; and gas is the state in which matter expands to occupy whatever volume is available.
The state or phase of a given set of matter can change depending on pressure
and temperature
conditions, transitioning to other phases as these conditions change to favor their existence; for example, solid transitions to liquid with an increase in temperature.
States of matter may also be defined in terms of phase transitions
. A phase transition indicates a change in structure and can be recognized by an abrupt change in properties. By this definition, a distinct state of matter is any set of states
distinguished from any other set of states by a phase transition
. Water can be said to have several distinct solid states. The appearance of superconductivity is associated with a phase transition, so there are superconductive
states. Likewise, ferromagnetic
states are demarcated by phase transitions and have distinctive properties.
When the change of state occurs in stages the intermediate steps are called mesophase
s. Such phases have been exploited by the introduction of liquid crystal
technology.
More recently, distinctions between states have been based on differences in molecular interrelationships. Solid is the state in which intermolecular attractions keep the molecules in fixed spatial relationships. Liquid is the state in which intermolecular attractions keep molecules in proximity, but do not keep the molecules in fixed relationships. Gas is that state in which the molecules are comparatively separated and intermolecular attractions have relatively little effect on their respective motions. Plasma
is a highly ionized gas that occurs at high temperatures. The intermolecular forces created by ionic attractions and repulsions give these compositions distinct properties, for which reason plasma is described as a fourth state of matter.
Forms of matter that are not composed of molecules and are organized by different forces can also be considered different states of matter. Superfluids (like Fermionic condensate
) and the quark–gluon plasma are examples.

The particles (ions, atoms or molecules) are packed closely together. The forces between particles
are strong enough so that the particles cannot move freely but can only vibrate. As a result, a solid has a stable, definite shape, and a definite volume. Solids can only change their shape by force, as when broken or cut.
In crystalline solids
, the particles (atoms, molecules, or ions) are packed in a regularly ordered, repeating pattern. There are many different crystal structure
s, and the same substance can have more than one structure (or solid phase). For example, iron
has a body-centred cubic
structure at temperatures below 912 °C, and a face-centred cubic
structure between 912 and 1394 °C. Ice
has fifteen known crystal structures, or fifteen solid phases which exist at various temperatures and pressures.
Glass
es and other non-crystalline, amorphous solid
s without long-range order
are not thermal equilibrium ground states; therefore they are described below as nonclassical states of matter.
Solids can be transformed into liquids by melting, and liquids can be transformed into solids by freezing. Solids can also change directly into gases through the process of sublimation.
A liquid is a nearly incompressible fluid
which is able to conform to the shape of its container but retains a (nearly) constant volume independent of pressure. The volume is definite if the temperature
and pressure
are constant. When a solid is heated above its melting point
, it becomes liquid, given that the pressure is higher than the triple point
of the substance. Intermolecular (or interatomic or interionic) forces are still important, but the molecules have enough energy to move relative to each other and the structure is mobile. This means that the shape of a liquid is not definite but is determined by its container. The volume is usually greater than that of the corresponding solid, the most well known exception being water, H2O. The highest temperature at which a given liquid can exist is its critical temperature.
In a gas, the molecules have enough kinetic energy
so that the effect of intermolecular forces is small (or zero for an ideal gas
), and the typical distahince between neighboring molecules is much greater than the molecular size. A gas has no definite shape or volume, but occupies the entire container in which it is confined. A liquid may be converted to a gas by heating at constant pressure to the boiling point
, or else by reducing the pressure at constant temperature.
At temperatures below its critical temperature, a gas is also called a vapor
, and can be liquefied by compression alone without cooling. A vapor can exist in equilibrium with a liquid (or solid), in which case the gas pressure equals the vapor pressure
of the liquid (or solid).
A supercritical fluid
(SCF) is a gas whose temperature and pressure are above the critical temperature and critical pressure respectively. In this state, the distinction between liquid and gas disappears. A supercritical fluid has the physical properties of a gas, but its high density confers solvent properties in some cases which lead to useful applications. For example, supercritical carbon dioxide
is used to extract
caffeine
in the manufacture of decaffeinated
coffee.
is a non-crystalline or amorphous solid
material that exhibits a glass transition
when heated towards the liquid state. Glasses can be made of quite different classes of materials: inorganic networks (such as window glass, made of silicate
plus additives), metallic alloys, ionic melts, aqueous solutions, molecular liquids, and polymers.
Thermodynamically, a glass is in a metastable state with respect to its crystalline counterpart. The conversion rate, however, is practically zero.
this degree of freedom is frozen in a quenched disordered
state.
Similarly, in a spin glass
magnetic disorder is frozen.
, which is nematic in the temperature range 118–136 °C. In this state
the molecules flow as in a liquid, but they all point in the same direction (within each domain) and cannot rotate freely.
Other types of liquid crystals are described in the main article on these states. Several types have technological importance, for example, in liquid crystal display
s.
atoms often have magnetic moment
s due to the net spin
of electrons which remain unpaired and do not form chemical bonds. In some solids the magnetic moments on different atoms are ordered and can form a ferromagnet, an antiferromagnet or a ferrimagnet.
In a ferromagnet
—for instance, solid iron
—the magnetic moment on each atom is aligned in the same direction (within a magnetic domain
). If the domains are also aligned, the solid is a permanent magnet
, which is magnetic even in the absence of an external magnetic field
. The magnetization
disappears when the magnet is heated to the Curie point
, which for iron is 768 °C.
An antiferromagnet
has two networks of equal and opposite magnetic moments which cancel each other out, so that the net magnetization is zero. For example, in nickel(II) oxide
(NiO), half the nickel atoms have moments aligned in one direction and half in the opposite direction.
In a ferrimagnet
, the two networks of magnetic moments are opposite but unequal, so that cancellation is incomplete and there is a non-zero net magnetization. An example is magnetite
(Fe3O4), which contains Fe2+ and Fe3+ ions with different magnetic moments.
 Copolymers can undergo microphase separation to form a diverse array of periodic nanostructures, as shown in the example of the styrene-butadiene-styrene block copolymer shown at right. Microphase separation can be understood by analogy to the phase separation between oil
Copolymers can undergo microphase separation to form a diverse array of periodic nanostructures, as shown in the example of the styrene-butadiene-styrene block copolymer shown at right. Microphase separation can be understood by analogy to the phase separation between oil
and water
. Due to chemical incompatibility between the blocks, block copolymers undergo a similar phase separation. However, because the blocks are covalently bonded
to each other, they cannot demix macroscopically as water and oil can, and so instead the blocks form nanometer-sized structures. Depending on the relative lengths of each block and the overall block topology of the polymer, many morphologies can be obtained, each its own phase of matter.
(or infinite fluidity; i.e., flowing without friction). This was discovered in 1937 for helium
which forms a superfluid below the lambda temperature
of 2.17 K. In this state it will attempt to "climb" out of its container. It also has infinite thermal conductivity
so that no temperature gradient
can form in a superfluid. Placing a super fluid in a spinning container will result in quantized vortices
.
These properties are explained by the theory that the common isotope helium-4
forms a Bose–Einstein condensate
(see next section) in the superfluid state. More recently, Fermionic condensate
superfluids have been formed at even lower temperatures by the rare isotope helium-3
and by lithium-6
.
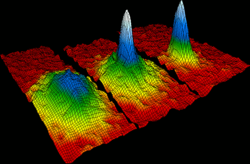
In 1924, Albert Einstein
and Satyendra Nath Bose
predicted the "Bose-Einstein condensate," (BEC) sometimes referred to as the fifth state of matter. In a BEC, matter stops behaving as independent particles, and collapses into a single quantum state that can be described with a single, uniform wavefunction.
In the gas phase, the Bose-Einstein condensate remained an unverified theoretical prediction for many years. In 1995 the research groups of Eric Cornell and Carl Wieman
, of JILA at the University of Colorado at Boulder
, produced the first such condensate experimentally. A Bose-Einstein condensate is "colder" than a solid. It may occur when atoms have very similar (or the same) quantum level
s, at temperatures very close to absolute zero
(−273.15 °C).
s. The Pauli exclusion principle
prevents fermions from entering the same quantum state, but a pair of fermions can behave as a boson, and multiple such pairs can then enter the same quantum state without restriction.
, which forms upon condensation of excited atom
s. These atoms can also turn into ion
s and electron
s if they reach a certain temperature. In April 2009, Nature
reported the creation of Rydberg molecules from a Rydberg atom and a ground state
atom, confirming that such a state of matter could exist. The experiment was performed using ultracold rubidium
atoms.
is a theoretical phase that may pave the way for the development of electronic devices that dissipate less energy and generate less heat. This is a derivation of the Quantum Hall state of matter.
es). May be stable at lower energy states once formed.
, and a star
such as our own sun
.
As a gas is heated, electrons begin to leave the atoms, resulting in the presence of free electrons, which are not bound to nuclei, and ions, which are chemical species
that contain unequal number of electrons and protons, and therefore possess an electrical charge. The free electric charges make the plasma electrically conductive so that it responds strongly to electromagnetic fields. At very high temperatures, such as those present in stars, it is assumed that essentially all electrons are "free," and that a very high-energy plasma is essentially bare nuclei swimming in a sea of electrons. Plasma is the most common state of non-dark matter
in the universe.
A plasma can be considered as a gas of highly ionized particles, but the powerful interionic forces lead to distinctly different properties, so that it is usually considered as a different phase or state of matter.
s become free and able to move independently (rather than being perpetually bound into particles) in a sea of gluon
s (subatomic particles that transmit the strong force
that binds quarks together); this is similar to splitting molecules into atoms. This state may be briefly attainable in particle accelerator
s, and allows scientists to observe the properties of individual quarks, and not just theorize. See also Strangeness production
.
Weakly symmetric matter: for up to 10−12 seconds after the Big Bang the strong, weak and electromagnetic forces were unified. Strongly symmetric matter
: for up to 10−36 seconds after the Big Bang
the energy density of the universe was so high that the four forces of nature
— strong
, weak
, electromagnetic
, and gravitation
al — are thought to have been unified into one single force. As the universe expanded, the temperature and density dropped and the gravitational force separated, a process called symmetry breaking
.
Quark-gluon plasma was discovered at CERN
in 2000.
predicted by general relativity
to exist at the center of a black hole
is not a phase of matter; it is not a material object at all (although the mass-energy of matter contributed to its creation) but rather a property of spacetime
at a location.
. In these conditions, the structure of matter is supported by the Pauli exclusion principle
. These are of great interest to astrophysicists, because these high-pressure conditions are believed to exist inside star
s that have used up their nuclear fusion
"fuel", such as white dwarf
s and neutron star
s.
Electron-degenerate matter
is found inside white dwarf
stars. Electrons remain bound to atoms but are able to transfer to adjacent atoms. Neutron-degenerate matter
is found in neutron star
s. Vast gravitational pressure compresses atoms so strongly that the electrons are forced to combine with protons via inverse beta-decay, resulting in a superdense conglomeration of neutrons. (Normally free neutrons
outside an atomic nucleus will decay
with a half life of just under 15 minutes, but in a neutron star, as in the nucleus of an atom, other effects stabilize the neutrons.)
ity and a frozen amorphous structure.
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
of matter
Matter
Matter is a general term for the substance of which all physical objects consist. Typically, matter includes atoms and other particles which have mass. A common way of defining matter is as anything that has mass and occupies volume...
take on. Solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
, liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
and gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
are the most common states of matter on Earth. However, much of the baryonic matter of the universe is in the form of hot plasma
Plasma (physics)
In physics and chemistry, plasma is a state of matter similar to gas in which a certain portion of the particles are ionized. Heating a gas may ionize its molecules or atoms , thus turning it into a plasma, which contains charged particles: positive ions and negative electrons or ions...
, both as rarefied interstellar medium
Interstellar medium
In astronomy, the interstellar medium is the matter that exists in the space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, dust, and cosmic rays. It fills interstellar space and blends smoothly into the surrounding intergalactic space...
and as dense star
Star
A star is a massive, luminous sphere of plasma held together by gravity. At the end of its lifetime, a star can also contain a proportion of degenerate matter. The nearest star to Earth is the Sun, which is the source of most of the energy on Earth...
s.
Historically, the distinction is made based on qualitative differences in bulk properties. Solid is the state in which matter maintains a fixed volume and shape; liquid is the state in which matter maintains a fixed volume but adapts to the shape of its container; and gas is the state in which matter expands to occupy whatever volume is available.
The state or phase of a given set of matter can change depending on pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
and temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
conditions, transitioning to other phases as these conditions change to favor their existence; for example, solid transitions to liquid with an increase in temperature.
States of matter may also be defined in terms of phase transitions
Phase diagram
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases can occur at equilibrium...
. A phase transition indicates a change in structure and can be recognized by an abrupt change in properties. By this definition, a distinct state of matter is any set of states
Thermodynamic state
A thermodynamic state is a set of values of properties of a thermodynamic system that must be specified to reproduce the system. The individual parameters are known as state variables, state parameters or thermodynamic variables. Once a sufficient set of thermodynamic variables have been...
distinguished from any other set of states by a phase transition
Phase diagram
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases can occur at equilibrium...
. Water can be said to have several distinct solid states. The appearance of superconductivity is associated with a phase transition, so there are superconductive
Superconductivity
Superconductivity is a phenomenon of exactly zero electrical resistance occurring in certain materials below a characteristic temperature. It was discovered by Heike Kamerlingh Onnes on April 8, 1911 in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum...
states. Likewise, ferromagnetic
Ferromagnetism
Ferromagnetism is the basic mechanism by which certain materials form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished...
states are demarcated by phase transitions and have distinctive properties.
When the change of state occurs in stages the intermediate steps are called mesophase
Mesophase
In physics, a mesophase is a state of matter intermediate between liquid and solid. Gelatin is a common example of a partially-ordered structure in a mesophase...
s. Such phases have been exploited by the introduction of liquid crystal
Liquid crystal
Liquid crystals are a state of matter that have properties between those of a conventional liquid and those of a solid crystal. For instance, an LC may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many different types of LC phases, which can be...
technology.
More recently, distinctions between states have been based on differences in molecular interrelationships. Solid is the state in which intermolecular attractions keep the molecules in fixed spatial relationships. Liquid is the state in which intermolecular attractions keep molecules in proximity, but do not keep the molecules in fixed relationships. Gas is that state in which the molecules are comparatively separated and intermolecular attractions have relatively little effect on their respective motions. Plasma
Plasma (physics)
In physics and chemistry, plasma is a state of matter similar to gas in which a certain portion of the particles are ionized. Heating a gas may ionize its molecules or atoms , thus turning it into a plasma, which contains charged particles: positive ions and negative electrons or ions...
is a highly ionized gas that occurs at high temperatures. The intermolecular forces created by ionic attractions and repulsions give these compositions distinct properties, for which reason plasma is described as a fourth state of matter.
Forms of matter that are not composed of molecules and are organized by different forces can also be considered different states of matter. Superfluids (like Fermionic condensate
Fermionic condensate
A fermionic condensate is a superfluid phase formed by fermionic particles at low temperatures. It is closely related to the Bose–Einstein condensate, a superfluid phase formed by bosonic atoms under similar conditions. Unlike the Bose–Einstein condensates, fermionic condensates are formed using...
) and the quark–gluon plasma are examples.
The three classical states
Each of the classical states of matter, unlike plasma for example, can transition directly into any of the other classical states.Solid

The particles (ions, atoms or molecules) are packed closely together. The forces between particles
Bonding in solids
Solids can be classified according to the nature of the bonding between their atomic or molecular components. The traditional classification distinguishes four kinds of bonding:...
are strong enough so that the particles cannot move freely but can only vibrate. As a result, a solid has a stable, definite shape, and a definite volume. Solids can only change their shape by force, as when broken or cut.
In crystalline solids
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
, the particles (atoms, molecules, or ions) are packed in a regularly ordered, repeating pattern. There are many different crystal structure
Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
s, and the same substance can have more than one structure (or solid phase). For example, iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
has a body-centred cubic
Cubic crystal system
In crystallography, the cubic crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals....
structure at temperatures below 912 °C, and a face-centred cubic
Cubic crystal system
In crystallography, the cubic crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals....
structure between 912 and 1394 °C. Ice
Ice
Ice is water frozen into the solid state. Usually ice is the phase known as ice Ih, which is the most abundant of the varying solid phases on the Earth's surface. It can appear transparent or opaque bluish-white color, depending on the presence of impurities or air inclusions...
has fifteen known crystal structures, or fifteen solid phases which exist at various temperatures and pressures.
Glass
Glass
Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
es and other non-crystalline, amorphous solid
Amorphous solid
In condensed matter physics, an amorphous or non-crystalline solid is a solid that lacks the long-range order characteristic of a crystal....
s without long-range order
Order and disorder (physics)
In physics, the terms order and disorder designate the presence or absence of some symmetry or correlation in a many-particle system.In condensed matter physics, systems typically are ordered at low temperatures; upon heating, they undergo one or several phase transitions into less ordered...
are not thermal equilibrium ground states; therefore they are described below as nonclassical states of matter.
Solids can be transformed into liquids by melting, and liquids can be transformed into solids by freezing. Solids can also change directly into gases through the process of sublimation.
Liquid
A liquid is a nearly incompressible fluid
Fluid
In physics, a fluid is a substance that continually deforms under an applied shear stress. Fluids are a subset of the phases of matter and include liquids, gases, plasmas and, to some extent, plastic solids....
which is able to conform to the shape of its container but retains a (nearly) constant volume independent of pressure. The volume is definite if the temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
and pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
are constant. When a solid is heated above its melting point
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
, it becomes liquid, given that the pressure is higher than the triple point
Triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases of that substance coexist in thermodynamic equilibrium...
of the substance. Intermolecular (or interatomic or interionic) forces are still important, but the molecules have enough energy to move relative to each other and the structure is mobile. This means that the shape of a liquid is not definite but is determined by its container. The volume is usually greater than that of the corresponding solid, the most well known exception being water, H2O. The highest temperature at which a given liquid can exist is its critical temperature.
Gas
A gas is a compressible fluid. Not only will a gas conform to the shape of its container but it will also expand to fill the container.In a gas, the molecules have enough kinetic energy
Kinetic energy
The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
so that the effect of intermolecular forces is small (or zero for an ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
), and the typical distahince between neighboring molecules is much greater than the molecular size. A gas has no definite shape or volume, but occupies the entire container in which it is confined. A liquid may be converted to a gas by heating at constant pressure to the boiling point
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
, or else by reducing the pressure at constant temperature.
At temperatures below its critical temperature, a gas is also called a vapor
Vapor
A vapor or vapour is a substance in the gas phase at a temperature lower than its critical point....
, and can be liquefied by compression alone without cooling. A vapor can exist in equilibrium with a liquid (or solid), in which case the gas pressure equals the vapor pressure
Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
of the liquid (or solid).
A supercritical fluid
Supercritical fluid
A supercritical fluid is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist. It can effuse through solids like a gas, and dissolve materials like a liquid...
(SCF) is a gas whose temperature and pressure are above the critical temperature and critical pressure respectively. In this state, the distinction between liquid and gas disappears. A supercritical fluid has the physical properties of a gas, but its high density confers solvent properties in some cases which lead to useful applications. For example, supercritical carbon dioxide
Supercritical carbon dioxide
Supercritical carbon dioxide is a fluid state of carbon dioxide where it is held at or above its critical temperature and critical pressure.Carbon dioxide usually behaves as a gas in air at STP or as a solid called dry ice when frozen...
is used to extract
Supercritical fluid extraction
Supercritical Fluid Extraction is the process of separating one component from another using supercritical fluids as the extracting solvent. Extraction is usually from a solid matrix, but can also be from liquids...
caffeine
Caffeine
Caffeine is a bitter, white crystalline xanthine alkaloid that acts as a stimulant drug. Caffeine is found in varying quantities in the seeds, leaves, and fruit of some plants, where it acts as a natural pesticide that paralyzes and kills certain insects feeding on the plants...
in the manufacture of decaffeinated
Decaffeination
Decaffeination is the act of removing caffeine from coffee beans, cocoa, tea leaves and other caffeine-containing materials. Despite removal of caffeine, many decaffeinated drinks still have around 1-2% of the...
coffee.
Glass
GlassGlass
Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
is a non-crystalline or amorphous solid
Amorphous solid
In condensed matter physics, an amorphous or non-crystalline solid is a solid that lacks the long-range order characteristic of a crystal....
material that exhibits a glass transition
Glass transition
The liquid-glass transition is the reversible transition in amorphous materials from a hard and relatively brittle state into a molten or rubber-like state. An amorphous solid that exhibits a glass transition is called a glass...
when heated towards the liquid state. Glasses can be made of quite different classes of materials: inorganic networks (such as window glass, made of silicate
Silicate
A silicate is a compound containing a silicon bearing anion. The great majority of silicates are oxides, but hexafluorosilicate and other anions are also included. This article focuses mainly on the Si-O anions. Silicates comprise the majority of the earth's crust, as well as the other...
plus additives), metallic alloys, ionic melts, aqueous solutions, molecular liquids, and polymers.
Thermodynamically, a glass is in a metastable state with respect to its crystalline counterpart. The conversion rate, however, is practically zero.
Crystals with some degree of disorder
A plastic crystal is a molecular solid with long-range positional order but with constituent molecules retaining rotational freedom; in an orientational glassOrientational glass
In solid-state physics, an orientational glass is a molecular solid in which crystalline long-range order coexists with quenched disorder in some rotational degree of freedom....
this degree of freedom is frozen in a quenched disordered
Order and disorder (physics)
In physics, the terms order and disorder designate the presence or absence of some symmetry or correlation in a many-particle system.In condensed matter physics, systems typically are ordered at low temperatures; upon heating, they undergo one or several phase transitions into less ordered...
state.
Similarly, in a spin glass
Spin glass
A spin glass is a magnet with frustrated interactions, augmented by stochastic disorder, where usually ferromagnetic and antiferromagnetic bonds are randomly distributed...
magnetic disorder is frozen.
Liquid crystal states
Liquid crystal states have properties intermediate between mobile liquids and ordered solids. Generally, they are able to flow like a liquid but exhibiting long-range order. For example, the nematic phase consists of long rod-like molecules such as para-azoxyanisolePara-Azoxyanisole
para-Azoxyanisole is an organic, aromatic compound. In a solid state, it appears as a white powder, but when heated it forms a liquid crystal. As one of the first known and most readily prepared liquid crystals, PAA has been played an important role in the development of liquid crystal...
, which is nematic in the temperature range 118–136 °C. In this state
the molecules flow as in a liquid, but they all point in the same direction (within each domain) and cannot rotate freely.
Other types of liquid crystals are described in the main article on these states. Several types have technological importance, for example, in liquid crystal display
Liquid crystal display
A liquid crystal display is a flat panel display, electronic visual display, or video display that uses the light modulating properties of liquid crystals . LCs do not emit light directly....
s.
Magnetically ordered
Transition metalTransition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
atoms often have magnetic moment
Magnetic moment
The magnetic moment of a magnet is a quantity that determines the force that the magnet can exert on electric currents and the torque that a magnetic field will exert on it...
s due to the net spin
Spin (physics)
In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
of electrons which remain unpaired and do not form chemical bonds. In some solids the magnetic moments on different atoms are ordered and can form a ferromagnet, an antiferromagnet or a ferrimagnet.
In a ferromagnet
Ferromagnetism
Ferromagnetism is the basic mechanism by which certain materials form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished...
—for instance, solid iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
—the magnetic moment on each atom is aligned in the same direction (within a magnetic domain
Magnetic domains
A magnetic domain describes a region within a magnetic material which has uniform magnetization. This means that the individual magnetic moments of the atoms are aligned with one another and they point in the same direction...
). If the domains are also aligned, the solid is a permanent magnet
Magnet
A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, and attracts or repels other magnets.A permanent magnet is an object...
, which is magnetic even in the absence of an external magnetic field
Magnetic field
A magnetic field is a mathematical description of the magnetic influence of electric currents and magnetic materials. The magnetic field at any given point is specified by both a direction and a magnitude ; as such it is a vector field.Technically, a magnetic field is a pseudo vector;...
. The magnetization
Magnetization
In classical electromagnetism, magnetization or magnetic polarization is the vector field that expresses the density of permanent or induced magnetic dipole moments in a magnetic material...
disappears when the magnet is heated to the Curie point
Curie point
In physics and materials science, the Curie temperature , or Curie point, is the temperature at which a ferromagnetic or a ferrimagnetic material becomes paramagnetic on heating; the effect is reversible. A magnet will lose its magnetism if heated above the Curie temperature...
, which for iron is 768 °C.
An antiferromagnet
Antiferromagnetism
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usuallyrelated to the spins of electrons, align in a regular pattern with neighboring spins pointing in opposite directions. This is, like ferromagnetism and ferrimagnetism, a manifestation of ordered magnetism...
has two networks of equal and opposite magnetic moments which cancel each other out, so that the net magnetization is zero. For example, in nickel(II) oxide
Nickel(II) oxide
Nickel oxide is the chemical compound with the formula NiO. It is notable as being the only well characterized oxide of nickel . The mineralogical form of NiO, bunsenite, is very rare. It is classified as a basic metal oxide...
(NiO), half the nickel atoms have moments aligned in one direction and half in the opposite direction.
In a ferrimagnet
Ferrimagnetism
In physics, a ferrimagnetic material is one in which the magnetic moments of the atoms on different sublattices are opposed, as in antiferromagnetism; however, in ferrimagnetic materials, the opposing moments are unequal and a spontaneous magnetization remains...
, the two networks of magnetic moments are opposite but unequal, so that cancellation is incomplete and there is a non-zero net magnetization. An example is magnetite
Magnetite
Magnetite is a ferrimagnetic mineral with chemical formula Fe3O4, one of several iron oxides and a member of the spinel group. The chemical IUPAC name is iron oxide and the common chemical name is ferrous-ferric oxide. The formula for magnetite may also be written as FeO·Fe2O3, which is one part...
(Fe3O4), which contains Fe2+ and Fe3+ ions with different magnetic moments.
Microphase-separated

Oil
An oil is any substance that is liquid at ambient temperatures and does not mix with water but may mix with other oils and organic solvents. This general definition includes vegetable oils, volatile essential oils, petrochemical oils, and synthetic oils....
and water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
. Due to chemical incompatibility between the blocks, block copolymers undergo a similar phase separation. However, because the blocks are covalently bonded
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
to each other, they cannot demix macroscopically as water and oil can, and so instead the blocks form nanometer-sized structures. Depending on the relative lengths of each block and the overall block topology of the polymer, many morphologies can be obtained, each its own phase of matter.
Superfluids
Close to absolute zero, some liquids form a second liquid state described as superfluid because it has zero viscosityViscosity
Viscosity is a measure of the resistance of a fluid which is being deformed by either shear or tensile stress. In everyday terms , viscosity is "thickness" or "internal friction". Thus, water is "thin", having a lower viscosity, while honey is "thick", having a higher viscosity...
(or infinite fluidity; i.e., flowing without friction). This was discovered in 1937 for helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
which forms a superfluid below the lambda temperature
Lambda point
The Lambda point is the temperature below which normal fluid helium transitions to superfluid helium II. More precisely, there is a lower lambda point at 2.172 K, 0.0497 atm, and an upper one at 1.76 K, 29.8 atm....
of 2.17 K. In this state it will attempt to "climb" out of its container. It also has infinite thermal conductivity
Thermal conductivity
In physics, thermal conductivity, k, is the property of a material's ability to conduct heat. It appears primarily in Fourier's Law for heat conduction....
so that no temperature gradient
Temperature gradient
A temperature gradient is a physical quantity that describes in which direction and at what rate the temperature changes the most rapidly around a particular location. The temperature gradient is a dimensional quantity expressed in units of degrees per unit length...
can form in a superfluid. Placing a super fluid in a spinning container will result in quantized vortices
Quantum vortex
In physics, a quantum vortex is a topological defect exhibited in superfluids and superconductors. Superfluids and superconductors are states of matter without friction. They exist only at very low temperatures. The existence of these quantum vortices was independently predicted by Richard Feynman...
.
These properties are explained by the theory that the common isotope helium-4
Helium-4
Helium-4 is a non-radioactive isotope of helium. It is by far the most abundant of the two naturally occurring isotopes of helium, making up about 99.99986% of the helium on earth. Its nucleus is the same as an alpha particle, consisting of two protons and two neutrons. Alpha decay of heavy...
forms a Bose–Einstein condensate
Bose–Einstein condensate
A Bose–Einstein condensate is a state of matter of a dilute gas of weakly interacting bosons confined in an external potential and cooled to temperatures very near absolute zero . Under such conditions, a large fraction of the bosons occupy the lowest quantum state of the external potential, at...
(see next section) in the superfluid state. More recently, Fermionic condensate
Fermionic condensate
A fermionic condensate is a superfluid phase formed by fermionic particles at low temperatures. It is closely related to the Bose–Einstein condensate, a superfluid phase formed by bosonic atoms under similar conditions. Unlike the Bose–Einstein condensates, fermionic condensates are formed using...
superfluids have been formed at even lower temperatures by the rare isotope helium-3
Helium-3
Helium-3 is a light, non-radioactive isotope of helium with two protons and one neutron. It is rare on Earth, and is sought for use in nuclear fusion research...
and by lithium-6
Isotopes of lithium
Naturally occurring lithium is composed of two stable isotopes, and , the latter being the more abundant...
.
Bose-Einstein condensates

In 1924, Albert Einstein
Albert Einstein
Albert Einstein was a German-born theoretical physicist who developed the theory of general relativity, effecting a revolution in physics. For this achievement, Einstein is often regarded as the father of modern physics and one of the most prolific intellects in human history...
and Satyendra Nath Bose
Satyendra Nath Bose
Satyendra Nath Bose FRS was an Indian mathematician and physicist noted for his collaboration with Albert Einstein in developing a theory regarding the gaslike qualities of electromagnetic radiation. He is best known for his work on quantum mechanics in the early 1920s, providing the foundation...
predicted the "Bose-Einstein condensate," (BEC) sometimes referred to as the fifth state of matter. In a BEC, matter stops behaving as independent particles, and collapses into a single quantum state that can be described with a single, uniform wavefunction.
In the gas phase, the Bose-Einstein condensate remained an unverified theoretical prediction for many years. In 1995 the research groups of Eric Cornell and Carl Wieman
Carl Wieman
Carl Edwin Wieman is an American physicist at the University of British Columbia and recipient of the Nobel Prize in Physics for the production, in 1995 with Eric Allin Cornell, of the first true Bose–Einstein condensate.-Biography:...
, of JILA at the University of Colorado at Boulder
University of Colorado at Boulder
The University of Colorado Boulder is a public research university located in Boulder, Colorado...
, produced the first such condensate experimentally. A Bose-Einstein condensate is "colder" than a solid. It may occur when atoms have very similar (or the same) quantum level
Quantum level
Quantum levels are fixed levels with a logarithmic, descending quantum pattern in the visible spectrum of light that can be observed through a spectrometer while looking at intense flows of electricity through the various halides on the periodic table in a vacuum tube...
s, at temperatures very close to absolute zero
Absolute zero
Absolute zero is the theoretical temperature at which entropy reaches its minimum value. The laws of thermodynamics state that absolute zero cannot be reached using only thermodynamic means....
(−273.15 °C).
Fermionic condensates
A fermionic condensate is similar to the Bose-Einstein condensate but composed of fermionFermion
In particle physics, a fermion is any particle which obeys the Fermi–Dirac statistics . Fermions contrast with bosons which obey Bose–Einstein statistics....
s. The Pauli exclusion principle
Pauli exclusion principle
The Pauli exclusion principle is the quantum mechanical principle that no two identical fermions may occupy the same quantum state simultaneously. A more rigorous statement is that the total wave function for two identical fermions is anti-symmetric with respect to exchange of the particles...
prevents fermions from entering the same quantum state, but a pair of fermions can behave as a boson, and multiple such pairs can then enter the same quantum state without restriction.
Rydberg molecules
One of the metastable states of strongly non-ideal plasma is Rydberg matterRydberg matter
Rydberg matter is a phase of matter formed by Rydberg atoms; it was predicted around 1980 by É. A. Manykin, M. I. Ozhovan and P. P. Poluéktov. It has been formed from various elements like caesium, potassium, hydrogen and nitrogen; studies have been conducted on theoretical possibilities like...
, which forms upon condensation of excited atom
Excited state
Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
s. These atoms can also turn into ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s and electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s if they reach a certain temperature. In April 2009, Nature
Nature (journal)
Nature, first published on 4 November 1869, is ranked the world's most cited interdisciplinary scientific journal by the Science Edition of the 2010 Journal Citation Reports...
reported the creation of Rydberg molecules from a Rydberg atom and a ground state
Ground state
The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
atom, confirming that such a state of matter could exist. The experiment was performed using ultracold rubidium
Rubidium
Rubidium is a chemical element with the symbol Rb and atomic number 37. Rubidium is a soft, silvery-white metallic element of the alkali metal group. Its atomic mass is 85.4678. Elemental rubidium is highly reactive, with properties similar to those of other elements in group 1, such as very rapid...
atoms.
Quantum Hall states
A quantum Hall state gives rise to quantized Hall voltage measured in the direction perpendicular to the current flow. A quantum spin Hall stateQuantum spin Hall effect
The quantum spin Hall state is a state of matter proposed to exist in special, two-dimensional, semiconductors with spin-orbit coupling. The quantum spin Hall state of matter is the cousin of the integer quantum Hall state, but, unlike the latter, it does not require the application of a large...
is a theoretical phase that may pave the way for the development of electronic devices that dissipate less energy and generate less heat. This is a derivation of the Quantum Hall state of matter.
Strange matter
Strange matter is a type of quark matter that may exist inside some neutron stars close to the Tolman–Oppenheimer–Volkoff limit (approximately 2–3 solar massSolar mass
The solar mass , , is a standard unit of mass in astronomy, used to indicate the masses of other stars and galaxies...
es). May be stable at lower energy states once formed.
Plasma (ionized gas)
Plasmas or ionized gases can exist at temperatures starting at several thousand degrees Celsius, where they consist of free charged particles, usually in equal numbers, such as ions and electrons. Plasma, like gas, is a state of matter that does not have definite shape or volume. Unlike gases, plasmas may self-generate magnetic fields and electric currents, and respond strongly and collectively to electromagnetic forces. The particles that make up plasmas have electric charges, so plasma can conduct electricity. Two examples of plasma are the charged air produced by lightningLightning
Lightning is an atmospheric electrostatic discharge accompanied by thunder, which typically occurs during thunderstorms, and sometimes during volcanic eruptions or dust storms...
, and a star
Star
A star is a massive, luminous sphere of plasma held together by gravity. At the end of its lifetime, a star can also contain a proportion of degenerate matter. The nearest star to Earth is the Sun, which is the source of most of the energy on Earth...
such as our own sun
Sun
The Sun is the star at the center of the Solar System. It is almost perfectly spherical and consists of hot plasma interwoven with magnetic fields...
.
As a gas is heated, electrons begin to leave the atoms, resulting in the presence of free electrons, which are not bound to nuclei, and ions, which are chemical species
Chemical species
Chemical species are atoms, molecules, molecular fragments, ions, etc., being subjected to a chemical process or to a measurement. Generally, a chemical species can be defined as an ensemble of chemically identical molecular entities that can explore the same set of molecular energy levels on a...
that contain unequal number of electrons and protons, and therefore possess an electrical charge. The free electric charges make the plasma electrically conductive so that it responds strongly to electromagnetic fields. At very high temperatures, such as those present in stars, it is assumed that essentially all electrons are "free," and that a very high-energy plasma is essentially bare nuclei swimming in a sea of electrons. Plasma is the most common state of non-dark matter
Dark matter
In astronomy and cosmology, dark matter is matter that neither emits nor scatters light or other electromagnetic radiation, and so cannot be directly detected via optical or radio astronomy...
in the universe.
A plasma can be considered as a gas of highly ionized particles, but the powerful interionic forces lead to distinctly different properties, so that it is usually considered as a different phase or state of matter.
Quark-gluon plasma
Quark-gluon plasma is a phase in which quarkQuark
A quark is an elementary particle and a fundamental constituent of matter. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. Due to a phenomenon known as color confinement, quarks are never directly...
s become free and able to move independently (rather than being perpetually bound into particles) in a sea of gluon
Gluon
Gluons are elementary particles which act as the exchange particles for the color force between quarks, analogous to the exchange of photons in the electromagnetic force between two charged particles....
s (subatomic particles that transmit the strong force
Strong interaction
In particle physics, the strong interaction is one of the four fundamental interactions of nature, the others being electromagnetism, the weak interaction and gravitation. As with the other fundamental interactions, it is a non-contact force...
that binds quarks together); this is similar to splitting molecules into atoms. This state may be briefly attainable in particle accelerator
Particle accelerator
A particle accelerator is a device that uses electromagnetic fields to propel charged particles to high speeds and to contain them in well-defined beams. An ordinary CRT television set is a simple form of accelerator. There are two basic types: electrostatic and oscillating field accelerators.In...
s, and allows scientists to observe the properties of individual quarks, and not just theorize. See also Strangeness production
Strangeness production
Strangeness production is a signature and a diagnostic tool of quark-gluon plasma formation and properties. Unlike up and down quarks, from which everyday matter is made, strange quarks are formed in pair production processes in collisions between constituents of the plasma...
.
Weakly symmetric matter: for up to 10−12 seconds after the Big Bang the strong, weak and electromagnetic forces were unified. Strongly symmetric matter
Strongly symmetric matter
Strongly symmetric matter: If the predictions of supersymmetry and more so, string theory are correct then during the time of the Planck Epoch all four fundamental forces were of equal strength and united into a single fundamental force...
: for up to 10−36 seconds after the Big Bang
Big Bang
The Big Bang theory is the prevailing cosmological model that explains the early development of the Universe. According to the Big Bang theory, the Universe was once in an extremely hot and dense state which expanded rapidly. This rapid expansion caused the young Universe to cool and resulted in...
the energy density of the universe was so high that the four forces of nature
Fundamental interaction
In particle physics, fundamental interactions are the ways that elementary particles interact with one another...
— strong
Strong interaction
In particle physics, the strong interaction is one of the four fundamental interactions of nature, the others being electromagnetism, the weak interaction and gravitation. As with the other fundamental interactions, it is a non-contact force...
, weak
Weak interaction
Weak interaction , is one of the four fundamental forces of nature, alongside the strong nuclear force, electromagnetism, and gravity. It is responsible for the radioactive decay of subatomic particles and initiates the process known as hydrogen fusion in stars...
, electromagnetic
Electromagnetism
Electromagnetism is one of the four fundamental interactions in nature. The other three are the strong interaction, the weak interaction and gravitation...
, and gravitation
Gravitation
Gravitation, or gravity, is a natural phenomenon by which physical bodies attract with a force proportional to their mass. Gravitation is most familiar as the agent that gives weight to objects with mass and causes them to fall to the ground when dropped...
al — are thought to have been unified into one single force. As the universe expanded, the temperature and density dropped and the gravitational force separated, a process called symmetry breaking
Symmetry breaking
Symmetry breaking in physics describes a phenomenon where small fluctuations acting on a system which is crossing a critical point decide the system's fate, by determining which branch of a bifurcation is taken. To an outside observer unaware of the fluctuations , the choice will appear arbitrary...
.
Quark-gluon plasma was discovered at CERN
CERN
The European Organization for Nuclear Research , known as CERN , is an international organization whose purpose is to operate the world's largest particle physics laboratory, which is situated in the northwest suburbs of Geneva on the Franco–Swiss border...
in 2000.
Very high energy states
The gravitational singularityGravitational singularity
A gravitational singularity or spacetime singularity is a location where the quantities that are used to measure the gravitational field become infinite in a way that does not depend on the coordinate system...
predicted by general relativity
General relativity
General relativity or the general theory of relativity is the geometric theory of gravitation published by Albert Einstein in 1916. It is the current description of gravitation in modern physics...
to exist at the center of a black hole
Black hole
A black hole is a region of spacetime from which nothing, not even light, can escape. The theory of general relativity predicts that a sufficiently compact mass will deform spacetime to form a black hole. Around a black hole there is a mathematically defined surface called an event horizon that...
is not a phase of matter; it is not a material object at all (although the mass-energy of matter contributed to its creation) but rather a property of spacetime
Spacetime
In physics, spacetime is any mathematical model that combines space and time into a single continuum. Spacetime is usually interpreted with space as being three-dimensional and time playing the role of a fourth dimension that is of a different sort from the spatial dimensions...
at a location.
Degenerate matter
Under extremely high pressure, ordinary matter undergoes a transition to a series of exotic states of matter collectively known as degenerate matterDegenerate matter
Degenerate matter is matter that has such extraordinarily high density that the dominant contribution to its pressure is attributable to the Pauli exclusion principle. The pressure maintained by a body of degenerate matter is called the degeneracy pressure, and arises because the Pauli principle...
. In these conditions, the structure of matter is supported by the Pauli exclusion principle
Pauli exclusion principle
The Pauli exclusion principle is the quantum mechanical principle that no two identical fermions may occupy the same quantum state simultaneously. A more rigorous statement is that the total wave function for two identical fermions is anti-symmetric with respect to exchange of the particles...
. These are of great interest to astrophysicists, because these high-pressure conditions are believed to exist inside star
Star
A star is a massive, luminous sphere of plasma held together by gravity. At the end of its lifetime, a star can also contain a proportion of degenerate matter. The nearest star to Earth is the Sun, which is the source of most of the energy on Earth...
s that have used up their nuclear fusion
Nuclear fusion
Nuclear fusion is the process by which two or more atomic nuclei join together, or "fuse", to form a single heavier nucleus. This is usually accompanied by the release or absorption of large quantities of energy...
"fuel", such as white dwarf
White dwarf
A white dwarf, also called a degenerate dwarf, is a small star composed mostly of electron-degenerate matter. They are very dense; a white dwarf's mass is comparable to that of the Sun and its volume is comparable to that of the Earth. Its faint luminosity comes from the emission of stored...
s and neutron star
Neutron star
A neutron star is a type of stellar remnant that can result from the gravitational collapse of a massive star during a Type II, Type Ib or Type Ic supernova event. Such stars are composed almost entirely of neutrons, which are subatomic particles without electrical charge and with a slightly larger...
s.
Electron-degenerate matter
Degenerate matter
Degenerate matter is matter that has such extraordinarily high density that the dominant contribution to its pressure is attributable to the Pauli exclusion principle. The pressure maintained by a body of degenerate matter is called the degeneracy pressure, and arises because the Pauli principle...
is found inside white dwarf
White dwarf
A white dwarf, also called a degenerate dwarf, is a small star composed mostly of electron-degenerate matter. They are very dense; a white dwarf's mass is comparable to that of the Sun and its volume is comparable to that of the Earth. Its faint luminosity comes from the emission of stored...
stars. Electrons remain bound to atoms but are able to transfer to adjacent atoms. Neutron-degenerate matter
Degenerate matter
Degenerate matter is matter that has such extraordinarily high density that the dominant contribution to its pressure is attributable to the Pauli exclusion principle. The pressure maintained by a body of degenerate matter is called the degeneracy pressure, and arises because the Pauli principle...
is found in neutron star
Neutron star
A neutron star is a type of stellar remnant that can result from the gravitational collapse of a massive star during a Type II, Type Ib or Type Ic supernova event. Such stars are composed almost entirely of neutrons, which are subatomic particles without electrical charge and with a slightly larger...
s. Vast gravitational pressure compresses atoms so strongly that the electrons are forced to combine with protons via inverse beta-decay, resulting in a superdense conglomeration of neutrons. (Normally free neutrons
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
outside an atomic nucleus will decay
Radioactive decay
Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
with a half life of just under 15 minutes, but in a neutron star, as in the nucleus of an atom, other effects stabilize the neutrons.)
Supersolid
A supersolid is a spatially ordered material (that is, a solid or crystal) with superfluid properties. Similar to a superfluid, a supersolid is able to move without friction but retains a rigid shape. Although a supersolid is a solid, it exhibits so many characteristic properties different from other solids that many argue it is another state of matter.String-net liquid
In a string-net liquid, atoms have apparently unstable arrangement, like a liquid, but are still consistent in overall pattern, like a solid. When in a normal solid state, the atoms of matter align themselves in a grid pattern, so that the spin of any electron is the opposite of the spin of all electrons touching it. But in a string-net liquid, atoms are arranged in some pattern which would require some electrons to have neighbors with the same spin. This gives rise to curious properties, as well as supporting some unusual proposals about the fundamental conditions of the universe itself.Superglass
A superglass is a phase of matter which is characterized at the same time by superfluidSuperfluid
Superfluidity is a state of matter in which the matter behaves like a fluid without viscosity and with extremely high thermal conductivity. The substance, which appears to be a normal liquid, will flow without friction past any surface, which allows it to continue to circulate over obstructions and...
ity and a frozen amorphous structure.
See also
- Condensed matter physicsCondensed matter physicsCondensed matter physics deals with the physical properties of condensed phases of matter. These properties appear when a number of atoms at the supramolecular and macromolecular scale interact strongly and adhere to each other or are otherwise highly concentrated in a system. The most familiar...
- Cooling curveCooling curveA cooling curve is a line graph that represents the change of phase of matter, typically from a gas to a solid or a liquid to a solid. The independent variable is time and the dependent variable is temperature...
- Phase (matter)Phase (matter)In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
- SupercoolingSupercoolingSupercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid....
- SuperheatingSuperheatingIn physics, superheating is the phenomenon in which a liquid is heated to a temperature higher than its boiling point, without boiling...
- EnthalpyEnthalpyEnthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
External links
- 2005-06-22, MIT News: MIT physicists create new form of matter Citat: "... They have become the first to create a new type of matter, a gas of atoms that shows high-temperature superfluidity."
- 2003-10-10, Science Daily: Metallic Phase For Bosons Implies New State Of Matter
- 2004-01-15, ScienceDaily: Probable Discovery Of A New, Supersolid, Phase Of Matter Citat: "...We apparently have observed, for the first time, a solid material with the characteristics of a superfluid...but because all its particles are in the identical quantum state, it remains a solid even though its component particles are continually flowing..."
- 2004-01-29, ScienceDaily: NIST/University Of Colorado Scientists Create New Form Of Matter: A Fermionic Condensate
- Short videos demonstrating of States of Matter, solids, liquids and gases by Prof. J M Murrell, University of Sussex

