Phase transition
Encyclopedia
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
system from one phase
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
or state of matter
State of matter
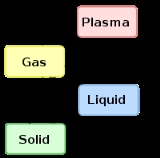
States of matter are the distinct forms that different phases of matter take on. Solid, liquid and gas are the most common states of matter on Earth. However, much of the baryonic matter of the universe is in the form of hot plasma, both as rarefied interstellar medium and as dense...
to another.
A phase of a thermodynamic system
Thermodynamic system
A thermodynamic system is a precisely defined macroscopic region of the universe, often called a physical system, that is studied using the principles of thermodynamics....
and the states of matter have uniform physical properties.
During a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some external condition, such as temperature, pressure, and others. For example, a liquid may become gas upon heating to the boiling point
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
, resulting in an abrupt change in volume
Volume
Volume is the quantity of three-dimensional space enclosed by some closed boundary, for example, the space that a substance or shape occupies or contains....
. The measurement of the external conditions at which the transformation occurs is termed the phase transition point.
Phase transitions are common occurrences observed in nature and many engineering techniques exploit certain types of phase transition.
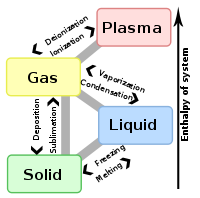
The term is most commonly used to describe transitions between solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
, liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
and gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
eous states of matter, in rare cases including plasma
Plasma (physics)
In physics and chemistry, plasma is a state of matter similar to gas in which a certain portion of the particles are ionized. Heating a gas may ionize its molecules or atoms , thus turning it into a plasma, which contains charged particles: positive ions and negative electrons or ions...
.
Types of phase transition
- The transitions between the solidSolidSolid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
, liquidLiquidLiquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
, and gasGasGas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
eous phases of a single component, due to the effects
- (see also vapor pressureVapor pressureVapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
and phase diagramPhase diagramA phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases can occur at equilibrium...
)

- A eutectic transformation, in which a two component single phase liquid is cooled and transforms into two solid phases. The same process, but beginning with a solid instead of a liquid is called a eutectoid transformation.
- A peritectic transformation, in which a two component single phase solid is heated and transforms into a solid phase and a liquid phase.
- A spinodal decompositionSpinodal decompositionSpinodal decomposition is a mechanism by which a solution of two or more components can separate into distinct regions with distinctly different chemical compositions and physical properties...
, in which a single phase is cooled and separates into two different compositions of that same phase. - Transition to a mesophaseMesophaseIn physics, a mesophase is a state of matter intermediate between liquid and solid. Gelatin is a common example of a partially-ordered structure in a mesophase...
between solid and liquid, such as one of the "liquid crystalLiquid crystalLiquid crystals are a state of matter that have properties between those of a conventional liquid and those of a solid crystal. For instance, an LC may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many different types of LC phases, which can be...
" phases. - The transition between the ferromagneticFerromagnetismFerromagnetism is the basic mechanism by which certain materials form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished...
and paramagneticParamagnetismParamagnetism is a form of magnetism whereby the paramagnetic material is only attracted when in the presence of an externally applied magnetic field. In contrast with this, diamagnetic materials are repulsive when placed in a magnetic field...
phases of magnetMagnetA magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, and attracts or repels other magnets.A permanent magnet is an object...
ic materials at the Curie pointCurie pointIn physics and materials science, the Curie temperature , or Curie point, is the temperature at which a ferromagnetic or a ferrimagnetic material becomes paramagnetic on heating; the effect is reversible. A magnet will lose its magnetism if heated above the Curie temperature...
. - The transition between differently ordered, commensurateANNNI modelIn statistical physics, the axial next-nearest-neighbor Ising model, usually known as the ANNNI model, is a variant of the Ising model in which competing ferromagnetic and...
or incommensurateCommensurability (mathematics)In mathematics, two non-zero real numbers a and b are said to be commensurable if a/b is a rational number.-History of the concept:...
, magnetic structures, such as in cerium antimonideAntimonideAntimonides are compounds of antimony with more electropositive elements. The antimonide ion is Sb3−.Many of them are flammable or decomposed by oxygen when heated since the antimonide ion is a reducing agent....
. - The martensitic transformation which occurs as one of the many phase transformations in carbon steel and stands as a model for displacive phase transformations.
- Changes in the crystallographic structure such as between ferriteFerrite (iron)Ferrite or alpha iron is a materials science term for iron, or a solid solution with iron as the main constituent, with a body centred cubic crystal structure. It is the component which gives steel and cast iron their magnetic properties, and is the classic example of a ferromagnetic material...
and austeniteAusteniteAustenite, also known as gamma phase iron, is a metallic non-magnetic allotrope of iron or a solid solution of iron, with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of ; other alloys of steel have different eutectoid temperatures...
of ironIronIron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
. - Order-disorder transitions such as in alpha-titanium aluminideTitanium aluminideTitanium aluminide, TiAl, is an intermetallic chemical compound. It is lightweight and resistant to oxidation and heat, however it suffers from low ductility. The density of gamma TiAl is about 4.0 g/cm³. It finds use in several applications including automobiles and aircraft...
s. - The emergence of superconductivitySuperconductivitySuperconductivity is a phenomenon of exactly zero electrical resistance occurring in certain materials below a characteristic temperature. It was discovered by Heike Kamerlingh Onnes on April 8, 1911 in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum...
in certain metalMetalA metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
s when cooled below a critical temperature. - The transition between different molecular structures (polymorphsPolymorphism (materials science)Polymorphism in materials science is the ability of a solid material to exist in more than one form or crystal structure. Polymorphism can potentially be found in any crystalline material including polymers, minerals, and metals, and is related to allotropy, which refers to chemical elements...
or allotropesAllotropyAllotropy or allotropism is the property of some chemical elements to exist in two or more different forms, known as allotropes of these elements...
), especially of solids, such as between an amorphousAmorphous solidIn condensed matter physics, an amorphous or non-crystalline solid is a solid that lacks the long-range order characteristic of a crystal....
structure and a crystalCrystalA crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
structure or between two different crystal structures. - Quantum condensation of bosonBosonIn particle physics, bosons are subatomic particles that obey Bose–Einstein statistics. Several bosons can occupy the same quantum state. The word boson derives from the name of Satyendra Nath Bose....
ic fluids, such as Bose-Einstein condensation and the superfluid transition in liquid heliumHeliumHelium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
. - The breaking of symmetriesSymmetrySymmetry generally conveys two primary meanings. The first is an imprecise sense of harmonious or aesthetically pleasing proportionality and balance; such that it reflects beauty or perfection...
in the laws of physics during the early history of the universe as its temperature cooled.
Phase transitions occur when the thermodynamic free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
of a system is non-analytic
Analytic function
In mathematics, an analytic function is a function that is locally given by a convergent power series. There exist both real analytic functions and complex analytic functions, categories that are similar in some ways, but different in others...
for some choice of thermodynamic variables (cf. phases
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
). This condition generally stems from the interactions of a large number of particles in a system, and does not appear in systems that are too small.
At the phase transition point (for instance, boiling point
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
) the two phases of a substance, liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
and vapor
Vapor
A vapor or vapour is a substance in the gas phase at a temperature lower than its critical point....
, have identical free energies and therefore are equally likely to exist. Below the boiling point, the liquid is the more stable state of the two, whereas above the gaseous form is preferred.
It is sometimes possible to change the state of a system diabatic
Diabatic
A diabatic process is one in which heat transfer takes place, which is the opposite of an adiabatic process. In quantum chemistry, the potential energy surfaces are obtained within the adiabatic or Born-Oppenheimer approximation...
ally (as opposed to adiabatic
Adiabatic invariant
An adiabatic invariant is a property of a physical system that stays constant when changes occur slowly.In thermodynamics, an adiabatic process is a change that occurs without heat flow, and slowly compared to the time to reach equilibrium. In an adiabatic process, the system is in equilibrium at...
ally) in such a way that it can be brought past a phase transition point without undergoing a phase transition. The resulting state is metastable, i.e. not theoretically stable, but quasistable. This occurs in superheating
Superheating
In physics, superheating is the phenomenon in which a liquid is heated to a temperature higher than its boiling point, without boiling...
, supercooling
Supercooling
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid....
and supersaturation
Supersaturation
The term supersaturation refers to a solution that contains more of the dissolved material than could be dissolved by the solvent under normal circumstances...
.
Ehrenfest classification
Paul EhrenfestPaul Ehrenfest
Paul Ehrenfest was an Austrian and Dutch physicist, who made major contributions to the field of statistical mechanics and its relations with quantum mechanics, including the theory of phase transition and the Ehrenfest theorem.- Biography :Paul Ehrenfest was born and grew up in Vienna in a Jewish...
classified phase transitions based on the behavior of the thermodynamic free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
as a function of other thermodynamic variables. Under this scheme, phase transitions were labeled by the lowest derivative of the free energy that is discontinuous at the transition. First-order phase transitions exhibit a discontinuity in the first derivative of the free energy with respect to some thermodynamic variable. The various solid/liquid/gas transitions are classified as first-order transitions because they involve a discontinuous change in density, which is the first derivative of the free energy with respect to chemical potential
Chemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
. Second-order phase transitions are continuous in the first derivative (the order parameter, which is the first derivative of the free energy with respect to the external field, is continuous across the transition) but exhibit discontinuity in a second derivative of the free energy. These include the ferromagnetic phase transition in materials such as iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
, where the magnetization
Magnetization
In classical electromagnetism, magnetization or magnetic polarization is the vector field that expresses the density of permanent or induced magnetic dipole moments in a magnetic material...
, which is the first derivative of the free energy with the applied magnetic field strength, increases continuously from zero as the temperature is lowered below the Curie temperature. The magnetic susceptibility
Magnetic susceptibility
In electromagnetism, the magnetic susceptibility \chi_m is a dimensionless proportionality constant that indicates the degree of magnetization of a material in response to an applied magnetic field...
, the second derivative of the free energy with the field, changes discontinuously. Under the Ehrenfest classification scheme, there could in principle be third, fourth, and higher-order phase transitions.
Though useful, Ehrenfest's classification has been found to be an inaccurate method of classifying phase transitions, for it does not take into account the case where a derivative
Derivative
In calculus, a branch of mathematics, the derivative is a measure of how a function changes as its input changes. Loosely speaking, a derivative can be thought of as how much one quantity is changing in response to changes in some other quantity; for example, the derivative of the position of a...
of free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
diverges (which is only possible in the thermodynamic limit
Thermodynamic limit
In thermodynamics, particularly statistical mechanics, the thermodynamic limit is reached as the number of particles in a system, N, approaches infinity...
). For instance, in the ferromagnetic transition, the heat capacity
Heat capacity
Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount...
diverges to infinity
Infinity
Infinity is a concept in many fields, most predominantly mathematics and physics, that refers to a quantity without bound or end. People have developed various ideas throughout history about the nature of infinity...
.
Modern classifications
In the modern classification scheme, phase transitions are divided into two broad categories, named similarly to the Ehrenfest classes:First-order phase transitions are those that involve a latent heat
Latent heat
Latent heat is the heat released or absorbed by a chemical substance or a thermodynamic system during a process that occurs without a change in temperature. A typical example is a change of state of matter, meaning a phase transition such as the melting of ice or the boiling of water. The term was...
. During such a transition, a system either absorbs or releases a fixed (and typically large) amount of energy. During this process, the temperature of the system will stay constant as heat is added: the system is in a "mixed-phase regime" in which some parts of the system have completed the transition and others have not. Familiar examples are the melting of ice or the boiling of water (the water does not instantly turn into vapor
Water vapor
Water vapor or water vapour , also aqueous vapor, is the gas phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Under typical atmospheric conditions, water vapor is continuously...
, but forms a turbulent
Turbulence
In fluid dynamics, turbulence or turbulent flow is a flow regime characterized by chaotic and stochastic property changes. This includes low momentum diffusion, high momentum convection, and rapid variation of pressure and velocity in space and time...
mixture of liquid water and vapor bubbles).
Second-order phase transitions are also called continuous phase transitions. They are characterized by a divergent susceptibility, an infinite correlation length, and a power-law decay of correlations near criticality. Examples of second-order phase transitions are the ferromagnetic transition, superconductor
Superconductor Insulator Transition
The Superconductor Insulator Transition is an example of a quantum phase transition, whereupon tuning some parameter in the Hamiltonian, a dramatic change in the behavior of the electrons occurs. The nature of how this transition occurs is disputed, and many studies seek to understand how the order...
and the superfluid
Superfluid
Superfluidity is a state of matter in which the matter behaves like a fluid without viscosity and with extremely high thermal conductivity. The substance, which appears to be a normal liquid, will flow without friction past any surface, which allows it to continue to circulate over obstructions and...
transition. Lev Landau
Lev Landau
Lev Davidovich Landau was a prominent Soviet physicist who made fundamental contributions to many areas of theoretical physics...
gave a phenomenological
Phenomenology (science)
The term phenomenology in science is used to describe a body of knowledge that relates empirical observations of phenomena to each other, in a way that is consistent with fundamental theory, but is not directly derived from theory. For example, we find the following definition in the Concise...
theory
Landau theory
Landau theory in physics was introduced by Lev Landau in an attempt to formulate a general theory of second-order phase transitions. He was motivated to suggest that the free energy of any system should obey two conditions: that the free energy is analytic, and that it obeys the symmetry of the...
of second order phase transitions.
Several transitions are known as the infinite-order phase transitions.
They are continuous but break no symmetries. The most famous example is the Kosterlitz–Thouless transition in the two-dimensional XY model
XY model
The classical XY model is a model of statistical mechanics. It is the special case of the n-vector model for n=2.-Definition:...
. Many quantum phase transition
Quantum phase transition
In physics, a quantum phase transition is a phase transition between different quantum phases . Contrary to classical phase transitions, quantum phase transitions can only be accessed by varying a physical parameter - such as magnetic field or pressure - at absolute zero temperature...
s in two-dimensional electron gases belong to this class.
The liquid-glass transition
Glass transition
The liquid-glass transition is the reversible transition in amorphous materials from a hard and relatively brittle state into a molten or rubber-like state. An amorphous solid that exhibits a glass transition is called a glass...
is observed in many polymers and other liquids that can be supercooled
Supercooling
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid....
far below the melting point of the crystalline phase. This is atypical in several respects. It is not a transition between thermodynamic ground states: it is widely believed that the true ground state is always crystalline. Glass is a quenched disorder state, and its entropy, density, and so on, depend on the thermal history. Therefore, the glass transition is primarily a dynamic phenomenon: on cooling a liquid, internal degrees of freedom successively fall out of equilibrium. However, there is a longstanding debate whether there is an underlying second-order phase transition in the hypothetical limit of infinitely long relaxation times.
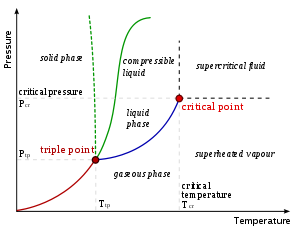
Critical points
In any system containing liquid and gaseous phases, there exists a special combination of pressure and temperature, known as the critical point, at which the transition between liquid and gas becomes a second-order transition. Near the critical point, the fluid is sufficiently hot and compressed that the distinction between the liquid and gaseous phases is almost non-existent. This is associated with the phenomenon of critical opalescenceCritical opalescence
Critical opalescence is a phenomenon which arises in the region of a continuous, or second-order, phase transition. Originally reported by Thomas Andrews in 1869 for the liquid-gas transition in carbon dioxide, many other examples have been discovered since. The phenomenon is most commonly...
, a milky appearance of the liquid due to density fluctuations at all possible wavelengths (including those of visible light).
Symmetry

Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
system from one phase
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
or state of matter
State of matter
States of matter are the distinct forms that different phases of matter take on. Solid, liquid and gas are the most common states of matter on Earth. However, much of the baryonic matter of the universe is in the form of hot plasma, both as rarefied interstellar medium and as dense...
to another.
A phase of a thermodynamic system
Thermodynamic system
A thermodynamic system is a precisely defined macroscopic region of the universe, often called a physical system, that is studied using the principles of thermodynamics....
and the states of matter have uniform physical properties.
During a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some external condition, such as temperature, pressure, and others. For example, a liquid may become gas upon heating to the boiling point
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
, resulting in an abrupt change in volume
Volume
Volume is the quantity of three-dimensional space enclosed by some closed boundary, for example, the space that a substance or shape occupies or contains....
. The measurement of the external conditions at which the transformation occurs is termed the phase transition point.
Phase transitions are common occurrences observed in nature and many engineering techniques exploit certain types of phase transition.
The term is most commonly used to describe transitions between solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
, liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
and gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
eous states of matter, in rare cases including plasma
Plasma (physics)
In physics and chemistry, plasma is a state of matter similar to gas in which a certain portion of the particles are ionized. Heating a gas may ionize its molecules or atoms , thus turning it into a plasma, which contains charged particles: positive ions and negative electrons or ions...
.
Types of phase transition
- The transitions between the solidSolidSolid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
, liquidLiquidLiquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
, and gasGasGas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
eous phases of a single component, due to the effects
- (see also vapor pressureVapor pressureVapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
and phase diagramPhase diagramA phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases can occur at equilibrium...
)


- A eutectic transformation, in which a two component single phase liquid is cooled and transforms into two solid phases. The same process, but beginning with a solid instead of a liquid is called a eutectoid transformation.
- A peritectic transformation, in which a two component single phase solid is heated and transforms into a solid phase and a liquid phase.
- A spinodal decompositionSpinodal decompositionSpinodal decomposition is a mechanism by which a solution of two or more components can separate into distinct regions with distinctly different chemical compositions and physical properties...
, in which a single phase is cooled and separates into two different compositions of that same phase. - Transition to a mesophaseMesophaseIn physics, a mesophase is a state of matter intermediate between liquid and solid. Gelatin is a common example of a partially-ordered structure in a mesophase...
between solid and liquid, such as one of the "liquid crystalLiquid crystalLiquid crystals are a state of matter that have properties between those of a conventional liquid and those of a solid crystal. For instance, an LC may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many different types of LC phases, which can be...
" phases. - The transition between the ferromagneticFerromagnetismFerromagnetism is the basic mechanism by which certain materials form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished...
and paramagneticParamagnetismParamagnetism is a form of magnetism whereby the paramagnetic material is only attracted when in the presence of an externally applied magnetic field. In contrast with this, diamagnetic materials are repulsive when placed in a magnetic field...
phases of magnetMagnetA magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, and attracts or repels other magnets.A permanent magnet is an object...
ic materials at the Curie pointCurie pointIn physics and materials science, the Curie temperature , or Curie point, is the temperature at which a ferromagnetic or a ferrimagnetic material becomes paramagnetic on heating; the effect is reversible. A magnet will lose its magnetism if heated above the Curie temperature...
. - The transition between differently ordered, commensurateANNNI modelIn statistical physics, the axial next-nearest-neighbor Ising model, usually known as the ANNNI model, is a variant of the Ising model in which competing ferromagnetic and...
or incommensurateCommensurability (mathematics)In mathematics, two non-zero real numbers a and b are said to be commensurable if a/b is a rational number.-History of the concept:...
, magnetic structures, such as in cerium antimonideAntimonideAntimonides are compounds of antimony with more electropositive elements. The antimonide ion is Sb3−.Many of them are flammable or decomposed by oxygen when heated since the antimonide ion is a reducing agent....
. - The martensitic transformation which occurs as one of the many phase transformations in carbon steel and stands as a model for displacive phase transformations.
- Changes in the crystallographic structure such as between ferriteFerrite (iron)Ferrite or alpha iron is a materials science term for iron, or a solid solution with iron as the main constituent, with a body centred cubic crystal structure. It is the component which gives steel and cast iron their magnetic properties, and is the classic example of a ferromagnetic material...
and austeniteAusteniteAustenite, also known as gamma phase iron, is a metallic non-magnetic allotrope of iron or a solid solution of iron, with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of ; other alloys of steel have different eutectoid temperatures...
of ironIronIron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
. - Order-disorder transitions such as in alpha-titanium aluminideTitanium aluminideTitanium aluminide, TiAl, is an intermetallic chemical compound. It is lightweight and resistant to oxidation and heat, however it suffers from low ductility. The density of gamma TiAl is about 4.0 g/cm³. It finds use in several applications including automobiles and aircraft...
s. - The emergence of superconductivitySuperconductivitySuperconductivity is a phenomenon of exactly zero electrical resistance occurring in certain materials below a characteristic temperature. It was discovered by Heike Kamerlingh Onnes on April 8, 1911 in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum...
in certain metalMetalA metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
s when cooled below a critical temperature. - The transition between different molecular structures (polymorphsPolymorphism (materials science)Polymorphism in materials science is the ability of a solid material to exist in more than one form or crystal structure. Polymorphism can potentially be found in any crystalline material including polymers, minerals, and metals, and is related to allotropy, which refers to chemical elements...
or allotropesAllotropyAllotropy or allotropism is the property of some chemical elements to exist in two or more different forms, known as allotropes of these elements...
), especially of solids, such as between an amorphousAmorphous solidIn condensed matter physics, an amorphous or non-crystalline solid is a solid that lacks the long-range order characteristic of a crystal....
structure and a crystalCrystalA crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
structure or between two different crystal structures. - Quantum condensation of bosonBosonIn particle physics, bosons are subatomic particles that obey Bose–Einstein statistics. Several bosons can occupy the same quantum state. The word boson derives from the name of Satyendra Nath Bose....
ic fluids, such as Bose-Einstein condensation and the superfluid transition in liquid heliumHeliumHelium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
. - The breaking of symmetriesSymmetrySymmetry generally conveys two primary meanings. The first is an imprecise sense of harmonious or aesthetically pleasing proportionality and balance; such that it reflects beauty or perfection...
in the laws of physics during the early history of the universe as its temperature cooled.
Phase transitions occur when the thermodynamic free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
of a system is non-analytic
Analytic function
In mathematics, an analytic function is a function that is locally given by a convergent power series. There exist both real analytic functions and complex analytic functions, categories that are similar in some ways, but different in others...
for some choice of thermodynamic variables (cf. phases
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
). This condition generally stems from the interactions of a large number of particles in a system, and does not appear in systems that are too small.
At the phase transition point (for instance, boiling point
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
) the two phases of a substance, liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
and vapor
Vapor
A vapor or vapour is a substance in the gas phase at a temperature lower than its critical point....
, have identical free energies and therefore are equally likely to exist. Below the boiling point, the liquid is the more stable state of the two, whereas above the gaseous form is preferred.
It is sometimes possible to change the state of a system diabatic
Diabatic
A diabatic process is one in which heat transfer takes place, which is the opposite of an adiabatic process. In quantum chemistry, the potential energy surfaces are obtained within the adiabatic or Born-Oppenheimer approximation...
ally (as opposed to adiabatic
Adiabatic invariant
An adiabatic invariant is a property of a physical system that stays constant when changes occur slowly.In thermodynamics, an adiabatic process is a change that occurs without heat flow, and slowly compared to the time to reach equilibrium. In an adiabatic process, the system is in equilibrium at...
ally) in such a way that it can be brought past a phase transition point without undergoing a phase transition. The resulting state is metastable, i.e. not theoretically stable, but quasistable. This occurs in superheating
Superheating
In physics, superheating is the phenomenon in which a liquid is heated to a temperature higher than its boiling point, without boiling...
, supercooling
Supercooling
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid....
and supersaturation
Supersaturation
The term supersaturation refers to a solution that contains more of the dissolved material than could be dissolved by the solvent under normal circumstances...
.
Ehrenfest classification
Paul EhrenfestPaul Ehrenfest
Paul Ehrenfest was an Austrian and Dutch physicist, who made major contributions to the field of statistical mechanics and its relations with quantum mechanics, including the theory of phase transition and the Ehrenfest theorem.- Biography :Paul Ehrenfest was born and grew up in Vienna in a Jewish...
classified phase transitions based on the behavior of the thermodynamic free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
as a function of other thermodynamic variables. Under this scheme, phase transitions were labeled by the lowest derivative of the free energy that is discontinuous at the transition. First-order phase transitions exhibit a discontinuity in the first derivative of the free energy with respect to some thermodynamic variable. The various solid/liquid/gas transitions are classified as first-order transitions because they involve a discontinuous change in density, which is the first derivative of the free energy with respect to chemical potential
Chemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
. Second-order phase transitions are continuous in the first derivative (the order parameter, which is the first derivative of the free energy with respect to the external field, is continuous across the transition) but exhibit discontinuity in a second derivative of the free energy. These include the ferromagnetic phase transition in materials such as iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
, where the magnetization
Magnetization
In classical electromagnetism, magnetization or magnetic polarization is the vector field that expresses the density of permanent or induced magnetic dipole moments in a magnetic material...
, which is the first derivative of the free energy with the applied magnetic field strength, increases continuously from zero as the temperature is lowered below the Curie temperature. The magnetic susceptibility
Magnetic susceptibility
In electromagnetism, the magnetic susceptibility \chi_m is a dimensionless proportionality constant that indicates the degree of magnetization of a material in response to an applied magnetic field...
, the second derivative of the free energy with the field, changes discontinuously. Under the Ehrenfest classification scheme, there could in principle be third, fourth, and higher-order phase transitions.
Though useful, Ehrenfest's classification has been found to be an inaccurate method of classifying phase transitions, for it does not take into account the case where a derivative
Derivative
In calculus, a branch of mathematics, the derivative is a measure of how a function changes as its input changes. Loosely speaking, a derivative can be thought of as how much one quantity is changing in response to changes in some other quantity; for example, the derivative of the position of a...
of free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
diverges (which is only possible in the thermodynamic limit
Thermodynamic limit
In thermodynamics, particularly statistical mechanics, the thermodynamic limit is reached as the number of particles in a system, N, approaches infinity...
). For instance, in the ferromagnetic transition, the heat capacity
Heat capacity
Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount...
diverges to infinity
Infinity
Infinity is a concept in many fields, most predominantly mathematics and physics, that refers to a quantity without bound or end. People have developed various ideas throughout history about the nature of infinity...
.
Modern classifications
In the modern classification scheme, phase transitions are divided into two broad categories, named similarly to the Ehrenfest classes:First-order phase transitions are those that involve a latent heat
Latent heat
Latent heat is the heat released or absorbed by a chemical substance or a thermodynamic system during a process that occurs without a change in temperature. A typical example is a change of state of matter, meaning a phase transition such as the melting of ice or the boiling of water. The term was...
. During such a transition, a system either absorbs or releases a fixed (and typically large) amount of energy. During this process, the temperature of the system will stay constant as heat is added: the system is in a "mixed-phase regime" in which some parts of the system have completed the transition and others have not. Familiar examples are the melting of ice or the boiling of water (the water does not instantly turn into vapor
Water vapor
Water vapor or water vapour , also aqueous vapor, is the gas phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Under typical atmospheric conditions, water vapor is continuously...
, but forms a turbulent
Turbulence
In fluid dynamics, turbulence or turbulent flow is a flow regime characterized by chaotic and stochastic property changes. This includes low momentum diffusion, high momentum convection, and rapid variation of pressure and velocity in space and time...
mixture of liquid water and vapor bubbles).
Second-order phase transitions are also called continuous phase transitions. They are characterized by a divergent susceptibility, an infinite correlation length, and a power-law decay of correlations near criticality. Examples of second-order phase transitions are the ferromagnetic transition, superconductor
Superconductor Insulator Transition
The Superconductor Insulator Transition is an example of a quantum phase transition, whereupon tuning some parameter in the Hamiltonian, a dramatic change in the behavior of the electrons occurs. The nature of how this transition occurs is disputed, and many studies seek to understand how the order...
and the superfluid
Superfluid
Superfluidity is a state of matter in which the matter behaves like a fluid without viscosity and with extremely high thermal conductivity. The substance, which appears to be a normal liquid, will flow without friction past any surface, which allows it to continue to circulate over obstructions and...
transition. Lev Landau
Lev Landau
Lev Davidovich Landau was a prominent Soviet physicist who made fundamental contributions to many areas of theoretical physics...
gave a phenomenological
Phenomenology (science)
The term phenomenology in science is used to describe a body of knowledge that relates empirical observations of phenomena to each other, in a way that is consistent with fundamental theory, but is not directly derived from theory. For example, we find the following definition in the Concise...
theory
Landau theory
Landau theory in physics was introduced by Lev Landau in an attempt to formulate a general theory of second-order phase transitions. He was motivated to suggest that the free energy of any system should obey two conditions: that the free energy is analytic, and that it obeys the symmetry of the...
of second order phase transitions.
Several transitions are known as the infinite-order phase transitions.
They are continuous but break no symmetries. The most famous example is the Kosterlitz–Thouless transition in the two-dimensional XY model
XY model
The classical XY model is a model of statistical mechanics. It is the special case of the n-vector model for n=2.-Definition:...
. Many quantum phase transition
Quantum phase transition
In physics, a quantum phase transition is a phase transition between different quantum phases . Contrary to classical phase transitions, quantum phase transitions can only be accessed by varying a physical parameter - such as magnetic field or pressure - at absolute zero temperature...
s in two-dimensional electron gases belong to this class.
The liquid-glass transition
Glass transition
The liquid-glass transition is the reversible transition in amorphous materials from a hard and relatively brittle state into a molten or rubber-like state. An amorphous solid that exhibits a glass transition is called a glass...
is observed in many polymers and other liquids that can be supercooled
Supercooling
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid....
far below the melting point of the crystalline phase. This is atypical in several respects. It is not a transition between thermodynamic ground states: it is widely believed that the true ground state is always crystalline. Glass is a quenched disorder state, and its entropy, density, and so on, depend on the thermal history. Therefore, the glass transition is primarily a dynamic phenomenon: on cooling a liquid, internal degrees of freedom successively fall out of equilibrium. However, there is a longstanding debate whether there is an underlying second-order phase transition in the hypothetical limit of infinitely long relaxation times.
Critical points
In any system containing liquid and gaseous phases, there exists a special combination of pressure and temperature, known as the critical point, at which the transition between liquid and gas becomes a second-order transition. Near the critical point, the fluid is sufficiently hot and compressed that the distinction between the liquid and gaseous phases is almost non-existent. This is associated with the phenomenon of critical opalescenceCritical opalescence
Critical opalescence is a phenomenon which arises in the region of a continuous, or second-order, phase transition. Originally reported by Thomas Andrews in 1869 for the liquid-gas transition in carbon dioxide, many other examples have been discovered since. The phenomenon is most commonly...
, a milky appearance of the liquid due to density fluctuations at all possible wavelengths (including those of visible light).
Symmetry

Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
system from one phase
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
or state of matter
State of matter
States of matter are the distinct forms that different phases of matter take on. Solid, liquid and gas are the most common states of matter on Earth. However, much of the baryonic matter of the universe is in the form of hot plasma, both as rarefied interstellar medium and as dense...
to another.
A phase of a thermodynamic system
Thermodynamic system
A thermodynamic system is a precisely defined macroscopic region of the universe, often called a physical system, that is studied using the principles of thermodynamics....
and the states of matter have uniform physical properties.
During a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some external condition, such as temperature, pressure, and others. For example, a liquid may become gas upon heating to the boiling point
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
, resulting in an abrupt change in volume
Volume
Volume is the quantity of three-dimensional space enclosed by some closed boundary, for example, the space that a substance or shape occupies or contains....
. The measurement of the external conditions at which the transformation occurs is termed the phase transition point.
Phase transitions are common occurrences observed in nature and many engineering techniques exploit certain types of phase transition.
The term is most commonly used to describe transitions between solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
, liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
and gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
eous states of matter, in rare cases including plasma
Plasma (physics)
In physics and chemistry, plasma is a state of matter similar to gas in which a certain portion of the particles are ionized. Heating a gas may ionize its molecules or atoms , thus turning it into a plasma, which contains charged particles: positive ions and negative electrons or ions...
.
Types of phase transition
- The transitions between the solidSolidSolid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
, liquidLiquidLiquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
, and gasGasGas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
eous phases of a single component, due to the effects
- (see also vapor pressureVapor pressureVapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
and phase diagramPhase diagramA phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases can occur at equilibrium...
)


- A eutectic transformation, in which a two component single phase liquid is cooled and transforms into two solid phases. The same process, but beginning with a solid instead of a liquid is called a eutectoid transformation.
- A peritectic transformation, in which a two component single phase solid is heated and transforms into a solid phase and a liquid phase.
- A spinodal decompositionSpinodal decompositionSpinodal decomposition is a mechanism by which a solution of two or more components can separate into distinct regions with distinctly different chemical compositions and physical properties...
, in which a single phase is cooled and separates into two different compositions of that same phase. - Transition to a mesophaseMesophaseIn physics, a mesophase is a state of matter intermediate between liquid and solid. Gelatin is a common example of a partially-ordered structure in a mesophase...
between solid and liquid, such as one of the "liquid crystalLiquid crystalLiquid crystals are a state of matter that have properties between those of a conventional liquid and those of a solid crystal. For instance, an LC may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many different types of LC phases, which can be...
" phases. - The transition between the ferromagneticFerromagnetismFerromagnetism is the basic mechanism by which certain materials form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished...
and paramagneticParamagnetismParamagnetism is a form of magnetism whereby the paramagnetic material is only attracted when in the presence of an externally applied magnetic field. In contrast with this, diamagnetic materials are repulsive when placed in a magnetic field...
phases of magnetMagnetA magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, and attracts or repels other magnets.A permanent magnet is an object...
ic materials at the Curie pointCurie pointIn physics and materials science, the Curie temperature , or Curie point, is the temperature at which a ferromagnetic or a ferrimagnetic material becomes paramagnetic on heating; the effect is reversible. A magnet will lose its magnetism if heated above the Curie temperature...
. - The transition between differently ordered, commensurateANNNI modelIn statistical physics, the axial next-nearest-neighbor Ising model, usually known as the ANNNI model, is a variant of the Ising model in which competing ferromagnetic and...
or incommensurateCommensurability (mathematics)In mathematics, two non-zero real numbers a and b are said to be commensurable if a/b is a rational number.-History of the concept:...
, magnetic structures, such as in cerium antimonideAntimonideAntimonides are compounds of antimony with more electropositive elements. The antimonide ion is Sb3−.Many of them are flammable or decomposed by oxygen when heated since the antimonide ion is a reducing agent....
. - The martensitic transformation which occurs as one of the many phase transformations in carbon steel and stands as a model for displacive phase transformations.
- Changes in the crystallographic structure such as between ferriteFerrite (iron)Ferrite or alpha iron is a materials science term for iron, or a solid solution with iron as the main constituent, with a body centred cubic crystal structure. It is the component which gives steel and cast iron their magnetic properties, and is the classic example of a ferromagnetic material...
and austeniteAusteniteAustenite, also known as gamma phase iron, is a metallic non-magnetic allotrope of iron or a solid solution of iron, with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of ; other alloys of steel have different eutectoid temperatures...
of ironIronIron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
. - Order-disorder transitions such as in alpha-titanium aluminideTitanium aluminideTitanium aluminide, TiAl, is an intermetallic chemical compound. It is lightweight and resistant to oxidation and heat, however it suffers from low ductility. The density of gamma TiAl is about 4.0 g/cm³. It finds use in several applications including automobiles and aircraft...
s. - The emergence of superconductivitySuperconductivitySuperconductivity is a phenomenon of exactly zero electrical resistance occurring in certain materials below a characteristic temperature. It was discovered by Heike Kamerlingh Onnes on April 8, 1911 in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum...
in certain metalMetalA metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
s when cooled below a critical temperature. - The transition between different molecular structures (polymorphsPolymorphism (materials science)Polymorphism in materials science is the ability of a solid material to exist in more than one form or crystal structure. Polymorphism can potentially be found in any crystalline material including polymers, minerals, and metals, and is related to allotropy, which refers to chemical elements...
or allotropesAllotropyAllotropy or allotropism is the property of some chemical elements to exist in two or more different forms, known as allotropes of these elements...
), especially of solids, such as between an amorphousAmorphous solidIn condensed matter physics, an amorphous or non-crystalline solid is a solid that lacks the long-range order characteristic of a crystal....
structure and a crystalCrystalA crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
structure or between two different crystal structures. - Quantum condensation of bosonBosonIn particle physics, bosons are subatomic particles that obey Bose–Einstein statistics. Several bosons can occupy the same quantum state. The word boson derives from the name of Satyendra Nath Bose....
ic fluids, such as Bose-Einstein condensation and the superfluid transition in liquid heliumHeliumHelium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
. - The breaking of symmetriesSymmetrySymmetry generally conveys two primary meanings. The first is an imprecise sense of harmonious or aesthetically pleasing proportionality and balance; such that it reflects beauty or perfection...
in the laws of physics during the early history of the universe as its temperature cooled.
Phase transitions occur when the thermodynamic free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
of a system is non-analytic
Analytic function
In mathematics, an analytic function is a function that is locally given by a convergent power series. There exist both real analytic functions and complex analytic functions, categories that are similar in some ways, but different in others...
for some choice of thermodynamic variables (cf. phases
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
). This condition generally stems from the interactions of a large number of particles in a system, and does not appear in systems that are too small.
At the phase transition point (for instance, boiling point
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
) the two phases of a substance, liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
and vapor
Vapor
A vapor or vapour is a substance in the gas phase at a temperature lower than its critical point....
, have identical free energies and therefore are equally likely to exist. Below the boiling point, the liquid is the more stable state of the two, whereas above the gaseous form is preferred.
It is sometimes possible to change the state of a system diabatic
Diabatic
A diabatic process is one in which heat transfer takes place, which is the opposite of an adiabatic process. In quantum chemistry, the potential energy surfaces are obtained within the adiabatic or Born-Oppenheimer approximation...
ally (as opposed to adiabatic
Adiabatic invariant
An adiabatic invariant is a property of a physical system that stays constant when changes occur slowly.In thermodynamics, an adiabatic process is a change that occurs without heat flow, and slowly compared to the time to reach equilibrium. In an adiabatic process, the system is in equilibrium at...
ally) in such a way that it can be brought past a phase transition point without undergoing a phase transition. The resulting state is metastable, i.e. not theoretically stable, but quasistable. This occurs in superheating
Superheating
In physics, superheating is the phenomenon in which a liquid is heated to a temperature higher than its boiling point, without boiling...
, supercooling
Supercooling
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid....
and supersaturation
Supersaturation
The term supersaturation refers to a solution that contains more of the dissolved material than could be dissolved by the solvent under normal circumstances...
.
Ehrenfest classification
Paul EhrenfestPaul Ehrenfest
Paul Ehrenfest was an Austrian and Dutch physicist, who made major contributions to the field of statistical mechanics and its relations with quantum mechanics, including the theory of phase transition and the Ehrenfest theorem.- Biography :Paul Ehrenfest was born and grew up in Vienna in a Jewish...
classified phase transitions based on the behavior of the thermodynamic free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
as a function of other thermodynamic variables. Under this scheme, phase transitions were labeled by the lowest derivative of the free energy that is discontinuous at the transition. First-order phase transitions exhibit a discontinuity in the first derivative of the free energy with respect to some thermodynamic variable. The various solid/liquid/gas transitions are classified as first-order transitions because they involve a discontinuous change in density, which is the first derivative of the free energy with respect to chemical potential
Chemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
. Second-order phase transitions are continuous in the first derivative (the order parameter, which is the first derivative of the free energy with respect to the external field, is continuous across the transition) but exhibit discontinuity in a second derivative of the free energy. These include the ferromagnetic phase transition in materials such as iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
, where the magnetization
Magnetization
In classical electromagnetism, magnetization or magnetic polarization is the vector field that expresses the density of permanent or induced magnetic dipole moments in a magnetic material...
, which is the first derivative of the free energy with the applied magnetic field strength, increases continuously from zero as the temperature is lowered below the Curie temperature. The magnetic susceptibility
Magnetic susceptibility
In electromagnetism, the magnetic susceptibility \chi_m is a dimensionless proportionality constant that indicates the degree of magnetization of a material in response to an applied magnetic field...
, the second derivative of the free energy with the field, changes discontinuously. Under the Ehrenfest classification scheme, there could in principle be third, fourth, and higher-order phase transitions.
Though useful, Ehrenfest's classification has been found to be an inaccurate method of classifying phase transitions, for it does not take into account the case where a derivative
Derivative
In calculus, a branch of mathematics, the derivative is a measure of how a function changes as its input changes. Loosely speaking, a derivative can be thought of as how much one quantity is changing in response to changes in some other quantity; for example, the derivative of the position of a...
of free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
diverges (which is only possible in the thermodynamic limit
Thermodynamic limit
In thermodynamics, particularly statistical mechanics, the thermodynamic limit is reached as the number of particles in a system, N, approaches infinity...
). For instance, in the ferromagnetic transition, the heat capacity
Heat capacity
Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount...
diverges to infinity
Infinity
Infinity is a concept in many fields, most predominantly mathematics and physics, that refers to a quantity without bound or end. People have developed various ideas throughout history about the nature of infinity...
.
Modern classifications
In the modern classification scheme, phase transitions are divided into two broad categories, named similarly to the Ehrenfest classes:First-order phase transitions are those that involve a latent heat
Latent heat
Latent heat is the heat released or absorbed by a chemical substance or a thermodynamic system during a process that occurs without a change in temperature. A typical example is a change of state of matter, meaning a phase transition such as the melting of ice or the boiling of water. The term was...
. During such a transition, a system either absorbs or releases a fixed (and typically large) amount of energy. During this process, the temperature of the system will stay constant as heat is added: the system is in a "mixed-phase regime" in which some parts of the system have completed the transition and others have not. Familiar examples are the melting of ice or the boiling of water (the water does not instantly turn into vapor
Water vapor
Water vapor or water vapour , also aqueous vapor, is the gas phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Under typical atmospheric conditions, water vapor is continuously...
, but forms a turbulent
Turbulence
In fluid dynamics, turbulence or turbulent flow is a flow regime characterized by chaotic and stochastic property changes. This includes low momentum diffusion, high momentum convection, and rapid variation of pressure and velocity in space and time...
mixture of liquid water and vapor bubbles).
Second-order phase transitions are also called continuous phase transitions. They are characterized by a divergent susceptibility, an infinite correlation length, and a power-law decay of correlations near criticality. Examples of second-order phase transitions are the ferromagnetic transition, superconductor
Superconductor Insulator Transition
The Superconductor Insulator Transition is an example of a quantum phase transition, whereupon tuning some parameter in the Hamiltonian, a dramatic change in the behavior of the electrons occurs. The nature of how this transition occurs is disputed, and many studies seek to understand how the order...
and the superfluid
Superfluid
Superfluidity is a state of matter in which the matter behaves like a fluid without viscosity and with extremely high thermal conductivity. The substance, which appears to be a normal liquid, will flow without friction past any surface, which allows it to continue to circulate over obstructions and...
transition. Lev Landau
Lev Landau
Lev Davidovich Landau was a prominent Soviet physicist who made fundamental contributions to many areas of theoretical physics...
gave a phenomenological
Phenomenology (science)
The term phenomenology in science is used to describe a body of knowledge that relates empirical observations of phenomena to each other, in a way that is consistent with fundamental theory, but is not directly derived from theory. For example, we find the following definition in the Concise...
theory
Landau theory
Landau theory in physics was introduced by Lev Landau in an attempt to formulate a general theory of second-order phase transitions. He was motivated to suggest that the free energy of any system should obey two conditions: that the free energy is analytic, and that it obeys the symmetry of the...
of second order phase transitions.
Several transitions are known as the infinite-order phase transitions.
They are continuous but break no symmetries. The most famous example is the Kosterlitz–Thouless transition in the two-dimensional XY model
XY model
The classical XY model is a model of statistical mechanics. It is the special case of the n-vector model for n=2.-Definition:...
. Many quantum phase transition
Quantum phase transition
In physics, a quantum phase transition is a phase transition between different quantum phases . Contrary to classical phase transitions, quantum phase transitions can only be accessed by varying a physical parameter - such as magnetic field or pressure - at absolute zero temperature...
s in two-dimensional electron gases belong to this class.
The liquid-glass transition
Glass transition
The liquid-glass transition is the reversible transition in amorphous materials from a hard and relatively brittle state into a molten or rubber-like state. An amorphous solid that exhibits a glass transition is called a glass...
is observed in many polymers and other liquids that can be supercooled
Supercooling
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid....
far below the melting point of the crystalline phase. This is atypical in several respects. It is not a transition between thermodynamic ground states: it is widely believed that the true ground state is always crystalline. Glass is a quenched disorder state, and its entropy, density, and so on, depend on the thermal history. Therefore, the glass transition is primarily a dynamic phenomenon: on cooling a liquid, internal degrees of freedom successively fall out of equilibrium. However, there is a longstanding debate whether there is an underlying second-order phase transition in the hypothetical limit of infinitely long relaxation times.
Critical points
In any system containing liquid and gaseous phases, there exists a special combination of pressure and temperature, known as the critical point, at which the transition between liquid and gas becomes a second-order transition. Near the critical point, the fluid is sufficiently hot and compressed that the distinction between the liquid and gaseous phases is almost non-existent. This is associated with the phenomenon of critical opalescenceCritical opalescence
Critical opalescence is a phenomenon which arises in the region of a continuous, or second-order, phase transition. Originally reported by Thomas Andrews in 1869 for the liquid-gas transition in carbon dioxide, many other examples have been discovered since. The phenomenon is most commonly...
, a milky appearance of the liquid due to density fluctuations at all possible wavelengths (including those of visible light).

