Resting potential
Encyclopedia
The relatively static membrane potential
of quiescent cells is called the resting membrane potential (or resting voltage), as opposed to the specific dynamic electrochemical phenomena called action potential
and graded membrane potential
.
Apart from the latter two, which occur in excitable cells
(neuron
s, muscle
s, and some secretory cells in gland
s), membrane voltage in the majority of non-excitable cells can also undergo changes in response to environmental or intracellular stimuli . In principle, there is no difference between resting membrane potential and dynamic voltage changes like action potential
from biophysical point of view: all these phenomena are caused by specific changes in membrane permeabilities for potassium
, sodium
, calcium
, and chloride
, which in turn result from concerted changes in functional activity of various ion channel
s, ion transporters, and exchangers. Conventionally, resting membrane potential can be defined as a relatively stable, ground value of transmembrane voltage in animal and plant cells.
Any voltage
is a difference in electric potential
between two points - for example, the separation of positive and negative electric charge
s on opposite sides of a resistive
barrier. The typical resting membrane potential of a cell arises from the separation of potassium
ions from intracellular, relatively immobile anions across the membrane of the cell. Because the membrane permeability for potassium is much higher than that for other ions (disregarding voltage-gated channels at this stage), and because of the strong chemical gradient for potassium, potassium ions flow from the cytosol into the extracellular space carrying out positive charge, until their movement is balanced by build-up of negative charge on the inner surface of the membrane. Again, because of the high relative permeability for potassium, the resulting membrane potential is almost always close to the potassium reversal potential
. But in order for this process to occur, a concentration gradient of potassium ions must first be set up. This work is done by the ion pumps/transporters and/or exchangers and generally is powered by ATP
.
In the case of the resting membrane potential across an animal cell's plasma membrane
, potassium (and sodium) gradients are established by the Na+/K+-ATPase
(sodium-potassium pump) which transports 2 potassium ions inside and 3 sodium ions outside at the cost of 1 ATP molecule. In other cases, for example, a membrane potential may be established by acidification of the inside of a membranous compartment (such as the proton pump that generates membrane potential across synaptic vesicle
membranes).
, electroneutrality is assumed; that is, that there is no measurable charge excess in any side of the membrane. So, although there is an electric potential across the membrane due to charge separation, there is no actual measurable difference in the global concentration of positive and negative ions across the membrane (as it is estimated below), that is, there is no actual measurable charge excess in either side. That occurs because the effect of charge on electrochemical potential is hugely greater than the effect of concentration so an undetectable change in concentration creates a great change on electric potential.
The resting voltage is the result of several ion-translocating enzymes (uniporters, cotransporters, and pumps) in the plasma membrane, steadily operating in parallel, whereby each ion-translocator has its characteristic electromotive force
(= reversal potential
= 'equilibrium voltage'), depending on the particular substrate concentrations inside and outside (internal ATP
included in case of some pumps). H+ exporting ATPase
render the membrane voltage in plants and fungi much more negative than in the more extensively investigated animal cells, where the resting voltage is mainly determined by selective ion channels.
In most neurons the resting potential has a value of approximately -70 mV. The resting potential is mostly determined by the concentrations of the ion
s in the fluids on both sides of the cell membrane
and the ion transport
protein
s that are in the cell membrane. How the concentrations of ions and the membrane transport proteins influence the value of the resting potential is outlined below.
The resting potential of a cell can be most thoroughly understood by thinking of it in terms of equilibrium potentials. In the example diagram here, the model cell was given only one permeant ion (potassium). In this case, the resting potential of this cell would be the same as the equilibrium potential for potassium.
However, a real cell is more complicated, having permeabilities to many ions, each of which contributes to the resting potential. To understand better, consider a cell with only two permeant ions, potassium and sodium. Consider a case where these two ions have equal concentration gradients directed in opposite directions, and that the membrane permeabilities to both ions are equal. K+ leaving the cell will tend to drag the membrane potential toward EK. Na+ entering the cell will tend to drag the membrane potential toward the reversal potential for sodium ENa. Since the permeabilities to both ions were set to be equal, the membrane potential will, at the end of the Na+/K+ tug-of-war, end up halfway between ENa and EK. As ENa and EK were equal but of opposite signs, halfway in between is zero, meaning that the membrane will rest at 0 mV.
Note that even though the membrane potential at 0 mV is stable, it is not an equilibrium condition because neither of the contributing ions are in equilibrium. Ions diffuse down their electrochemical gradients through ion channels, but the membrane potential is upheld by continual K+ influx and Na+ efflux via ion transporters. Such situation with similar permeabilities for counter-acting ions, like potassium and sodium in animal cells, can be extremely costly for the cell if these permeabilities are relatively large, as it takes a lot of ATP
energy to pump the ions back. Because no real cell can afford such equal and large ionic permeabilities at rest, resting potential of animal cells is determined by predominant high permeability to potassium and adjusted to the required value by modulating sodium and chloride permeabilities and gradients.
In a healthy animal cell Na+ permeability is about 5% of the K permeability or even less, whereas the respective reversal potential
s are +60 mV for sodium (ENa)and -80 mV for potassium (EK). Thus the membrane potential will not be right at EK, but rather depolarized from EK by an amount of approximately 5% of the 140 mV difference between EK and ENa. Thus, the cell's resting potential will be about −73 mV.
In a more formal notation, the membrane potential is the weighted average
of each contributing ion's equilibrium potential (Goldman equation
). The size of each weight is the relative permeability of each ion. In the normal case, where three ions contribute to the membrane potential: ,
,
where
s and ion transporters. Ion channel proteins create paths across cell membranes through which ions can passively diffuse
without direct expenditure of metabolic energy. They have selectivity for certain ions, thus, there are potassium-
, chloride-, and sodium-selective ion channels
. Different cells and even different parts of one cell (dendrite
s, cell bodies, nodes of Ranvier) will have different amounts of various ion transport proteins. Typically, the amount of certain potassium channels is most important for control of the resting potential (see below). Some ion pumps such as the Na+/K+-ATPase
are electrogenic, that is, they produce charge imbalance across the cell membrane and can also contribute directly to the membrane potential. Most pumps use metabolic energy (ATP) to function.
ions (K+) are the most important for the resting potential. Due to the active transport
of potassium ions, the concentration of potassium is higher inside cells than outside. Most cells have potassium-selective ion channel proteins that remain open all the time. There will be net movement of positively-charged potassium ions through these potassium channels with a resulting accumulation of excess negative charge inside of the cell. The outward movement of positively-charged potassium ions is due to random molecular motion (diffusion
) and continues until enough excess negative charge accumulates inside the cell to form a membrane potential which can balance the difference in concentration of potassium between inside and outside the cell. "Balance" means that the electrical force (potential
) that results from the build-up of ionic charge
, and which impedes outward diffusion, increases until it is equal in magnitude but opposite in direction to the tendency for outward diffusive movement of potassium. This balance point is an equilibrium potential as the net transmembrane flux (or current) of K+ is zero. The equilibrium potential for a given ion depends only upon the concentrations on either side of the membrane and the temperature. It can be calculated using the Nernst equation
:
where
Potassium equilibrium potentials of around -80 millivolts (inside negative) are common. Differences are observed in different species, different tissues within the same animal, and the same tissues under different environmental conditions. Applying the Nernst Equation above, one may account for these differences by changes in relative K+ concentration or differences in temperature.
For common usage the Nernst equation is often given in a simplified form by assuming typical human body temperature (37 C), reducing the constants and switching to Log base 10. (The units used for concentration are unimportant as they will cancel out into a ratio). For Potassium at normal body temperature one may calculate the equilibrium potential in millivolts as:

Likewise the equilibrium potential for sodium (Na+) at normal human body temperature is calculated using the same simplified constant. You can calculate E assuming an outside concentration, [K+]o, of 10mM and an inside concentration, [K+]i, of 100mM. For chloride ions (Cl-) the sign of the constant must be reversed (-61.54 mV). If calculating the equilibrium potential for calcium (Ca2+) the 2+ charge halves the simplified constant to 30.77 mV. If working at room temperature, about 21 C, the calculated constants are approximately 58 mV for K+ and Na+, - 58 mV for Cl- and 29 mV for Ca2+. At physiological temperature, about 29.5 C, and physiological concentrations (which vary for each ion), the calculated potentials are approximately 67 mV for Na+, -90mV for K+, -86 mV for Cl- and 123 mV for Ca2+.
using the concentrations of ions as for the equilibrium potential while also including the relative permeabilities
, or conductances, of each ionic species. Under normal conditions, it is safe to assume that only potassium, sodium
(Na+) and chloride
(Cl-) ions play large roles for the resting potential:

This equation resembles the Nernst equation, but has a term for each permeant ion. Also, z has been inserted into the equation, causing the intracellular and extracellular concentrations of Cl- to be reversed relative to K+ and Na+, as chloride's negative charge is handled by inverting the fraction inside the logarithmic term. *Em is the membrane potential, measured in volts *R, T, and F are as above *PX is the relative permeability of ion X in arbitrary units (e.g. siemens
for electrical conductance) *[X]Y is the concentration of ion X in compartment Y as above. Another way to view the membrane potential is using the Millman equation:
or reformulated

where Ptot is the combined permeability of all ionic species, again in arbitrary units. The latter equation portrays the resting membrane potential as a weighted average
of the reversal potentials of the system, where the weights are the relative permeabilites across the membranes (PX/Ptot). During the action potential, these weights change. If the permeabilities of Na+ and Cl- are zero, the membrane potential reduces to the Nernst potential for K+ (as PK+ = Ptot). Normally, under resting conditions PNa+ and PCl- are not zero, but they are much smaller than PK+, which renders Em close to Eeq,K+. Medical conditions such as hyperkalemia
in which blood
serum
potassium (which governs [K+]o) is changed are very dangerous since they offset Eeq,K+, thus affecting Em. This may cause arrhythmias and cardiac arrest
. The use of a bolus
injection of potassium chloride in executions by lethal injection stops the heart by shifting the resting potential to a more positive value, which depolarizes and contracts the cardiac cells permanently, not allowing the heart to repolarize
and thus enter diastole
to be refilled with blood.
). For such cells there is never any “rest” and the “resting potential” is a theoretical concept. Other cells with little in the way of membrane transport functions that change with time have a resting membrane potential that can be measured by inserting an electrode into the cell. Transmembrane potentials can also be measured optically with dyes that change their optical properties according to the membrane potential.
Membrane potential
Membrane potential is the difference in electrical potential between the interior and exterior of a biological cell. All animal cells are surrounded by a plasma membrane composed of a lipid bilayer with a variety of types of proteins embedded in it...
of quiescent cells is called the resting membrane potential (or resting voltage), as opposed to the specific dynamic electrochemical phenomena called action potential
Action potential
In physiology, an action potential is a short-lasting event in which the electrical membrane potential of a cell rapidly rises and falls, following a consistent trajectory. Action potentials occur in several types of animal cells, called excitable cells, which include neurons, muscle cells, and...
and graded membrane potential
Membrane potential
Membrane potential is the difference in electrical potential between the interior and exterior of a biological cell. All animal cells are surrounded by a plasma membrane composed of a lipid bilayer with a variety of types of proteins embedded in it...
.
Apart from the latter two, which occur in excitable cells
Membrane potential
Membrane potential is the difference in electrical potential between the interior and exterior of a biological cell. All animal cells are surrounded by a plasma membrane composed of a lipid bilayer with a variety of types of proteins embedded in it...
(neuron
Neuron
A neuron is an electrically excitable cell that processes and transmits information by electrical and chemical signaling. Chemical signaling occurs via synapses, specialized connections with other cells. Neurons connect to each other to form networks. Neurons are the core components of the nervous...
s, muscle
Muscle
Muscle is a contractile tissue of animals and is derived from the mesodermal layer of embryonic germ cells. Muscle cells contain contractile filaments that move past each other and change the size of the cell. They are classified as skeletal, cardiac, or smooth muscles. Their function is to...
s, and some secretory cells in gland
Gland
A gland is an organ in an animal's body that synthesizes a substance for release of substances such as hormones or breast milk, often into the bloodstream or into cavities inside the body or its outer surface .- Types :...
s), membrane voltage in the majority of non-excitable cells can also undergo changes in response to environmental or intracellular stimuli . In principle, there is no difference between resting membrane potential and dynamic voltage changes like action potential
Action potential
In physiology, an action potential is a short-lasting event in which the electrical membrane potential of a cell rapidly rises and falls, following a consistent trajectory. Action potentials occur in several types of animal cells, called excitable cells, which include neurons, muscle cells, and...
from biophysical point of view: all these phenomena are caused by specific changes in membrane permeabilities for potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
, sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
, calcium
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
, and chloride
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
, which in turn result from concerted changes in functional activity of various ion channel
Ion channel
Ion channels are pore-forming proteins that help establish and control the small voltage gradient across the plasma membrane of cells by allowing the flow of ions down their electrochemical gradient. They are present in the membranes that surround all biological cells...
s, ion transporters, and exchangers. Conventionally, resting membrane potential can be defined as a relatively stable, ground value of transmembrane voltage in animal and plant cells.
Any voltage
Voltage
Voltage, otherwise known as electrical potential difference or electric tension is the difference in electric potential between two points — or the difference in electric potential energy per unit charge between two points...
is a difference in electric potential
Electric potential
In classical electromagnetism, the electric potential at a point within a defined space is equal to the electric potential energy at that location divided by the charge there...
between two points - for example, the separation of positive and negative electric charge
Electric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
s on opposite sides of a resistive
Electrical resistance
The electrical resistance of an electrical element is the opposition to the passage of an electric current through that element; the inverse quantity is electrical conductance, the ease at which an electric current passes. Electrical resistance shares some conceptual parallels with the mechanical...
barrier. The typical resting membrane potential of a cell arises from the separation of potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
ions from intracellular, relatively immobile anions across the membrane of the cell. Because the membrane permeability for potassium is much higher than that for other ions (disregarding voltage-gated channels at this stage), and because of the strong chemical gradient for potassium, potassium ions flow from the cytosol into the extracellular space carrying out positive charge, until their movement is balanced by build-up of negative charge on the inner surface of the membrane. Again, because of the high relative permeability for potassium, the resulting membrane potential is almost always close to the potassium reversal potential
Reversal potential
In a biological membrane, the reversal potential of an ion is the membrane potential at which there is no net flow of that particular ion from one side of the membrane to the other...
. But in order for this process to occur, a concentration gradient of potassium ions must first be set up. This work is done by the ion pumps/transporters and/or exchangers and generally is powered by ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
.
In the case of the resting membrane potential across an animal cell's plasma membrane
Cell membrane
The cell membrane or plasma membrane is a biological membrane that separates the interior of all cells from the outside environment. The cell membrane is selectively permeable to ions and organic molecules and controls the movement of substances in and out of cells. It basically protects the cell...
, potassium (and sodium) gradients are established by the Na+/K+-ATPase
Na+/K+-ATPase
Na+/K+-ATPase is an enzyme located in the plasma membrane in all animals.- Sodium-potassium pumps :Active transport is responsible for cells containing relatively high...
(sodium-potassium pump) which transports 2 potassium ions inside and 3 sodium ions outside at the cost of 1 ATP molecule. In other cases, for example, a membrane potential may be established by acidification of the inside of a membranous compartment (such as the proton pump that generates membrane potential across synaptic vesicle
Synaptic vesicle
In a neuron, synaptic vesicles store various neurotransmitters that are released at the synapse. The release is regulated by a voltage-dependent calcium channel. Vesicles are essential for propagating nerve impulses between neurons and are constantly recreated by the cell...
membranes).
Electroneutrality
In most quantitative treatments of membrane potential, such as the derivation of Goldman equationGoldman equation
The Goldman–Hodgkin–Katz voltage equation, more commonly known as the Goldman equation is used in cell membrane physiology to determine the equilibrium potential across a cell's membrane taking into account all of the ions that are permeant through that membrane.The discoverers of this are David E...
, electroneutrality is assumed; that is, that there is no measurable charge excess in any side of the membrane. So, although there is an electric potential across the membrane due to charge separation, there is no actual measurable difference in the global concentration of positive and negative ions across the membrane (as it is estimated below), that is, there is no actual measurable charge excess in either side. That occurs because the effect of charge on electrochemical potential is hugely greater than the effect of concentration so an undetectable change in concentration creates a great change on electric potential.
Generation of the resting potential
Cell membranes are typically permeable to only a subset of ions. These usually include potassium ions, chloride ions, bicarbonate ions, and others. To simplify the description of the ionic basis of the resting membrane potential, it is most useful to consider only one ionic species at first, and consider the others later. Since trans-plasma-membrane potentials are almost always determined primarily by potassium permeability, that is where to start.
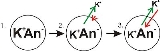
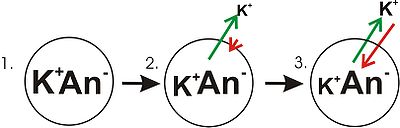
- Panel 1 of the diagram shows a digramatic representation of a simple cell where a concentration gradient has already been established. This panel is drawn as if the membrane has no permeability to any ion. There is no membrane potential, because despite there being a concentration gradient for potassium, there is no net charge imbalance across the membrane. If the membrane were to become permeable to a type of ion that is more concentrated on one side of the membrane, then that ion would contribute to membrane voltage because the permeant ions would move across the membrane with net movement of that ion type down the concentration gradient. There would be net movement from the side of the membrane with a higher concentration of the ion to the side with lower concentration. Such a movement of one ion across the membrane would result in a net imbalance of charge across the membrane and a membrane potential. This is a common mechanism by which many cells establish a membrane potential.
- In panel 2 of the diagram, the cell membrane has been made permeable to potassium ions, but not the anions (An-) inside the cell. These anions are mostly contributed by protein. There is energy stored in the potassium ion concentration gradient that can be converted into an electrical gradient when potassium (K) ions move out of the cell. Note that K ions can move across the membrane in both directions but by the purely statistical process that arises from the higher concentration of K inside the cell, there will be more K ions moving out of the cell. Because there is a higher concentration of K ions inside the cells, their random molecular motion is more likely to encounter the permeability pore (ion channelIon channelIon channels are pore-forming proteins that help establish and control the small voltage gradient across the plasma membrane of cells by allowing the flow of ions down their electrochemical gradient. They are present in the membranes that surround all biological cells...
) than is the case for the K ions that are outside and at a lower concentration. An internal K+ is simply "more likely" to leave the cell than an extracellular K+ is to enter it. It is a matter of simple diffusionDiffusionMolecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
doing work by dissipating the concentration gradient. As potassium leaves the cell, it is leaving behind the anions. Therefore a charge separation is developing as K+ leaves the cell. This charge separation creates a transmembrane voltage. This transmembrane voltage is the membrane potential. As potassium continues to leave the cell, separating more charges, the membrane potential will continue to grow. The length of the arrows (green indicating concentration gradient, red indicating voltage), represents the magnitude of potassium ion movement due to each form of energy. The direction of the arrow indicates the direction in which that particular force is applied. Thus, the building membrane voltage is an increasing force that acts counter to the tendency for net movement of K ions down the potassium concentration gradient. - In Panel 3, the membrane voltage has grown to the extent that its "strength" now matches the concentration gradient's. Since these forces (which are applied to K+ ions) are now the same strength and oriented in opposite directions, the system is now in equilibrium. Put another way, the tendency of potassium to leave the cell by running down its concentration gradient is now matched by the tendency of the membrane voltage to pull potassium ions back into the cell. K+ continues to move across the membrane, but the rate at which it enters and leaves the cell are the same, thus, there is no net potassium current. Because the K+ is at equilibrium, membrane potential is stable, or "resting".
The resting voltage is the result of several ion-translocating enzymes (uniporters, cotransporters, and pumps) in the plasma membrane, steadily operating in parallel, whereby each ion-translocator has its characteristic electromotive force
Electromotive force
In physics, electromotive force, emf , or electromotance refers to voltage generated by a battery or by the magnetic force according to Faraday's Law, which states that a time varying magnetic field will induce an electric current.It is important to note that the electromotive "force" is not a...
(= reversal potential
Reversal potential
In a biological membrane, the reversal potential of an ion is the membrane potential at which there is no net flow of that particular ion from one side of the membrane to the other...
= 'equilibrium voltage'), depending on the particular substrate concentrations inside and outside (internal ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
included in case of some pumps). H+ exporting ATPase
ATPase
ATPases are a class of enzymes that catalyze the decomposition of adenosine triphosphate into adenosine diphosphate and a free phosphate ion. This dephosphorylation reaction releases energy, which the enzyme harnesses to drive other chemical reactions that would not otherwise occur...
render the membrane voltage in plants and fungi much more negative than in the more extensively investigated animal cells, where the resting voltage is mainly determined by selective ion channels.
In most neurons the resting potential has a value of approximately -70 mV. The resting potential is mostly determined by the concentrations of the ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s in the fluids on both sides of the cell membrane
Cell membrane
The cell membrane or plasma membrane is a biological membrane that separates the interior of all cells from the outside environment. The cell membrane is selectively permeable to ions and organic molecules and controls the movement of substances in and out of cells. It basically protects the cell...
and the ion transport
Membrane transport
In cellular biology the term membrane transport refers to the collection of mechanisms that regulate the passage of solutes such as ions and small molecules through biological membranes namely lipid bilayers that contain proteins embedded in them...
protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s that are in the cell membrane. How the concentrations of ions and the membrane transport proteins influence the value of the resting potential is outlined below.
The resting potential of a cell can be most thoroughly understood by thinking of it in terms of equilibrium potentials. In the example diagram here, the model cell was given only one permeant ion (potassium). In this case, the resting potential of this cell would be the same as the equilibrium potential for potassium.
However, a real cell is more complicated, having permeabilities to many ions, each of which contributes to the resting potential. To understand better, consider a cell with only two permeant ions, potassium and sodium. Consider a case where these two ions have equal concentration gradients directed in opposite directions, and that the membrane permeabilities to both ions are equal. K+ leaving the cell will tend to drag the membrane potential toward EK. Na+ entering the cell will tend to drag the membrane potential toward the reversal potential for sodium ENa. Since the permeabilities to both ions were set to be equal, the membrane potential will, at the end of the Na+/K+ tug-of-war, end up halfway between ENa and EK. As ENa and EK were equal but of opposite signs, halfway in between is zero, meaning that the membrane will rest at 0 mV.
Note that even though the membrane potential at 0 mV is stable, it is not an equilibrium condition because neither of the contributing ions are in equilibrium. Ions diffuse down their electrochemical gradients through ion channels, but the membrane potential is upheld by continual K+ influx and Na+ efflux via ion transporters. Such situation with similar permeabilities for counter-acting ions, like potassium and sodium in animal cells, can be extremely costly for the cell if these permeabilities are relatively large, as it takes a lot of ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
energy to pump the ions back. Because no real cell can afford such equal and large ionic permeabilities at rest, resting potential of animal cells is determined by predominant high permeability to potassium and adjusted to the required value by modulating sodium and chloride permeabilities and gradients.
In a healthy animal cell Na+ permeability is about 5% of the K permeability or even less, whereas the respective reversal potential
Reversal potential
In a biological membrane, the reversal potential of an ion is the membrane potential at which there is no net flow of that particular ion from one side of the membrane to the other...
s are +60 mV for sodium (ENa)and -80 mV for potassium (EK). Thus the membrane potential will not be right at EK, but rather depolarized from EK by an amount of approximately 5% of the 140 mV difference between EK and ENa. Thus, the cell's resting potential will be about −73 mV.
In a more formal notation, the membrane potential is the weighted average
Weighted mean
The weighted mean is similar to an arithmetic mean , where instead of each of the data points contributing equally to the final average, some data points contribute more than others...
of each contributing ion's equilibrium potential (Goldman equation
Goldman equation
The Goldman–Hodgkin–Katz voltage equation, more commonly known as the Goldman equation is used in cell membrane physiology to determine the equilibrium potential across a cell's membrane taking into account all of the ions that are permeant through that membrane.The discoverers of this are David E...
). The size of each weight is the relative permeability of each ion. In the normal case, where three ions contribute to the membrane potential:
 ,
,where
- Em is the membrane potential, measured in volts
- EX is the equilibrium potential for ion X, also in volts
- PX is the relative permeability of ion X in arbitrary units (e.g. siemensSiemens (unit)The siemens is the SI derived unit of electric conductance and electric admittance. Conductance and admittance are the reciprocals of resistance and impedance respectively, hence one siemens is equal to the reciprocal of one ohm, and is sometimes referred to as the mho. In English, the term...
for electrical conductance) - Ptot is the total permeability of all permeant ions, in this case PK+ + PNa+ + PCl-
Membrane transport proteins
For determination of membrane potentials, the two most important types of membrane ion transport proteins are ion channelIon channel
Ion channels are pore-forming proteins that help establish and control the small voltage gradient across the plasma membrane of cells by allowing the flow of ions down their electrochemical gradient. They are present in the membranes that surround all biological cells...
s and ion transporters. Ion channel proteins create paths across cell membranes through which ions can passively diffuse
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
without direct expenditure of metabolic energy. They have selectivity for certain ions, thus, there are potassium-
Potassium channel
In the field of cell biology, potassium channels are the most widely distributed type of ion channel and are found in virtually all living organisms. They form potassium-selective pores that span cell membranes...
, chloride-, and sodium-selective ion channels
Sodium ion channel
Sodium channels are integral membrane proteins that form ion channels, conducting sodium ions through a cell's plasma membrane. They are classified according to the trigger that opens the channel for such ions, i.e...
. Different cells and even different parts of one cell (dendrite
Dendrite
Dendrites are the branched projections of a neuron that act to conduct the electrochemical stimulation received from other neural cells to the cell body, or soma, of the neuron from which the dendrites project...
s, cell bodies, nodes of Ranvier) will have different amounts of various ion transport proteins. Typically, the amount of certain potassium channels is most important for control of the resting potential (see below). Some ion pumps such as the Na+/K+-ATPase
Na+/K+-ATPase
Na+/K+-ATPase is an enzyme located in the plasma membrane in all animals.- Sodium-potassium pumps :Active transport is responsible for cells containing relatively high...
are electrogenic, that is, they produce charge imbalance across the cell membrane and can also contribute directly to the membrane potential. Most pumps use metabolic energy (ATP) to function.
Equilibrium potentials
For most animal cells potassiumPotassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
ions (K+) are the most important for the resting potential. Due to the active transport
Active transport
Active transport is the movement of a substance against its concentration gradient . In all cells, this is usually concerned with accumulating high concentrations of molecules that the cell needs, such as ions, glucose, and amino acids. If the process uses chemical energy, such as from adenosine...
of potassium ions, the concentration of potassium is higher inside cells than outside. Most cells have potassium-selective ion channel proteins that remain open all the time. There will be net movement of positively-charged potassium ions through these potassium channels with a resulting accumulation of excess negative charge inside of the cell. The outward movement of positively-charged potassium ions is due to random molecular motion (diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
) and continues until enough excess negative charge accumulates inside the cell to form a membrane potential which can balance the difference in concentration of potassium between inside and outside the cell. "Balance" means that the electrical force (potential
Electric field
In physics, an electric field surrounds electrically charged particles and time-varying magnetic fields. The electric field depicts the force exerted on other electrically charged objects by the electrically charged particle the field is surrounding...
) that results from the build-up of ionic charge
Charge (physics)
In physics, a charge may refer to one of many different quantities, such as the electric charge in electromagnetism or the color charge in quantum chromodynamics. Charges are associated with conserved quantum numbers.-Formal definition:...
, and which impedes outward diffusion, increases until it is equal in magnitude but opposite in direction to the tendency for outward diffusive movement of potassium. This balance point is an equilibrium potential as the net transmembrane flux (or current) of K+ is zero. The equilibrium potential for a given ion depends only upon the concentrations on either side of the membrane and the temperature. It can be calculated using the Nernst equation
Nernst equation
In electrochemistry, the Nernst equation is an equation that can be used to determine the equilibrium reduction potential of a half-cell in an electrochemical cell. It can also be used to determine the total voltage for a full electrochemical cell...
:

where
- Eeq,K+ is the equilibrium potential for potassium, measured in voltVoltThe volt is the SI derived unit for electric potential, electric potential difference, and electromotive force. The volt is named in honor of the Italian physicist Alessandro Volta , who invented the voltaic pile, possibly the first chemical battery.- Definition :A single volt is defined as the...
s - R is the universal gas constantGas constantThe gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
, equal to 8.314 jouleJouleThe joule ; symbol J) is a derived unit of energy or work in the International System of Units. It is equal to the energy expended in applying a force of one newton through a distance of one metre , or in passing an electric current of one ampere through a resistance of one ohm for one second...
s·K−1·mol−1 - T is the absolute temperature, measured in kelvinKelvinThe kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
s (= K = degrees Celsius + 273.15) - z is the number of elementary chargeElementary chargeThe elementary charge, usually denoted as e, is the electric charge carried by a single proton, or equivalently, the absolute value of the electric charge carried by a single electron. This elementary charge is a fundamental physical constant. To avoid confusion over its sign, e is sometimes called...
s of the ion in question involved in the reaction - F is the Faraday constant, equal to 96,485 coulombs·mol−1 or J·V−1·mol−1
- [K+]o is the extracellular concentration of potassium, measured in molMole (unit)The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
·m−3 or mmol·l−1 - [K+]i is likewise the intracellular concentration of potassium
Potassium equilibrium potentials of around -80 millivolts (inside negative) are common. Differences are observed in different species, different tissues within the same animal, and the same tissues under different environmental conditions. Applying the Nernst Equation above, one may account for these differences by changes in relative K+ concentration or differences in temperature.
For common usage the Nernst equation is often given in a simplified form by assuming typical human body temperature (37 C), reducing the constants and switching to Log base 10. (The units used for concentration are unimportant as they will cancel out into a ratio). For Potassium at normal body temperature one may calculate the equilibrium potential in millivolts as:

Likewise the equilibrium potential for sodium (Na+) at normal human body temperature is calculated using the same simplified constant. You can calculate E assuming an outside concentration, [K+]o, of 10mM and an inside concentration, [K+]i, of 100mM. For chloride ions (Cl-) the sign of the constant must be reversed (-61.54 mV). If calculating the equilibrium potential for calcium (Ca2+) the 2+ charge halves the simplified constant to 30.77 mV. If working at room temperature, about 21 C, the calculated constants are approximately 58 mV for K+ and Na+, - 58 mV for Cl- and 29 mV for Ca2+. At physiological temperature, about 29.5 C, and physiological concentrations (which vary for each ion), the calculated potentials are approximately 67 mV for Na+, -90mV for K+, -86 mV for Cl- and 123 mV for Ca2+.
Resting potentials
The resting membrane potential is not an equilibrium potential as it relies on the constant expenditure of energy (for ionic pumps as mentioned above) for its maintenance. It is a dynamic diffusion potential that takes this mechanism into account—wholly unlike the equilibrium potential, which is true no matter the nature of the system under consideration. The resting membrane potential is dominated by the ionic species in the system that has the greatest conductance across the membrane. For most cells this is potassium. As potassium is also the ion with the most negative equilibrium potential, usually the resting potential can be no more negative than the potassium equilibrium potential. The resting potential can be calculated with the Goldman-Hodgkin-Katz voltage equationGoldman equation
The Goldman–Hodgkin–Katz voltage equation, more commonly known as the Goldman equation is used in cell membrane physiology to determine the equilibrium potential across a cell's membrane taking into account all of the ions that are permeant through that membrane.The discoverers of this are David E...
using the concentrations of ions as for the equilibrium potential while also including the relative permeabilities
Semipermeable membrane
A semipermeable membrane, also termed a selectively permeable membrane, a partially permeable membrane or a differentially permeable membrane, is a membrane that will allow certain molecules or ions to pass through it by diffusion and occasionally specialized "facilitated diffusion".The rate of...
, or conductances, of each ionic species. Under normal conditions, it is safe to assume that only potassium, sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
(Na+) and chloride
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
(Cl-) ions play large roles for the resting potential:

This equation resembles the Nernst equation, but has a term for each permeant ion. Also, z has been inserted into the equation, causing the intracellular and extracellular concentrations of Cl- to be reversed relative to K+ and Na+, as chloride's negative charge is handled by inverting the fraction inside the logarithmic term. *Em is the membrane potential, measured in volts *R, T, and F are as above *PX is the relative permeability of ion X in arbitrary units (e.g. siemens
Siemens (unit)
The siemens is the SI derived unit of electric conductance and electric admittance. Conductance and admittance are the reciprocals of resistance and impedance respectively, hence one siemens is equal to the reciprocal of one ohm, and is sometimes referred to as the mho. In English, the term...
for electrical conductance) *[X]Y is the concentration of ion X in compartment Y as above. Another way to view the membrane potential is using the Millman equation:

or reformulated

where Ptot is the combined permeability of all ionic species, again in arbitrary units. The latter equation portrays the resting membrane potential as a weighted average
Weighted mean
The weighted mean is similar to an arithmetic mean , where instead of each of the data points contributing equally to the final average, some data points contribute more than others...
of the reversal potentials of the system, where the weights are the relative permeabilites across the membranes (PX/Ptot). During the action potential, these weights change. If the permeabilities of Na+ and Cl- are zero, the membrane potential reduces to the Nernst potential for K+ (as PK+ = Ptot). Normally, under resting conditions PNa+ and PCl- are not zero, but they are much smaller than PK+, which renders Em close to Eeq,K+. Medical conditions such as hyperkalemia
Hyperkalemia
Hyperkalemia refers to the condition in which the concentration of the electrolyte potassium in the blood is elevated...
in which blood
Blood
Blood is a specialized bodily fluid in animals that delivers necessary substances such as nutrients and oxygen to the cells and transports metabolic waste products away from those same cells....
serum
Blood plasma
Blood plasma is the straw-colored liquid component of blood in which the blood cells in whole blood are normally suspended. It makes up about 55% of the total blood volume. It is the intravascular fluid part of extracellular fluid...
potassium (which governs [K+]o) is changed are very dangerous since they offset Eeq,K+, thus affecting Em. This may cause arrhythmias and cardiac arrest
Cardiac arrest
Cardiac arrest, is the cessation of normal circulation of the blood due to failure of the heart to contract effectively...
. The use of a bolus
Bolus (medicine)
In medicine, a bolus is the administration of a medication, drug or other compound that is given to raise its concentration in blood to an effective level...
injection of potassium chloride in executions by lethal injection stops the heart by shifting the resting potential to a more positive value, which depolarizes and contracts the cardiac cells permanently, not allowing the heart to repolarize
Repolarization
In neuroscience, repolarization refers to the change in membrane potential that returns the membrane potential to a negative value after the depolarization phase of an action potential has just previously changed the membrane potential to a positive value. Repolarization results from the movement...
and thus enter diastole
Diastole
Diastole is the period of time when the heart fills with blood after systole . Ventricular diastole is the period during which the ventricles are relaxing, while atrial diastole is the period during which the atria are relaxing...
to be refilled with blood.
Measuring resting potentials
In some cells, the membrane potential is always changing (such as cardiac pacemaker cellsCardiac pacemaker
right|thumb|350px|Image showing the cardiac pacemaker which is the SA nodeThe contraction of heart muscle in all animals with hearts is initiated by chemical impulses. The rate at which these impulses fire controls the heart rate...
). For such cells there is never any “rest” and the “resting potential” is a theoretical concept. Other cells with little in the way of membrane transport functions that change with time have a resting membrane potential that can be measured by inserting an electrode into the cell. Transmembrane potentials can also be measured optically with dyes that change their optical properties according to the membrane potential.
Summary of resting potential values in different types of cells
The resting membrane potential in different cell types are approximately:- Skeletal muscleSkeletal muscleSkeletal muscle is a form of striated muscle tissue existing under control of the somatic nervous system- i.e. it is voluntarily controlled. It is one of three major muscle types, the others being cardiac and smooth muscle...
cells: −95 mV - Smooth muscle cells: -60mV
- Astroglia: -80/-90mV
- Neurons: -60mV
See also
- action potentialAction potentialIn physiology, an action potential is a short-lasting event in which the electrical membrane potential of a cell rapidly rises and falls, following a consistent trajectory. Action potentials occur in several types of animal cells, called excitable cells, which include neurons, muscle cells, and...
- depolarizationDepolarizationIn biology, depolarization is a change in a cell's membrane potential, making it more positive, or less negative. In neurons and some other cells, a large enough depolarization may result in an action potential...
- hyperpolarizationHyperpolarizationHyperpolarization has several meanings:* Hyperpolarization occurs when the strength of the electric field across the width of a cell membrane increases...
- membrane potentialMembrane potentialMembrane potential is the difference in electrical potential between the interior and exterior of a biological cell. All animal cells are surrounded by a plasma membrane composed of a lipid bilayer with a variety of types of proteins embedded in it...
External links
- Neuroscience - online textbook by Purves, et al.
- Basic Neurochemistry Molecular, Cellular, and Medical Aspects by Siegel, et al.
- Bertil HilleBertil HilleBertil Hille is a professor in the Department of Physiology and Biophysics at the University of Washington. He is particularly well known for his research and expertise on cell signalling by ion channels.-Early life and education:...
Ion channels of excitable membranes, 3rd ed., Sinauer Associates, Sunderland, MA (2001). ISBN 0-87893-321-2 - Generation of resting membrane potential Wright, S.H., Advances in Physiology Education, 28(1-4): 139-142, 2004,

