Delocalized electron
Encyclopedia
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s in a molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
, ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
or solid metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
that are not associated with a single atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
or one covalent bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
.
Delocalized electrons are contained within an orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
that extends over several adjacent atoms. Classically, delocalized electrons can be found in conjugated system
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
s and mesoionic
Mesoionic
Mesoionic chemical compounds are dipolar five- or six- membered heterocyclic compounds in which both the negative and the positive charges are delocalized...
compounds. It is increasingly appreciated that electrons in sigma bonding levels are also delocalized. For example, in methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
, the bonding electrons are shared by all five atoms equally. Pervasive existence of delocalization is implicit in molecular orbital theory
Molecular orbital theory
In chemistry, molecular orbital theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule...
.
Examples
In the simple aromatic ringSimple aromatic ring
Simple aromatic rings, also known as simple arenes or simple aromatics, are aromatic organic compounds that consist only of a conjugated planar ring system with delocalized pi electron clouds. Many simple aromatic rings have trivial names. They are usually found as substructures of more complex...
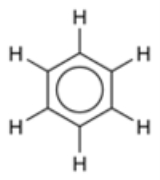
of benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
the delocalization of six π electrons
Pi bond
In chemistry, pi bonds are covalent chemical bonds where two lobes of one involved atomic orbital overlap two lobes of the other involved atomic orbital...
over the C6 ring is often graphically indicated by a circle. The fact that the six C-C bonds are equidistant is one indication of this delocalization. In valence bond theory
Valence bond theory
In chemistry, valence bond theory is one of two basic theories, along with molecular orbital theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds...
, delocalization in benzene is represented by resonance structures
Resonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula...
.
Delocalized electrons also exist in the structure of solid metals, where the d-subshell interferes with the above s-subshell. Metallic structure consist of aligned positive ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s (cations) in a "sea" of delocalized electrons. This means that the electrons are free to move throughout the structure, and gives rise to properties such as conductivity.
In diamond
Diamond
In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
all four outer electrons of each carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atom are 'localized' between the atoms in covalent bonding. The movement of electrons is restricted and diamond does not conduct an electric current. In graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
, each carbon atom uses only 3 of its 4 outer energy level electrons in covalently bonding to three other carbon atoms in a plane. Each carbon atom contributes one electron to a delocalized system of electrons that is also a part of the chemical bonding. The delocalized electrons are free to move throughout the plane. For this reason, graphite conducts electricity along the planes of carbon atoms, but does not conduct in a direction at right angle
Right angle
In geometry and trigonometry, a right angle is an angle that bisects the angle formed by two halves of a straight line. More precisely, if a ray is placed so that its endpoint is on a line and the adjacent angles are equal, then they are right angles...
s to the plane.
In the case of ions it is common to speak about delocalized charge (charge delocalization) when meaning delocalized electrons. An example of delocalized electrons (delcocalized charge) in ions can be found in the carboxylate group, wherein the negative charge is centered equally on the two oxygen atoms. Charge delocalization in anions is an important factor determining their reactivity (generally: the higher the extent of delocalization the lower the reactivity) and, specifically, the acid strength of their conjugate acids. As a general rule, the better delocalized is the charge in an anion the stronger is its conjugate acid
Conjugate acid
Within the Brønsted–Lowry acid-base theory , a conjugate acid is the acid member, HX, of a pair of two compounds that transform into each other by gain or loss of a proton. A conjugate acid can also be seen as the chemical substance that releases, or donates, a proton in the forward chemical...
. For example, the negative charge in perchlorate
Perchlorate
Perchlorates are the salts derived from perchloric acid . They occur both naturally and through manufacturing. They have been used as a medicine for more than 50 years to treat thyroid gland disorders. They are used extensively within the pyrotechnics industry, and ammonium perchlorate is also a...
anion (ClO4-) is evenly distributed among the symmetrically oriented oxygen atoms (and a part of it is also kept by the central chlorine atom). This excellent charge delocalization combined with the high number of oxygen atoms (four) and high electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
of the central chlorine atom leads to perchloric acid
Perchloric acid
Perchloric acid is the inorganic compound with the formula HClO4. Usually encountered as an aqueous solution, this colourless compound is a strong acid comparable in strength to sulfuric and nitric acids. It is a powerful oxidizer, but its aqueous solutions up to appr. 70% are remarkably inert,...
being one of the strongest known acids (with pKa values cited in the range of -7 .. -10). The extent of charge delocalization in an anion can be quantitatively expressed via the WAPS parameter.
Delocalization in reactions
Delocalized electrons are important for several reasons. One, an expected chemical reaction may not occur because the electrons delocalize to a more stable configuration, resulting in a reaction that happens at a different location. An example attempting the Friedel–Crafts alkylationAlkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
of benzene with 1-chloro-2-methylpropane; the carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
rearranges to a tert-butyl
Butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula -C4H9, derived from either of the two isomers of butane....
group stabilized by hyperconjugation
Hyperconjugation
In organic chemistry, hyperconjugation is the interaction of the electrons in a sigma bond with an adjacent empty non-bonding p-orbital or antibonding π orbital or filled π orbital, to give an extended molecular orbital that increases the stability of the system...
, a particular form of delocalization.

