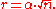Atomic diffusion
Encyclopedia
Atomic diffusion is a diffusion
process whereby the random thermally-activated movement of atom
s in a solid
results in the net transport of atoms. For example, helium
atoms inside a balloon can diffuse through the wall of the balloon and escape, resulting in the balloon slowly deflating. Other air molecule
s (e.g. oxygen
, nitrogen
) have lower mobilities and thus diffuse more slowly through the balloon wall. There is a concentration gradient in the balloon wall, because the balloon was initially filled with helium, and thus there is plenty of helium on the inside, but there is relatively little helium on the outside (helium is not a major component of air). The rate of transport is governed by the diffusivity and the concentration gradient.
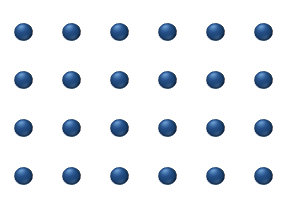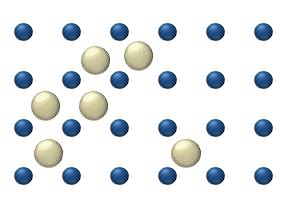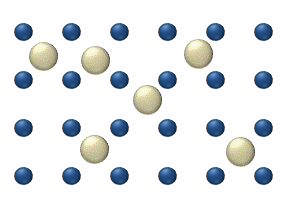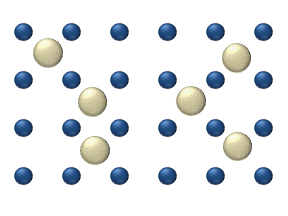 In the crystal solid state, diffusion within the crystal lattice occurs by either interstitial
In the crystal solid state, diffusion within the crystal lattice occurs by either interstitial
or substitutional mechanisms and is referred to as lattice diffusion
. In interstitial lattice diffusion, a diffusant (such as C in an iron alloy), will diffuse in between the lattice structure of another crystalline element. In substitutional lattice diffusion (self-diffusion
for example), the atom can only move by substituting place with another atom. Substitutional lattice diffusion is often contingent upon the availability of point vacancies
throughout the crystal lattice. Diffusing particles migrate from point vacancy to point vacancy by the rapid, essentially random jumping about
(jump diffusion).
Since the prevalence of point vacancies increases in accordance with the Arrhenius equation
, the rate of crystal solid state diffusion increases with temperature.
For a single atom in a defect-free crystal, the movement can be described by the "random walk" model. In 3-dimensions it can be shown that after jumps of length
jumps of length  the atom will have moved, on average, a distance of:
the atom will have moved, on average, a distance of:
If the jump frequency is given by (in jumps per second) and time is given by
(in jumps per second) and time is given by  , then
, then  is proportional to the square root of
is proportional to the square root of  :
:
Diffusion in polycrystalline
materials can involve short circuit diffusion mechanisms. For example, along the grain boundaries and certain crystalline defects such as dislocations there is more open space, thereby allowing for a lower activation energy for diffusion. Atomic diffusion in polycrystalline materials is therefore often modeled using an effective diffusion coefficient
, which is a combination of lattice, and grain boundary diffusion coefficient
s. In general, surface diffusion occurs much faster than grain boundary diffusion, and grain boundary diffusion occurs much faster than lattice diffusion.
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
process whereby the random thermally-activated movement of atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s in a solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
results in the net transport of atoms. For example, helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
atoms inside a balloon can diffuse through the wall of the balloon and escape, resulting in the balloon slowly deflating. Other air molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s (e.g. oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
) have lower mobilities and thus diffuse more slowly through the balloon wall. There is a concentration gradient in the balloon wall, because the balloon was initially filled with helium, and thus there is plenty of helium on the inside, but there is relatively little helium on the outside (helium is not a major component of air). The rate of transport is governed by the diffusivity and the concentration gradient.
In crystals
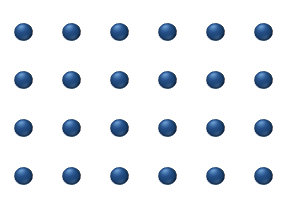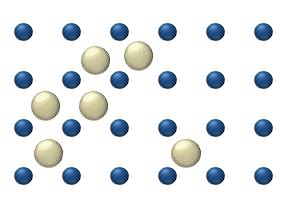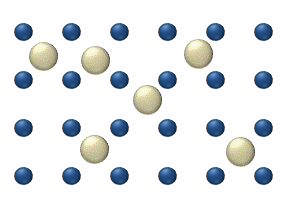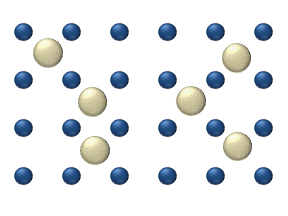
Interstitial defect
Interstitials are a variety of crystallographic defects, i.e. atoms which occupy a site in the crystal structure at which there is usually not an atom, or two or more atoms sharing one or more lattice sites such that the number of atoms is larger than the number of lattice sites.They are generally...
or substitutional mechanisms and is referred to as lattice diffusion
Lattice diffusion coefficient
Lattice diffusion refers to atomic diffusion within a crystalline lattice . Diffusion within the crystal lattice occurs by either interstitial or substitutional mechanisms and is referred to as lattice diffusion...
. In interstitial lattice diffusion, a diffusant (such as C in an iron alloy), will diffuse in between the lattice structure of another crystalline element. In substitutional lattice diffusion (self-diffusion
Self-diffusion
According to IUPAC definition, self-diffusion coefficient is the diffusion coefficient D_i^* of species i when the chemical potential gradient equals zero. It is linked to the diffusion coefficient D_i by the equation:...
for example), the atom can only move by substituting place with another atom. Substitutional lattice diffusion is often contingent upon the availability of point vacancies
Crystallographic defect
Crystalline solids exhibit a periodic crystal structure. The positions of atoms or molecules occur on repeating fixed distances, determined by the unit cell parameters. However, the arrangement of atom or molecules in most crystalline materials is not perfect...
throughout the crystal lattice. Diffusing particles migrate from point vacancy to point vacancy by the rapid, essentially random jumping about
(jump diffusion).
Since the prevalence of point vacancies increases in accordance with the Arrhenius equation
Arrhenius equation
The Arrhenius equation is a simple, but remarkably accurate, formula for the temperature dependence of the reaction rate constant, and therefore, rate of a chemical reaction. The equation was first proposed by the Dutch chemist J. H. van 't Hoff in 1884; five years later in 1889, the Swedish...
, the rate of crystal solid state diffusion increases with temperature.
For a single atom in a defect-free crystal, the movement can be described by the "random walk" model. In 3-dimensions it can be shown that after
 jumps of length
jumps of length  the atom will have moved, on average, a distance of:
the atom will have moved, on average, a distance of:If the jump frequency is given by
 (in jumps per second) and time is given by
(in jumps per second) and time is given by  , then
, then  is proportional to the square root of
is proportional to the square root of  :
:Diffusion in polycrystalline
Polycrystalline
Polycrystalline materials are solids that are composed of many crystallites of varying size and orientation. The variation in direction can be random or directed, possibly due to growth and processing conditions. Fiber texture is an example of the latter.Almost all common metals, and many ceramics...
materials can involve short circuit diffusion mechanisms. For example, along the grain boundaries and certain crystalline defects such as dislocations there is more open space, thereby allowing for a lower activation energy for diffusion. Atomic diffusion in polycrystalline materials is therefore often modeled using an effective diffusion coefficient
Effective diffusion coefficient
The effective diffusion coefficient of a diffusant in atomic diffusion of solid polycrystalline materials like metal alloys is often represented as a weighted average of the grain boundary diffusion coefficient and the lattice diffusion coefficient...
, which is a combination of lattice, and grain boundary diffusion coefficient
Grain boundary diffusion coefficient
The grain boundary diffusion coefficient is the diffusion coefficient of a diffusant along a grain boundary in a polycrystalline solid. At lower temperatures in metals and metal alloys, this diffusion term may dominate the effective diffusion rate.- See also :* Kirkendall effect* Phase...
s. In general, surface diffusion occurs much faster than grain boundary diffusion, and grain boundary diffusion occurs much faster than lattice diffusion.