
Nuclear reprocessing
Encyclopedia
Nuclear reprocessing technology was developed to chemically separate and recover fissionable plutonium from irradiated nuclear fuel. Reprocessing serves multiple purposes, whose relative importance has changed over time. Originally reprocessing was used solely to extract plutonium for producing nuclear weapons. With the commercialization of nuclear power
, the reprocessed plutonium was recycled back into MOX nuclear fuel for thermal reactor
s. The reprocessed uranium
, which constitutes the bulk of the spent fuel material, can in principle also be re-used as fuel, but that is only economic when uranium prices are high. Finally, the breeder reactor
can employ not only the recycled plutonium and uranium in spent fuel, but all the actinide
s, closing the nuclear fuel cycle
and potentially multiplying the energy
extracted from natural uranium
by more than 60 times.
Nuclear reprocessing reduces the volume of high-level waste, but by itself does not reduce radioactivity or heat generation and therefore does not eliminate the need for a geological waste repository. Reprocessing has been politically controversial because of the potential to contribute to nuclear proliferation
, the potential vulnerability to nuclear terrorism
, the political challenges of repository siting (a problem that applies equally to direct disposal of spent fuel), and because of its high cost compared to the once-through fuel cycle. The Obama administration stepped back from President Bush's plans for commercial-scale reprocessing and reverted to a program focused on reprocessing-related scientific research.
). The lighter elements
components include fission products, activation products, and cladding.
. These reactors were designed for the production of plutonium for use in nuclear weapon
s. The only reprocessing required, therefore, was the extraction of the plutonium
(free of fission-product
contamination) from the spent natural uranium
fuel. In 1943, several methods were proposed for separating the relatively small quantity of plutonium from the uranium and fission products. The first method selected, a precipitation process called the Bismuth
Phosphate
process, was developed and tested at the Oak Ridge National Laboratory
(ORNL) in the 1943-1945 period to produce quantities of plutonium for evaluation and use in weapons programs
. ORNL produced the first macroscopic quantities (grams) of separated plutonium with these processes.
The Bismuth Phosphate process was first operated on a large scale at the Hanford Site
, in the latter part of 1944. It was successful for plutonium separation in the emergency situation existing then, but it had a significant weakness: the inability to recover uranium.
The first successful solvent extraction process for the recovery of pure uranium and plutonium was developed at ORNL in 1949. The PUREX
process is the current method of extraction. Separation plants were also constructed at Savannah River Site
and a smaller plant at West Valley, New York
which closed by 1972 because of its inability to meet new regulatory requirements.
Reprocessing of civilian fuel has long been employed in Europe, at the COGEMA La Hague site
in France, the Sellafield
site in the United Kingdom, the Mayak
Chemical Combine in Russia, and at sites such as the Tokai plant in Japan, the Tarapur plant in India, and briefly at the West Valley Reprocessing Plant
in the United States.
In October 1976, fear of nuclear weapons proliferation (especially after India
demonstrated nuclear weapons capabilities using reprocessing technology) led President Gerald Ford
to issue a Presidential directive
to indefinitely suspend the commercial reprocessing and recycling of plutonium in the U.S. On April 7, 1977 , President Jimmy Carter
banned the reprocessing of commercial reactor spent nuclear fuel
. The key issue driving this policy was the serious threat of nuclear weapons proliferation
by diversion of plutonium from the civilian fuel cycle, and to encourage other nations to follow the USA lead.
. After that, only countries that already had large investments in reprocessing infrastructure continued to reprocess spent nuclear fuel. President Reagan lifted the ban in 1981, but did not provide the substantial subsidy that would have been necessary to start up commercial reprocessing.
In March 1999, the U.S. Department of Energy (DOE) reversed its own policy and signed a contract with a consortium
of Duke Energy
, COGEMA
, and Stone & Webster
(DCS) to design and operate a Mixed Oxide (MOX) fuel
fabrication facility. Site preparation at the Savannah River Site (South Carolina) began in October 2005.
method used to reprocess spent nuclear fuel
, in order to extract uranium
and plutonium
, independent of each other, from the fission
products. This is the most developed and widely used process in the industry at present.
When used on fuel from commercial power reactors the plutonium extracted typically contains too much Pu-240 to be useful in a nuclear weapon. However, reactors that are capable of refuelling frequently can be used to produce weapon-grade
plutonium, which can later be recovered using PUREX. Because of this, PUREX chemicals are monitored.
The PUREX process can be modified to make a UREX (URanium EXtraction) process which could be used to save space inside high level nuclear waste disposal sites, such as the Yucca Mountain nuclear waste repository, by removing the uranium which makes up the vast majority of the mass and volume of used fuel and recycling it as reprocessed uranium
.
The UREX process is a PUREX process which has been modified to prevent the plutonium from being extracted. This can be done by adding a plutonium reductant before the first metal extraction step. In the UREX process, ~99.9% of the uranium and >95% of technetium
are separated from each other and the other fission products and actinide
s. The key is the addition of acetohydroxamic acid
(AHA) to the extraction and scrub sections of the process. The addition of AHA greatly diminishes the extractability of plutonium and neptunium
, providing greater proliferation resistance than with the plutonium extraction stage of the PUREX process.
Adding a second extraction agent, octyl(phenyl)-N, N-dibutyl carbamoylmethyl phosphine oxide(CMPO) in combination with tributylphosphate, (TBP), the PUREX process can be turned into the TRUEX (TRansUranic EXtraction) process. TRUEX was invented in the USA by Argonne National Laboratory and is designed to remove the transuranic metals (Am/Cm) from waste. The idea is that by lowering the alpha activity of the waste, the majority of the waste can then be disposed of with greater ease. In common with PUREX this process operates by a solvation
mechanism.
As an alternative to TRUEX, an extraction process using a malondiamide has been devised. The DIAMEX (DIAMideEXtraction) process has the advantage of avoiding the formation of organic waste which contains elements other than carbon
, hydrogen
, nitrogen
, and oxygen
. Such an organic waste can be burned without the formation of acidic gases which could contribute to acid rain
. The DIAMEX process is being worked on in Europe
by the French CEA
. The process is sufficiently mature that an industrial plant could be constructed with the existing knowledge of the process. In common with PUREX this process operates by a solvation mechanism.
Selective ActiNide EXtraction. As part of the management of minor actinides it has been proposed that the lanthanides and trivalent minor actinides should be removed from the PUREX raffinate
by a process such as DIAMEX or TRUEX. In order to allow the actinides such as americium to be either reused in industrial sources or used as fuel, the lanthanides must be removed. The lanthanides have large neutron cross sections and hence they would poison a neutron driven nuclear reaction. To date the extraction system for the SANEX process has not been defined, but currently several different research groups are working towards a process. For instance the French CEA
is working on a bis-triazinyl pyridine (BTP) based process.
Other systems such as the dithiophosphinic acids are being worked on by some other workers.
The UNiversal EXtraction process was developed in Russia
and the Czech Republic
; it is designed to completely remove the most troublesome radioisotopes (Sr, Cs and minor actinides
) from the raffinate remaining after the extraction of uranium and plutonium from used nuclear fuel
. The chemistry is based upon the interaction of caesium
and strontium
with polyethylene glycol
) and a cobalt
carborane
anion (known as chlorinated cobalt dicarbollide). The actinides are extracted by CMPO, and the diluent
is a polar aromatic such as nitrobenzene
. Other dilents such as meta-nitrobenzotrifluoride
and phenyl trifluoromethyl sulfone
have been suggested as well.
and ion exchange
in ammonium
carbonate
has been reported.
The bismuth phosphate process
is an obsolete process that adds significant unnecessary material to the final radioactive waste. The bismuth phosphate process has been replaced by solvent extraction processes. The bismuth phosphate process was designed to extract plutonium
from aluminium
-clad nuclear fuel rods, containing uranium. The fuel was declad by boiling it in caustic soda. After decladding, the uranium metal was dissolved in nitric acid
.
The plutonium at this point is in the +4 oxidation state. It was then precipitated out of the solution by the addition of bismuth
nitrate and phosphoric acid
to form the bismuth phosphate. The plutonium was coprecipitated
with this. The supernatant liquid (containing many of the fission products) was separated from the solid. The precipitate was then dissolved in nitric acid before the addition of an oxidant such as potassium permanganate
which converted the plutonium to PuO22+ (Pu VI), then a dichromate salt was added to maintain the plutonium in the +6 oxidation state.
The bismuth phosphate was next re-precipitated leaving the plutonium in solution. Then an iron
(II) salt such as ferrous sulfate was added, and the plutonium re-precipitated again using a bismuth phosphate carrier precipitate. Then lanthanum
salts and fluoride
were added to create solid lanthanum fluoride which acted as a carrier for the plutonium. This was converted to the oxide by the action of an alkali
. The lanthanum plutonium oxide was next collected and extracted with nitric acid to form plutonium nitrate.
This is a liquid-liquid extraction process which uses methyl isobutyl ketone
as the extractant. The extraction is by a solvation mechanism. This process has the disadvantage of requiring the use of a salting-out reagent (aluminium
nitrate
) to increase the nitrate concentration in the aqueous phase to obtain a reasonable distribution ratio (D value). Also, hexone is degraded by concentrated nitric acid. This process has been replaced by the PUREX process.
Pu4+ + 4NO3- + 2S --> [Pu(NO3)4S2]
A process based on a solvation extraction process using the triether extractant named above. This process has the disadvantage of requiring the use of a salting-out reagent (aluminium
nitrate
) to increase the nitrate concentration in the aqueous phase to obtain a reasonable distribution ratio. This process was used at Windscale many years ago. This process has been replaced by PUREX.
is a generic term for high-temperature methods. Solvents are molten salt
s (e.g. LiCl+KCl or LiF+CaF2) and molten metals (e.g. cadmium, bismuth, magnesium) rather than water and organic compounds. Electrorefining, distillation
, and solvent-solvent extraction are common steps.
These processes are not currently in significant use worldwide, but they have been researched and developed at Argonne National Laboratory
and elsewhere.
Advantages
Disadvantages
These processes were developed by Argonne National Laboratory
and used in the Integral Fast Reactor
project.
PYRO-A is a means of separating actinides (elements within the actinide
family, generally heavier than U-235) from non-actinides. The spent fuel is placed in an anode
basket
which is immersed in a molten salt electrolyte. An electrical current is applied, causing the uranium metal (or sometimes oxide, depending on the spent fuel) to plate out on a solid metal cathode while the other actinides (and the rare earths) can be absorbed into a liquid cadmium
cathode. Many of the fission products (such as caesium
, zirconium
and strontium
) remain in the salt. As alternatives to the molten cadmium electrode it is possible to use a molten bismuth
cathode, or a solid aluminium cathode.
As an alternative to electrowinning, the wanted metal
can be isolated by using a molten
alloy
of an electropositive metal and a less reactive metal.
Since the majority of the long term radioactivity, and volume, of spent fuel comes from actinides, removing the actinides produces waste that is more compact, and not nearly as dangerous over the long term. The radioactivity of this waste will then drop to the level of various naturally occurring minerals and ores within a few hundred, rather than thousands of, years.
The mixed actinides produced by pyrometallic processing can be used again as nuclear fuel, as they are virtually all either fissile
, or fertile
, though many of these materials would require a fast breeder reactor in order to be burned efficiently. In a thermal neutron spectrum, the concentrations of several heavy actinides (curium-242
and plutonium-240
) can become quite high, creating fuel that is substantially different from the usual uranium or mixed uranium-plutonium oxides (MOX) that most current reactors were designed to use.
Another pyrochemical process, the PYRO-B process, has been developed for the processing and recycling of fuel from a transmuter reactor ( a fast breeder reactor designed to convert transuranic nuclear waste into fission products ). A typical transmuter fuel is free from uranium and contains recovered transuranics in an inert matrix such as metallic zirconium
. In the PYRO-B processing of such fuel, an electrorefining step is used to separate the residual transuranic elements from the fission products and recycle the transuranics to the reactor for fissioning. Newly-generated technetium and iodine are extracted for incorporation into transmutation targets, and the other fission products are sent to waste.
to uranium trioxide
with decomposition by heating back to triuranium octoxide. A major purpose is to capture tritium
as tritiated water vapor before further processing where it would be difficult to retain the tritium. Other volatile elements leave the fuel and must be recovered, especially iodine
, technetium
, and carbon-14
. Voloxidation also breaks up the fuel or increases its surface area to enhance penetration of reagents in following reprocessing steps.
which remains).
The estimated overall mass balance for 20,000 grams of processed fuel with 2,000 grams of cladding is:
Tritium is not mentioned in this paper.
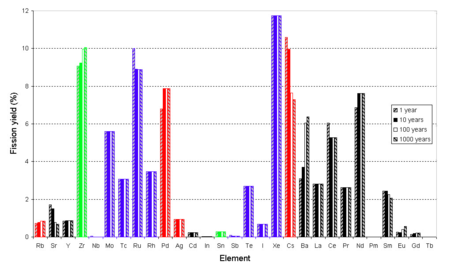 In the fluoride volatility process, fluorine
In the fluoride volatility process, fluorine
is reacted with the fuel. Fluorine is so much more reactive than even oxygen
that small particles of ground oxide fuel will burst into flame when dropped into a chamber full of fluorine. This is known as flame fluorination; the heat produced helps the reaction proceed. Most of the uranium
, which makes up the bulk of the fuel, is converted to uranium hexafluoride
, the form of uranium used in uranium enrichment, which has a very low boiling point. Technetium
, the main long-lived fission product
, is also efficiently converted to its volatile hexafluoride. A few other elements also form similarly volatile hexafluorides, pentafluorides, or heptafluorides. The volatile fluorides can be separated from excess fluorine by condensation, then separated from each other by fractional distillation
or selective reduction
. Uranium hexafluoride
and technetium hexafluoride have very similar boiling points and vapor pressures, which makes complete separation more difficult.
Many of the fission product
s volatilized are the same ones volatilized in non-fluorinated, higher-temperature volatilization, such as iodine
, tellurium and molybdenum
; notable differences are that technetium
is volatilized, but caesium
is not.
Some transuranium elements such as plutonium
, neptunium
and americium
can form volatile fluorides, but these compounds are not stable when the fluorine partial pressure is decreased. Most of the plutonium and some of the uranium will initially remain in ash which drops to the bottom of the flame fluorinator. The plutonium-uranium ratio in the ash may even approximate the composition needed for fast neutron reactor
fuel. Further fluorination of the ash can remove all the uranium, neptunium
, and plutonium as volatile fluorides; however, some other minor actinides
may not form volatile fluorides and instead remain with the alkaline fission products. Some noble metals may not form fluorides at all, but remain in metallic form; however ruthenium
hexafluoride is relatively stable and volatile.
Distillation of the residue at higher temperatures can separate lower-boiling transition metal
fluorides and alkali metal
(Cs, Rb) fluorides from higher-boiling lanthanide
and alkaline earth metal
(Sr, Ba) and yttrium
fluorides. The temperatures involved are much higher, but can be lowered somewhat by distilling in a vacuum. If a carrier salt like lithium fluoride
or sodium fluoride
is being used as a solvent, high-temperature distillation is a way to separate the carrier salt for reuse.
Molten salt reactor
designs carry out fluoride volatility reprocessing continuously or at frequent intervals. The goal is to return actinide
s to the molten fuel mixture for eventual fission, while removing fission product
s that are neutron poisons, or that can be more securely stored outside the reactor core while awaiting eventual transfer to permanent storage.
fluorides will also form volatile high-valence chlorides. Chlorination and distillation is another possible method for separation. The sequence of separation may differ usefully from the sequence for fluorides; for example, zirconium tetrachloride and tin tetrachloride have relatively low boiling points of 331°C and 114.1°C. Chlorination has even been proposed as a method for removing zirconium fuel cladding, instead of mechanical decladding.
Chlorides are likely to be easier than fluorides to later convert back to other compounds, such as oxides.
Chlorides remaining after volatilization may also be separated by solubility in water. Chlorides of alkaline elements like americium
, curium
, lanthanides, strontium
, caesium
are more soluble than those of uranium
, neptunium
, plutonium
, and zirconium
.
of reprocessing-waste disposal and interim storage-direct disposal has been the focus of much debate over the past ten years. Studies
have modeled the total fuel cycle costs of a reprocessing-recycling system based on one-time recycling of plutonium in existing thermal reactor
s (as opposed to the proposed breeder reactor
cycle) and compare this to the total costs of an open fuel cycle with direct disposal. The range of results produced by these studies is very wide, but all are agreed that under current (2005) economic conditions the reprocessing-recycle option is the more costly.
If reprocessing is undertaken only to reduce the radioactivity level of spent fuel it should be taken into account that spent nuclear fuel becomes less radioactive over time. After 40 years its radioactivity drops by 99.9%, though it still takes over a thousand years for the level of radioactivity to approach that of natural uranium. However the level of transuranic elements,
including plutonium-239
, remains high for over 100,000 years, so if not reused as nuclear fuel, then those elements need secure disposal because of nuclear proliferation
reasons as well as radiation hazard.
On 25 October 2011 a commission of the Japanese Atomic Energy Commission revealed during a meeting calculations about the costs of recycling nuclear fuel for power generation. These costs could be twice the costs of direct geological disposal of spent fuel: the cost of extracting plutonium and handling spent fuel was estimated at 1.98 to 2.14 yen per kilowatt-hour of electricity generated. Discarding the spent fuel as waste would cost only 1 to 1.35 yen per kilowatt-hour.
Nuclear power
Nuclear power is the use of sustained nuclear fission to generate heat and electricity. Nuclear power plants provide about 6% of the world's energy and 13–14% of the world's electricity, with the U.S., France, and Japan together accounting for about 50% of nuclear generated electricity...
, the reprocessed plutonium was recycled back into MOX nuclear fuel for thermal reactor
Thermal reactor
A thermal reactor is a nuclear reactor that uses slow or thermal neutrons. Most power reactors are of this type. These type of reactors use a neutron moderator to slow neutrons until they approach the average kinetic energy of the surrounding particles, that is, to reduce the speed of the neutrons...
s. The reprocessed uranium
Reprocessed uranium
Reprocessed uranium is the uranium recovered from nuclear reprocessing, as done commercially in France, the UK and Japan and by nuclear weapons states' military plutonium production programs. This uranium actually makes up the bulk of the material separated during reprocessing...
, which constitutes the bulk of the spent fuel material, can in principle also be re-used as fuel, but that is only economic when uranium prices are high. Finally, the breeder reactor
Breeder reactor
A breeder reactor is a nuclear reactor capable of generating more fissile material than it consumes because its neutron economy is high enough to breed fissile from fertile material like uranium-238 or thorium-232. Breeders were at first considered superior because of their superior fuel economy...
can employ not only the recycled plutonium and uranium in spent fuel, but all the actinide
Actinide
The actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium.The actinide series derives its name from the group 3 element actinium...
s, closing the nuclear fuel cycle
Nuclear fuel cycle
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the front end, which are the preparation of the fuel, steps in the service period in which the fuel is used during reactor operation, and steps in...
and potentially multiplying the energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
extracted from natural uranium
Natural uranium
Natural uranium refers to refined uranium with the same isotopic ratio as found in nature. It contains 0.7 % uranium-235, 99.3 % uranium-238, and a trace of uranium-234 by weight. In terms of the amount of radioactivity, approximately 2.2 % comes from uranium-235, 48.6 % uranium-238, and 49.2 %...
by more than 60 times.
Nuclear reprocessing reduces the volume of high-level waste, but by itself does not reduce radioactivity or heat generation and therefore does not eliminate the need for a geological waste repository. Reprocessing has been politically controversial because of the potential to contribute to nuclear proliferation
Nuclear proliferation
Nuclear proliferation is a term now used to describe the spread of nuclear weapons, fissile material, and weapons-applicable nuclear technology and information, to nations which are not recognized as "Nuclear Weapon States" by the Treaty on the Nonproliferation of Nuclear Weapons, also known as the...
, the potential vulnerability to nuclear terrorism
Nuclear terrorism
Nuclear terrorism denotes the use, or threat of the use, of nuclear weapons or radiological weapons in acts of terrorism, includingattacks against facilities where radioactive materials are present...
, the political challenges of repository siting (a problem that applies equally to direct disposal of spent fuel), and because of its high cost compared to the once-through fuel cycle. The Obama administration stepped back from President Bush's plans for commercial-scale reprocessing and reverted to a program focused on reprocessing-related scientific research.
Separated components and disposition
The potentially useful components dealt with in nuclear reprocessing comprise specific actinides (plutonium, uranium, and some minor actinidesMinor actinides
The minor actinides are the actinide elements in used nuclear fuel other than uranium and plutonium, which are termed the major actinides. The minor actinides include neptunium, americium, curium, berkelium, californium, einsteinium, and fermium...
). The lighter elements
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
components include fission products, activation products, and cladding.
| material | disposition |
|---|---|
| plutonium, minor actinides, reprocessed uranium Reprocessed uranium Reprocessed uranium is the uranium recovered from nuclear reprocessing, as done commercially in France, the UK and Japan and by nuclear weapons states' military plutonium production programs. This uranium actually makes up the bulk of the material separated during reprocessing... |
fission Nuclear fission In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy... in fast, fusion, or subcritical reactor Subcritical reactor A subcritical reactor is a nuclear fission reactor that produces fission without achieving criticality. Instead of a sustaining chain reaction, a subcritical reactor uses additional neutrons from an outside source... |
| reprocessed uranium, cladding, filters | less stringent storage as intermediate-level waste |
| long-lived fission and activation product Activation product Activation products are materials made radioactive by neutron activation.Fission products and actinides produced by neutron absorption of nuclear fuel itself are normally referred to by those specific names, and activation product reserved for products of neutron capture by other materials, such as... s |
nuclear transmutation Nuclear transmutation Nuclear transmutation is the conversion of one chemical element or isotope into another. In other words, atoms of one element can be changed into atoms of other element by 'transmutation'... or geological repository |
| medium-lived fission products 137Cs and 90Sr Strontium-90 Strontium-90 is a radioactive isotope of strontium, with a half-life of 28.8 years.-Radioactivity:Natural strontium is nonradioactive and nontoxic, but 90Sr is a radioactivity hazard... |
medium-term storage as high-level waste |
| useful radionuclides and noble metal Noble metal Noble metals are metals that are resistant to corrosion and oxidation in moist air, unlike most base metals. They tend to be precious, often due to their rarity in the Earth's crust... s |
industrial and medical uses |
History
The first large-scale nuclear reactors were built during World War IIWorld War II
World War II, or the Second World War , was a global conflict lasting from 1939 to 1945, involving most of the world's nations—including all of the great powers—eventually forming two opposing military alliances: the Allies and the Axis...
. These reactors were designed for the production of plutonium for use in nuclear weapon
Nuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission or a combination of fission and fusion. Both reactions release vast quantities of energy from relatively small amounts of matter. The first fission bomb test released the same amount...
s. The only reprocessing required, therefore, was the extraction of the plutonium
Plutonium
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
(free of fission-product
Fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus fissions. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons and a large release of energy in the form of heat , gamma rays and neutrinos. The...
contamination) from the spent natural uranium
Natural uranium
Natural uranium refers to refined uranium with the same isotopic ratio as found in nature. It contains 0.7 % uranium-235, 99.3 % uranium-238, and a trace of uranium-234 by weight. In terms of the amount of radioactivity, approximately 2.2 % comes from uranium-235, 48.6 % uranium-238, and 49.2 %...
fuel. In 1943, several methods were proposed for separating the relatively small quantity of plutonium from the uranium and fission products. The first method selected, a precipitation process called the Bismuth
Bismuth
Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead...
Phosphate
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
process, was developed and tested at the Oak Ridge National Laboratory
Oak Ridge National Laboratory
Oak Ridge National Laboratory is a multiprogram science and technology national laboratory managed for the United States Department of Energy by UT-Battelle. ORNL is the DOE's largest science and energy laboratory. ORNL is located in Oak Ridge, Tennessee, near Knoxville...
(ORNL) in the 1943-1945 period to produce quantities of plutonium for evaluation and use in weapons programs
History of nuclear weapons
The history of nuclear weapons chronicles the development of nuclear weapons. Nuclear weapons possess enormous destructive potential derived from nuclear fission or nuclear fusion reactions...
. ORNL produced the first macroscopic quantities (grams) of separated plutonium with these processes.
The Bismuth Phosphate process was first operated on a large scale at the Hanford Site
Hanford Site
The Hanford Site is a mostly decommissioned nuclear production complex on the Columbia River in the U.S. state of Washington, operated by the United States federal government. The site has been known by many names, including Hanford Works, Hanford Engineer Works or HEW, Hanford Nuclear Reservation...
, in the latter part of 1944. It was successful for plutonium separation in the emergency situation existing then, but it had a significant weakness: the inability to recover uranium.
The first successful solvent extraction process for the recovery of pure uranium and plutonium was developed at ORNL in 1949. The PUREX
PUREX
PUREX is an acronym standing for Plutonium - URanium EXtraction — de facto standard aqueous nuclear reprocessing method for the recovery of uranium and plutonium from used nuclear fuel. It is based on liquid-liquid extraction ion-exchange.The PUREX process was invented by Herbert H. Anderson and...
process is the current method of extraction. Separation plants were also constructed at Savannah River Site
Savannah River Site
The Savannah River Site is a nuclear reservation in the United States in the state of South Carolina, located on land in Aiken, Allendale and Barnwell Counties adjacent to the Savannah River, southeast of Augusta, Georgia. The site was built during the 1950s to refine nuclear materials for...
and a smaller plant at West Valley, New York
West Valley, New York
West Valley is a hamlet located within the town of Ashford in Cattaraugus County, New York, United States. Located at the intersection of Cattaraugus County Route 53 and State Route 240, the hamlet is home to West Valley Central School and the West Valley Demonstration Project, a nuclear cleanup...
which closed by 1972 because of its inability to meet new regulatory requirements.
Reprocessing of civilian fuel has long been employed in Europe, at the COGEMA La Hague site
COGEMA La Hague site
The AREVA NC La Hague site is a nuclear fuel reprocessing plant of AREVA in La Hague on the French Cotentin Peninsula that currently has nearly half of the world's light water reactor spent nuclear fuel reprocessing capacity. It has been in operation since 1976, and has a capacity of about 1700...
in France, the Sellafield
Sellafield
Sellafield is a nuclear reprocessing site, close to the village of Seascale on the coast of the Irish Sea in Cumbria, England. The site is served by Sellafield railway station. Sellafield is an off-shoot from the original nuclear reactor site at Windscale which is currently undergoing...
site in the United Kingdom, the Mayak
Mayak
Mayak Production Association refers to an industrial complex that is one of the biggest nuclear facilities in the Russian Federation. It housed plutonium production reactors and a reprocessing plant...
Chemical Combine in Russia, and at sites such as the Tokai plant in Japan, the Tarapur plant in India, and briefly at the West Valley Reprocessing Plant
West Valley Reprocessing Plant
West Valley Reprocessing Plant was a formerly operational plant for the reprocessing of used nuclear fuel at West Valley, New York, USA. It was operated from 1966-72. During this time period, 600,000 gallons of highly radioactive waste accumulated in an underground waste tank...
in the United States.
In October 1976, fear of nuclear weapons proliferation (especially after India
India
India , officially the Republic of India , is a country in South Asia. It is the seventh-largest country by geographical area, the second-most populous country with over 1.2 billion people, and the most populous democracy in the world...
demonstrated nuclear weapons capabilities using reprocessing technology) led President Gerald Ford
Gerald Ford
Gerald Rudolph "Jerry" Ford, Jr. was the 38th President of the United States, serving from 1974 to 1977, and the 40th Vice President of the United States serving from 1973 to 1974...
to issue a Presidential directive
Presidential directive
Presidential Directives, better known as Presidential Decision Directives or PDD are a form of an executive order issued by the President of the United States with the advice and consent of the National Security Council...
to indefinitely suspend the commercial reprocessing and recycling of plutonium in the U.S. On April 7, 1977 , President Jimmy Carter
Jimmy Carter
James Earl "Jimmy" Carter, Jr. is an American politician who served as the 39th President of the United States and was the recipient of the 2002 Nobel Peace Prize, the only U.S. President to have received the Prize after leaving office...
banned the reprocessing of commercial reactor spent nuclear fuel
Spent nuclear fuel
Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor...
. The key issue driving this policy was the serious threat of nuclear weapons proliferation
Nuclear proliferation
Nuclear proliferation is a term now used to describe the spread of nuclear weapons, fissile material, and weapons-applicable nuclear technology and information, to nations which are not recognized as "Nuclear Weapon States" by the Treaty on the Nonproliferation of Nuclear Weapons, also known as the...
by diversion of plutonium from the civilian fuel cycle, and to encourage other nations to follow the USA lead.
. After that, only countries that already had large investments in reprocessing infrastructure continued to reprocess spent nuclear fuel. President Reagan lifted the ban in 1981, but did not provide the substantial subsidy that would have been necessary to start up commercial reprocessing.
In March 1999, the U.S. Department of Energy (DOE) reversed its own policy and signed a contract with a consortium
Consortium
A consortium is an association of two or more individuals, companies, organizations or governments with the objective of participating in a common activity or pooling their resources for achieving a common goal....
of Duke Energy
Duke Energy
Duke Energy , headquartered in Charlotte, North Carolina, is an energy company with assets in the United States, Canada and Latin America.-Overview:...
, COGEMA
Areva NC
Areva NC, formerly Cogema is a French company, created in 1976 from the production division of the French government's CEA It is an industrial group active in all stages of the uranium fuel cycle, including uranium mining, conversion, enrichment, spent fuel reprocessing, and recycling...
, and Stone & Webster
Stone & Webster
Stone & Webster is an American engineering services company based in Stoughton, Massachusetts. Stone & Webster was founded as an electrical testing lab and consulting firm by electrical engineers Charles Stone and Edwin Webster in 1889. It was acquired by The Shaw Group in 2000. The company...
(DCS) to design and operate a Mixed Oxide (MOX) fuel
MOX fuel
Mixed oxide fuel, commonly referred to as MOX fuel, is nuclear fuel that contains more than one oxide of fissile material. MOX fuel contains plutonium blended with natural uranium, reprocessed uranium, or depleted uranium. MOX fuel is an alternative to the low-enriched uranium fuel used in the...
fabrication facility. Site preparation at the Savannah River Site (South Carolina) began in October 2005.
PUREX
PUREX, the current standard method, is an acronym standing for Plutonium and Uranium Recovery by EXtraction. The PUREX process is a liquid-liquid extractionLiquid-liquid extraction
Liquid–liquid extraction, also known as solvent extraction and partitioning, is a method to separate compounds based on their relative solubilities in two different immiscible liquids, usually water and an organic solvent. It is an extraction of a substance from one liquid phase into another liquid...
method used to reprocess spent nuclear fuel
Nuclear fuel
Nuclear fuel is a material that can be 'consumed' by fission or fusion to derive nuclear energy. Nuclear fuels are the most dense sources of energy available...
, in order to extract uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
and plutonium
Plutonium
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
, independent of each other, from the fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
products. This is the most developed and widely used process in the industry at present.
When used on fuel from commercial power reactors the plutonium extracted typically contains too much Pu-240 to be useful in a nuclear weapon. However, reactors that are capable of refuelling frequently can be used to produce weapon-grade
Nuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission or a combination of fission and fusion. Both reactions release vast quantities of energy from relatively small amounts of matter. The first fission bomb test released the same amount...
plutonium, which can later be recovered using PUREX. Because of this, PUREX chemicals are monitored.
UREX
The PUREX process can be modified to make a UREX (URanium EXtraction) process which could be used to save space inside high level nuclear waste disposal sites, such as the Yucca Mountain nuclear waste repository, by removing the uranium which makes up the vast majority of the mass and volume of used fuel and recycling it as reprocessed uranium
Reprocessed uranium
Reprocessed uranium is the uranium recovered from nuclear reprocessing, as done commercially in France, the UK and Japan and by nuclear weapons states' military plutonium production programs. This uranium actually makes up the bulk of the material separated during reprocessing...
.
The UREX process is a PUREX process which has been modified to prevent the plutonium from being extracted. This can be done by adding a plutonium reductant before the first metal extraction step. In the UREX process, ~99.9% of the uranium and >95% of technetium
Technetium
Technetium is the chemical element with atomic number 43 and symbol Tc. It is the lowest atomic number element without any stable isotopes; every form of it is radioactive. Nearly all technetium is produced synthetically and only minute amounts are found in nature...
are separated from each other and the other fission products and actinide
Actinide
The actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium.The actinide series derives its name from the group 3 element actinium...
s. The key is the addition of acetohydroxamic acid
Acetohydroxamic acid
Acetohydroxamic acid is a drug that is a potent and irreversible inhibitor of bacterial and plant urease usually used for urinary tract infections. The molecule is similar to urea but is not hydrolyzable by the urease enzyme ....
(AHA) to the extraction and scrub sections of the process. The addition of AHA greatly diminishes the extractability of plutonium and neptunium
Neptunium
Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive metal, neptunium is the first transuranic element and belongs to the actinide series. Its most stable isotope, 237Np, is a by-product of nuclear reactors and plutonium production and it can be used as a...
, providing greater proliferation resistance than with the plutonium extraction stage of the PUREX process.
TRUEX
Adding a second extraction agent, octyl(phenyl)-N, N-dibutyl carbamoylmethyl phosphine oxide(CMPO) in combination with tributylphosphate, (TBP), the PUREX process can be turned into the TRUEX (TRansUranic EXtraction) process. TRUEX was invented in the USA by Argonne National Laboratory and is designed to remove the transuranic metals (Am/Cm) from waste. The idea is that by lowering the alpha activity of the waste, the majority of the waste can then be disposed of with greater ease. In common with PUREX this process operates by a solvation
Solvation
Solvation, also sometimes called dissolution, is the process of attraction and association of molecules of a solvent with molecules or ions of a solute...
mechanism.
DIAMEX
As an alternative to TRUEX, an extraction process using a malondiamide has been devised. The DIAMEX (DIAMideEXtraction) process has the advantage of avoiding the formation of organic waste which contains elements other than carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
, hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
, and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
. Such an organic waste can be burned without the formation of acidic gases which could contribute to acid rain
Acid rain
Acid rain is a rain or any other form of precipitation that is unusually acidic, meaning that it possesses elevated levels of hydrogen ions . It can have harmful effects on plants, aquatic animals, and infrastructure. Acid rain is caused by emissions of carbon dioxide, sulfur dioxide and nitrogen...
. The DIAMEX process is being worked on in Europe
Europe
Europe is, by convention, one of the world's seven continents. Comprising the westernmost peninsula of Eurasia, Europe is generally 'divided' from Asia to its east by the watershed divides of the Ural and Caucasus Mountains, the Ural River, the Caspian and Black Seas, and the waterways connecting...
by the French CEA
Commissariat à l'Énergie Atomique
The Commissariat à l'énergie atomique et aux énergies alternatives or CEA, is a French “public establishment related to industrial and commercial activities” whose mission is to develop all applications of nuclear power, both civilian and military...
. The process is sufficiently mature that an industrial plant could be constructed with the existing knowledge of the process. In common with PUREX this process operates by a solvation mechanism.
SANEX
Selective ActiNide EXtraction. As part of the management of minor actinides it has been proposed that the lanthanides and trivalent minor actinides should be removed from the PUREX raffinate
Raffinate
Raffinating something is a technique used in metallurgy to remove impurities from liquid material. There are many different kinds of raffination, for example you can use vacuum to extract hydrogen from metals....
by a process such as DIAMEX or TRUEX. In order to allow the actinides such as americium to be either reused in industrial sources or used as fuel, the lanthanides must be removed. The lanthanides have large neutron cross sections and hence they would poison a neutron driven nuclear reaction. To date the extraction system for the SANEX process has not been defined, but currently several different research groups are working towards a process. For instance the French CEA
Commissariat à l'Énergie Atomique
The Commissariat à l'énergie atomique et aux énergies alternatives or CEA, is a French “public establishment related to industrial and commercial activities” whose mission is to develop all applications of nuclear power, both civilian and military...
is working on a bis-triazinyl pyridine (BTP) based process.
Other systems such as the dithiophosphinic acids are being worked on by some other workers.
UNEX
The UNiversal EXtraction process was developed in Russia
Russia
Russia or , officially known as both Russia and the Russian Federation , is a country in northern Eurasia. It is a federal semi-presidential republic, comprising 83 federal subjects...
and the Czech Republic
Czech Republic
The Czech Republic is a landlocked country in Central Europe. The country is bordered by Poland to the northeast, Slovakia to the east, Austria to the south, and Germany to the west and northwest....
; it is designed to completely remove the most troublesome radioisotopes (Sr, Cs and minor actinides
Minor actinides
The minor actinides are the actinide elements in used nuclear fuel other than uranium and plutonium, which are termed the major actinides. The minor actinides include neptunium, americium, curium, berkelium, californium, einsteinium, and fermium...
) from the raffinate remaining after the extraction of uranium and plutonium from used nuclear fuel
Nuclear fuel
Nuclear fuel is a material that can be 'consumed' by fission or fusion to derive nuclear energy. Nuclear fuels are the most dense sources of energy available...
. The chemistry is based upon the interaction of caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
and strontium
Strontium
Strontium is a chemical element with the symbol Sr and the atomic number 38. An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when exposed to air. It occurs naturally in the minerals celestine and...
with polyethylene glycol
Polyethylene glycol
Polyethylene glycol is a polyether compound with many applications from industrial manufacturing to medicine. It has also been known as polyethylene oxide or polyoxyethylene , depending on its molecular weight, and under the tradename Carbowax.-Available forms:PEG, PEO, or POE refers to an...
) and a cobalt
Cobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
carborane
Carborane
A carborane is a cluster composed of boron and carbon atoms. Like many of the related boranes, these clusters are polyhedra and are similarly classified as closo-, nido-, arachno-, hypho-, etc...
anion (known as chlorinated cobalt dicarbollide). The actinides are extracted by CMPO, and the diluent
Diluent
A diluent is a diluting agent.Certain fluids are too viscous to be pumped easily or too dense to flow from one particular point to the other. This can be problematic, because it might not be economically feasible to transport such fluids in this state.To ease this restricted movement, diluents...
is a polar aromatic such as nitrobenzene
Nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale as a precursor to aniline. Although occasionally used as a flavoring or perfume...
. Other dilents such as meta-nitrobenzotrifluoride
Fluoride
Fluoride is the anion F−, the reduced form of fluorine when as an ion and when bonded to another element. Both organofluorine compounds and inorganic fluorine containing compounds are called fluorides. Fluoride, like other halides, is a monovalent ion . Its compounds often have properties that are...
and phenyl trifluoromethyl sulfone
Sulfone
A sulfone is a chemical compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.-IUPAC name and...
have been suggested as well.
Electrochemical methods
An exotic method using electrochemistryElectrochemistry
Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
and ion exchange
Ion exchange
Ion exchange is an exchange of ions between two electrolytes or between an electrolyte solution and a complex. In most cases the term is used to denote the processes of purification, separation, and decontamination of aqueous and other ion-containing solutions with solid polymeric or mineralic 'ion...
in ammonium
Ammonium
The ammonium cation is a positively charged polyatomic cation with the chemical formula NH. It is formed by the protonation of ammonia...
carbonate
Carbonate
In chemistry, a carbonate is a salt of carbonic acid, characterized by the presence of the carbonate ion, . The name may also mean an ester of carbonic acid, an organic compound containing the carbonate group C2....
has been reported.
Bismuth phosphate
The bismuth phosphate process
Bismuth phosphate process
Bismuth-phosphate process was a process used to extract plutonium from used nuclear fuel taken from nuclear reactors. This process was used to produce all the plutonium of the atomic bomb dropped on Nagasaki in 1945. In 1952 this process was replaced by the Redox and PUREX processes.-References:*...
is an obsolete process that adds significant unnecessary material to the final radioactive waste. The bismuth phosphate process has been replaced by solvent extraction processes. The bismuth phosphate process was designed to extract plutonium
Plutonium
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
from aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
-clad nuclear fuel rods, containing uranium. The fuel was declad by boiling it in caustic soda. After decladding, the uranium metal was dissolved in nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
.
The plutonium at this point is in the +4 oxidation state. It was then precipitated out of the solution by the addition of bismuth
Bismuth
Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead...
nitrate and phosphoric acid
Phosphoric acid
Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way...
to form the bismuth phosphate. The plutonium was coprecipitated
Coprecipitation
In chemistry, coprecipitation or co-precipitation is the carrying down by a precipitate of substances normally soluble under the conditions employed...
with this. The supernatant liquid (containing many of the fission products) was separated from the solid. The precipitate was then dissolved in nitric acid before the addition of an oxidant such as potassium permanganate
Potassium permanganate
Potassium permanganate is an inorganic chemical compound with the formula KMnO4. It is a salt consisting of K+ and MnO4− ions. Formerly known as permanganate of potash or Condy's crystals, it is a strong oxidizing agent. It dissolves in water to give intensely purple solutions, the...
which converted the plutonium to PuO22+ (Pu VI), then a dichromate salt was added to maintain the plutonium in the +6 oxidation state.
The bismuth phosphate was next re-precipitated leaving the plutonium in solution. Then an iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
(II) salt such as ferrous sulfate was added, and the plutonium re-precipitated again using a bismuth phosphate carrier precipitate. Then lanthanum
Lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57.Lanthanum is a silvery white metallic element that belongs to group 3 of the periodic table and is the first element of the lanthanide series. It is found in some rare-earth minerals, usually in combination with cerium and...
salts and fluoride
Fluoride
Fluoride is the anion F−, the reduced form of fluorine when as an ion and when bonded to another element. Both organofluorine compounds and inorganic fluorine containing compounds are called fluorides. Fluoride, like other halides, is a monovalent ion . Its compounds often have properties that are...
were added to create solid lanthanum fluoride which acted as a carrier for the plutonium. This was converted to the oxide by the action of an alkali
Alkali
In chemistry, an alkali is a basic, ionic salt of an alkali metal or alkaline earth metal element. Some authors also define an alkali as a base that dissolves in water. A solution of a soluble base has a pH greater than 7. The adjective alkaline is commonly used in English as a synonym for base,...
. The lanthanum plutonium oxide was next collected and extracted with nitric acid to form plutonium nitrate.
Hexone or Redox
This is a liquid-liquid extraction process which uses methyl isobutyl ketone
Methyl isobutyl ketone
Methyl isobutyl ketone is the organic compound with the formula 2CHCH2CCH3. This colourless liquid, a ketone, is widely used as a solvent.-Production:...
as the extractant. The extraction is by a solvation mechanism. This process has the disadvantage of requiring the use of a salting-out reagent (aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
nitrate
Nitrate
The nitrate ion is a polyatomic ion with the molecular formula NO and a molecular mass of 62.0049 g/mol. It is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically-bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a...
) to increase the nitrate concentration in the aqueous phase to obtain a reasonable distribution ratio (D value). Also, hexone is degraded by concentrated nitric acid. This process has been replaced by the PUREX process.
Pu4+ + 4NO3- + 2S --> [Pu(NO3)4S2]
Butex, β,β'-dibutyoxydiethyl ether
A process based on a solvation extraction process using the triether extractant named above. This process has the disadvantage of requiring the use of a salting-out reagent (aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
nitrate
Nitrate
The nitrate ion is a polyatomic ion with the molecular formula NO and a molecular mass of 62.0049 g/mol. It is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically-bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a...
) to increase the nitrate concentration in the aqueous phase to obtain a reasonable distribution ratio. This process was used at Windscale many years ago. This process has been replaced by PUREX.
Pyroprocessing
PyroprocessingPyroprocessing
Pyroprocessing is a process in which materials are subjected to high temperatures in order to bring about a chemical or physical change. Pyroprocessing includes such terms as ore-roasting, calcination and sintering...
is a generic term for high-temperature methods. Solvents are molten salt
Molten salt
Molten salt refers to a salt that is in the liquid phase that is normally a solid at standard temperature and pressure . A salt which is normally liquid at STP is usually called a room temperature ionic liquid, although technically molten salts are a class of ionic liquids.-Uses:Molten salts have...
s (e.g. LiCl+KCl or LiF+CaF2) and molten metals (e.g. cadmium, bismuth, magnesium) rather than water and organic compounds. Electrorefining, distillation
Distillation
Distillation is a method of separating mixtures based on differences in volatilities of components in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process, and not a chemical reaction....
, and solvent-solvent extraction are common steps.
These processes are not currently in significant use worldwide, but they have been researched and developed at Argonne National Laboratory
Argonne National Laboratory
Argonne National Laboratory is the first science and engineering research national laboratory in the United States, receiving this designation on July 1, 1946. It is the largest national laboratory by size and scope in the Midwest...
and elsewhere.
Advantages
- The principles behind them are well understood, and no significant technical barriers exist to their adoption.
- Readily applied to high-burnupBurnupIn nuclear power technology, burnup is a measure of how much energy is extracted from a primary nuclear fuel source...
spent fuel and requires little cooling time, since the operating temperatureOperating temperatureAn operating temperature is the temperature at which an electrical or mechanical device operates. The device will operate effectively within a specified temperature range which varies based on the device function and application context, and ranges from the minimum operating temperature to the...
s are high already. - Does not use solvents containing hydrogen and carbon, which are neutron moderatorNeutron moderatorIn nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, thereby turning them into thermal neutrons capable of sustaining a nuclear chain reaction involving uranium-235....
s creating risk of criticality accidentCriticality accidentA criticality accident, sometimes referred to as an excursion or a power excursion, is an accidental increase of nuclear chain reactions in a fissile material, such as enriched uranium or plutonium...
s and can absorb the fission productFission productNuclear fission products are the atomic fragments left after a large atomic nucleus fissions. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons and a large release of energy in the form of heat , gamma rays and neutrinos. The...
tritiumTritiumTritium is a radioactive isotope of hydrogen. The nucleus of tritium contains one proton and two neutrons, whereas the nucleus of protium contains one proton and no neutrons...
and the activation productActivation productActivation products are materials made radioactive by neutron activation.Fission products and actinides produced by neutron absorption of nuclear fuel itself are normally referred to by those specific names, and activation product reserved for products of neutron capture by other materials, such as...
carbon-14Carbon-14Carbon-14, 14C, or radiocarbon, is a radioactive isotope of carbon with a nucleus containing 6 protons and 8 neutrons. Its presence in organic materials is the basis of the radiocarbon dating method pioneered by Willard Libby and colleagues , to date archaeological, geological, and hydrogeological...
in dilute solutions that cannot be separated later.- Alternatively, voloxidation can remove 99% of the tritium from used fuel and recover it in the form of a strong solution suitable for use as a supply of tritium.
- More compact than aqueous methods, allowing on-site reprocessing at the reactor site, which avoids transportation of spent fuel and its security issues, instead storing a much smaller volume of fission productFission productNuclear fission products are the atomic fragments left after a large atomic nucleus fissions. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons and a large release of energy in the form of heat , gamma rays and neutrinos. The...
s on site as high-level waste until decommissioningNuclear decommissioningNuclear decommissioning is the dismantling of a nuclear power plant and decontamination of the site to a state no longer requiring protection from radiation for the general public...
. For example, the Integral Fast ReactorIntegral Fast ReactorThe Integral Fast Reactor is a design for a nuclear reactor using fast neutrons and no neutron moderator . IFR is distinguished by a nuclear fuel cycle that uses reprocessing via electrorefining at the reactor site.The U.S...
and Molten Salt ReactorMolten salt reactorA molten salt reactor is a type of nuclear fission reactor in which the primary coolant, or even the fuel itself is a molten salt mixture...
fuel cycles are based on on-site pyroprocessing. - It can separate many or even all actinideActinideThe actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium.The actinide series derives its name from the group 3 element actinium...
s at once and produce highly radioactive fuel which is harder to manipulate for theft or making nuclear weapons. (However, the difficulty has been questioned.) In contrast the PUREX process was designed to separate plutonium only for weapons, and it also leaves the minor actinides (americiumAmericiumAmericium is a synthetic element that has the symbol Am and atomic number 95. This transuranic element of the actinide series is located in the periodic table below the lanthanide element europium, and thus by analogy was named after another continent, America.Americium was first produced in 1944...
and curiumCuriumCurium is a synthetic chemical element with the symbol Cm and atomic number 96. This radioactive transuranic element of the actinide series was named after Marie Skłodowska-Curie and her husband Pierre Curie. Curium was first intentionally produced and identified in summer 1944 by the group of...
) behind, producing waste with more long-lived radioactivity. - Most of the radioactivity in roughly 102 to 105 years after the use of the nuclear fuel is produced by the actinides, since there are no fission products with half-lives in this range. These actinides can fuel fast reactors, so extracting and reusing (fissioning) them reduces the long-term radioactivity of the wastes.
Disadvantages
- Reprocessing as a whole is not currently (2005) in favor, and places that do reprocess already have PUREX plants constructed. Consequently, there is little demand for new pyrometalurgical systems, although there could be if the Generation IV reactorGeneration IV reactorGeneration IV reactors are a set of theoretical nuclear reactor designs currently being researched. Most of these designs are generally not expected to be available for commercial construction before 2030...
programs become reality. - The used salt from pyroprocessing is less suitable for conversion into glass than the waste materials produced by the PUREX process.
- If the goal is to reduce the longevity of spent nuclear fuel in burner reactors, then better recovery rates of the minor actinides need to be achieved.
PYRO-A and -B for IFR
These processes were developed by Argonne National Laboratory
Argonne National Laboratory
Argonne National Laboratory is the first science and engineering research national laboratory in the United States, receiving this designation on July 1, 1946. It is the largest national laboratory by size and scope in the Midwest...
and used in the Integral Fast Reactor
Integral Fast Reactor
The Integral Fast Reactor is a design for a nuclear reactor using fast neutrons and no neutron moderator . IFR is distinguished by a nuclear fuel cycle that uses reprocessing via electrorefining at the reactor site.The U.S...
project.
PYRO-A is a means of separating actinides (elements within the actinide
Actinide
The actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium.The actinide series derives its name from the group 3 element actinium...
family, generally heavier than U-235) from non-actinides. The spent fuel is placed in an anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
basket
Basket
A basket is a container which is traditionally constructed from stiff fibres, which can be made from a range of materials, including wood splints, runners, and cane. While most baskets are made from plant materials, other materials such as horsehair, baleen, or metal wire can be used. Baskets are...
which is immersed in a molten salt electrolyte. An electrical current is applied, causing the uranium metal (or sometimes oxide, depending on the spent fuel) to plate out on a solid metal cathode while the other actinides (and the rare earths) can be absorbed into a liquid cadmium
Cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low...
cathode. Many of the fission products (such as caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
, zirconium
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
and strontium
Strontium
Strontium is a chemical element with the symbol Sr and the atomic number 38. An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when exposed to air. It occurs naturally in the minerals celestine and...
) remain in the salt. As alternatives to the molten cadmium electrode it is possible to use a molten bismuth
Bismuth
Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead...
cathode, or a solid aluminium cathode.
As an alternative to electrowinning, the wanted metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
can be isolated by using a molten
Mölten
Mölten is a comune in South Tyrol in the Italian region Trentino-Alto Adige/Südtirol, located about 60 km north of Trento and about 12 km northwest of Bolzano .-Geography:...
alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
of an electropositive metal and a less reactive metal.
Since the majority of the long term radioactivity, and volume, of spent fuel comes from actinides, removing the actinides produces waste that is more compact, and not nearly as dangerous over the long term. The radioactivity of this waste will then drop to the level of various naturally occurring minerals and ores within a few hundred, rather than thousands of, years.
The mixed actinides produced by pyrometallic processing can be used again as nuclear fuel, as they are virtually all either fissile
Fissile
In nuclear engineering, a fissile material is one that is capable of sustaining a chain reaction of nuclear fission. By definition, fissile materials can sustain a chain reaction with neutrons of any energy. The predominant neutron energy may be typified by either slow neutrons or fast neutrons...
, or fertile
Fertile material
Fertile material is a term used to describe nuclides which generally themselves do not undergo induced fission but from which fissile material is generated by neutron absorption and subsequent nuclei conversions...
, though many of these materials would require a fast breeder reactor in order to be burned efficiently. In a thermal neutron spectrum, the concentrations of several heavy actinides (curium-242
Curium
Curium is a synthetic chemical element with the symbol Cm and atomic number 96. This radioactive transuranic element of the actinide series was named after Marie Skłodowska-Curie and her husband Pierre Curie. Curium was first intentionally produced and identified in summer 1944 by the group of...
and plutonium-240
Plutonium-240
Plutonium-240 is an isotope of the metal plutonium formed when plutonium-239 captures a neutron. About 62% to 73% of the time when Pu-239 captures a neutron it undergoes fission; the rest of the time it forms Pu-240. The longer a nuclear fuel element remains in a nuclear reactor the greater the...
) can become quite high, creating fuel that is substantially different from the usual uranium or mixed uranium-plutonium oxides (MOX) that most current reactors were designed to use.
Another pyrochemical process, the PYRO-B process, has been developed for the processing and recycling of fuel from a transmuter reactor ( a fast breeder reactor designed to convert transuranic nuclear waste into fission products ). A typical transmuter fuel is free from uranium and contains recovered transuranics in an inert matrix such as metallic zirconium
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
. In the PYRO-B processing of such fuel, an electrorefining step is used to separate the residual transuranic elements from the fission products and recycle the transuranics to the reactor for fissioning. Newly-generated technetium and iodine are extracted for incorporation into transmutation targets, and the other fission products are sent to waste.
Voloxidation
Voloxidation (for volumetric oxidation) involves heating oxide fuel with oxygen, sometimes with alternating oxidation and reduction, or alternating oxidation by ozoneOzone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
to uranium trioxide
Uranium trioxide
Uranium trioxide , also called uranyl oxide, uranium oxide, and uranic oxide, is the hexavalent oxide of uranium. The solid may be obtained by heating uranyl nitrate to 400 °C. Its most commonly encountered polymorph, γ-UO3, is a yellow-orange powder.-Production and use:There are three methods...
with decomposition by heating back to triuranium octoxide. A major purpose is to capture tritium
Tritium
Tritium is a radioactive isotope of hydrogen. The nucleus of tritium contains one proton and two neutrons, whereas the nucleus of protium contains one proton and no neutrons...
as tritiated water vapor before further processing where it would be difficult to retain the tritium. Other volatile elements leave the fuel and must be recovered, especially iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
, technetium
Technetium
Technetium is the chemical element with atomic number 43 and symbol Tc. It is the lowest atomic number element without any stable isotopes; every form of it is radioactive. Nearly all technetium is produced synthetically and only minute amounts are found in nature...
, and carbon-14
Carbon-14
Carbon-14, 14C, or radiocarbon, is a radioactive isotope of carbon with a nucleus containing 6 protons and 8 neutrons. Its presence in organic materials is the basis of the radiocarbon dating method pioneered by Willard Libby and colleagues , to date archaeological, geological, and hydrogeological...
. Voloxidation also breaks up the fuel or increases its surface area to enhance penetration of reagents in following reprocessing steps.
Volatilization in isolation
Simply heating spent oxide fuel in an inert atmosphere or vacuum at a temperature between 700°C and 1000°C as a first reprocessing step can remove several volatile elements, including caesium whose isotope cesium-137 emits about half of the heat produced by the spent fuel over the following 100 years of cooling (however, most of the other half is from strontium-90Strontium-90
Strontium-90 is a radioactive isotope of strontium, with a half-life of 28.8 years.-Radioactivity:Natural strontium is nonradioactive and nontoxic, but 90Sr is a radioactivity hazard...
which remains).
The estimated overall mass balance for 20,000 grams of processed fuel with 2,000 grams of cladding is:
| Input | Residue | Zeolite Zeolite Zeolites are microporous, aluminosilicate minerals commonly used as commercial adsorbents. The term zeolite was originally coined in 1756 by Swedish mineralogist Axel Fredrik Cronstedt, who observed that upon rapidly heating the material stilbite, it produced large amounts of steam from water that... filter | Carbon filter | Particle filters |
|
|---|---|---|---|---|---|
| Palladium Palladium Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired... |
28 | 14 | 14 | ||
| Tellurium | 10 | 5 | 5 | ||
| Molybdenum Molybdenum Molybdenum , is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek , meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores... |
70 | 70 | |||
| Caesium Caesium Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature... |
46 | 46 | |||
| Rubidium Rubidium Rubidium is a chemical element with the symbol Rb and atomic number 37. Rubidium is a soft, silvery-white metallic element of the alkali metal group. Its atomic mass is 85.4678. Elemental rubidium is highly reactive, with properties similar to those of other elements in group 1, such as very rapid... |
8 | 8 | |||
| Silver Silver Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal... |
2 | 2 | |||
| Iodine Iodine Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor.... |
4 | 4 | |||
| Cladding | 2000 | 2000 | |||
| Uranium Uranium Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons... |
19218 | 19218 | ? | ||
| Others | 614 | 614 | ? | ||
| Total | 22000 | 21851 | 145 | 4 | 0 |
Tritium is not mentioned in this paper.
Fluoride volatility

Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
is reacted with the fuel. Fluorine is so much more reactive than even oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
that small particles of ground oxide fuel will burst into flame when dropped into a chamber full of fluorine. This is known as flame fluorination; the heat produced helps the reaction proceed. Most of the uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
, which makes up the bulk of the fuel, is converted to uranium hexafluoride
Uranium hexafluoride
Uranium hexafluoride , referred to as "hex" in the nuclear industry, is a compound used in the uranium enrichment process that produces fuel for nuclear reactors and nuclear weapons. It forms solid grey crystals at standard temperature and pressure , is highly toxic, reacts violently with water...
, the form of uranium used in uranium enrichment, which has a very low boiling point. Technetium
Technetium
Technetium is the chemical element with atomic number 43 and symbol Tc. It is the lowest atomic number element without any stable isotopes; every form of it is radioactive. Nearly all technetium is produced synthetically and only minute amounts are found in nature...
, the main long-lived fission product
Long-lived fission product
Long-lived fission products are radioactive materials with a long half-life produced by nuclear fission.-Evolution of radioactivity in nuclear waste:...
, is also efficiently converted to its volatile hexafluoride. A few other elements also form similarly volatile hexafluorides, pentafluorides, or heptafluorides. The volatile fluorides can be separated from excess fluorine by condensation, then separated from each other by fractional distillation
Fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions, such as in separating chemical compounds by their boiling point by heating them to a temperature at which several fractions of the compound will evaporate. It is a special type of distillation...
or selective reduction
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
. Uranium hexafluoride
Uranium hexafluoride
Uranium hexafluoride , referred to as "hex" in the nuclear industry, is a compound used in the uranium enrichment process that produces fuel for nuclear reactors and nuclear weapons. It forms solid grey crystals at standard temperature and pressure , is highly toxic, reacts violently with water...
and technetium hexafluoride have very similar boiling points and vapor pressures, which makes complete separation more difficult.
Many of the fission product
Fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus fissions. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons and a large release of energy in the form of heat , gamma rays and neutrinos. The...
s volatilized are the same ones volatilized in non-fluorinated, higher-temperature volatilization, such as iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
, tellurium and molybdenum
Molybdenum
Molybdenum , is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek , meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores...
; notable differences are that technetium
Technetium
Technetium is the chemical element with atomic number 43 and symbol Tc. It is the lowest atomic number element without any stable isotopes; every form of it is radioactive. Nearly all technetium is produced synthetically and only minute amounts are found in nature...
is volatilized, but caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
is not.
Some transuranium elements such as plutonium
Plutonium
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
, neptunium
Neptunium
Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive metal, neptunium is the first transuranic element and belongs to the actinide series. Its most stable isotope, 237Np, is a by-product of nuclear reactors and plutonium production and it can be used as a...
and americium
Americium
Americium is a synthetic element that has the symbol Am and atomic number 95. This transuranic element of the actinide series is located in the periodic table below the lanthanide element europium, and thus by analogy was named after another continent, America.Americium was first produced in 1944...
can form volatile fluorides, but these compounds are not stable when the fluorine partial pressure is decreased. Most of the plutonium and some of the uranium will initially remain in ash which drops to the bottom of the flame fluorinator. The plutonium-uranium ratio in the ash may even approximate the composition needed for fast neutron reactor
Fast neutron reactor
A fast neutron reactor or simply a fast reactor is a category of nuclear reactor in which the fission chain reaction is sustained by fast neutrons...
fuel. Further fluorination of the ash can remove all the uranium, neptunium
Neptunium
Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive metal, neptunium is the first transuranic element and belongs to the actinide series. Its most stable isotope, 237Np, is a by-product of nuclear reactors and plutonium production and it can be used as a...
, and plutonium as volatile fluorides; however, some other minor actinides
Minor actinides
The minor actinides are the actinide elements in used nuclear fuel other than uranium and plutonium, which are termed the major actinides. The minor actinides include neptunium, americium, curium, berkelium, californium, einsteinium, and fermium...
may not form volatile fluorides and instead remain with the alkaline fission products. Some noble metals may not form fluorides at all, but remain in metallic form; however ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
hexafluoride is relatively stable and volatile.
Distillation of the residue at higher temperatures can separate lower-boiling transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
fluorides and alkali metal
Alkali metal
The alkali metals are a series of chemical elements in the periodic table. In the modern IUPAC nomenclature, the alkali metals comprise the group 1 elements, along with hydrogen. The alkali metals are lithium , sodium , potassium , rubidium , caesium , and francium...
(Cs, Rb) fluorides from higher-boiling lanthanide
Lanthanide
The lanthanide or lanthanoid series comprises the fifteen metallic chemical elements with atomic numbers 57 through 71, from lanthanum through lutetium...
and alkaline earth metal
Alkaline earth metal
The alkaline earth metals are a group in the periodic table. In the modern IUPAC nomenclature, the alkaline earth metals are called the group 2 elements. Previously, they were called the Group IIA elements . The alkaline earth metals contain beryllium , magnesium , calcium , strontium , barium and...
(Sr, Ba) and yttrium
Yttrium
Yttrium is a chemical element with symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and it has often been classified as a "rare earth element". Yttrium is almost always found combined with the lanthanides in rare earth minerals and is...
fluorides. The temperatures involved are much higher, but can be lowered somewhat by distilling in a vacuum. If a carrier salt like lithium fluoride
Lithium fluoride
Lithium fluoride is an inorganic compound with the formula LiF. It is the lithium salt of hydrofluoric acid. This white solid is a simple ionic compound. Its structure is analogous to that of sodium chloride, but it is much less soluble in water. It is mainly used as a component of molten...
or sodium fluoride
Sodium fluoride
Sodium fluoride is an inorganic chemical compound with the formula NaF. A colorless solid, it is a source of the fluoride ion in diverse applications. Sodium fluoride is less expensive and less hygroscopic than the related salt potassium fluoride....
is being used as a solvent, high-temperature distillation is a way to separate the carrier salt for reuse.
Molten salt reactor
Molten salt reactor
A molten salt reactor is a type of nuclear fission reactor in which the primary coolant, or even the fuel itself is a molten salt mixture...
designs carry out fluoride volatility reprocessing continuously or at frequent intervals. The goal is to return actinide
Actinide
The actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium.The actinide series derives its name from the group 3 element actinium...
s to the molten fuel mixture for eventual fission, while removing fission product
Fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus fissions. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons and a large release of energy in the form of heat , gamma rays and neutrinos. The...
s that are neutron poisons, or that can be more securely stored outside the reactor core while awaiting eventual transfer to permanent storage.
Chloride volatility and solubility
Many of the elements that form volatile high-valenceValence (chemistry)
In chemistry, valence, also known as valency or valence number, is a measure of the number of bonds formed by an atom of a given element. "Valence" can be defined as the number of valence bonds...
fluorides will also form volatile high-valence chlorides. Chlorination and distillation is another possible method for separation. The sequence of separation may differ usefully from the sequence for fluorides; for example, zirconium tetrachloride and tin tetrachloride have relatively low boiling points of 331°C and 114.1°C. Chlorination has even been proposed as a method for removing zirconium fuel cladding, instead of mechanical decladding.
Chlorides are likely to be easier than fluorides to later convert back to other compounds, such as oxides.
Chlorides remaining after volatilization may also be separated by solubility in water. Chlorides of alkaline elements like americium
Americium
Americium is a synthetic element that has the symbol Am and atomic number 95. This transuranic element of the actinide series is located in the periodic table below the lanthanide element europium, and thus by analogy was named after another continent, America.Americium was first produced in 1944...
, curium
Curium
Curium is a synthetic chemical element with the symbol Cm and atomic number 96. This radioactive transuranic element of the actinide series was named after Marie Skłodowska-Curie and her husband Pierre Curie. Curium was first intentionally produced and identified in summer 1944 by the group of...
, lanthanides, strontium
Strontium
Strontium is a chemical element with the symbol Sr and the atomic number 38. An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when exposed to air. It occurs naturally in the minerals celestine and...
, caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
are more soluble than those of uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
, neptunium
Neptunium
Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive metal, neptunium is the first transuranic element and belongs to the actinide series. Its most stable isotope, 237Np, is a by-product of nuclear reactors and plutonium production and it can be used as a...
, plutonium
Plutonium
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
, and zirconium
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
.
Economics
The relative economicsEconomics
Economics is the social science that analyzes the production, distribution, and consumption of goods and services. The term economics comes from the Ancient Greek from + , hence "rules of the house"...
of reprocessing-waste disposal and interim storage-direct disposal has been the focus of much debate over the past ten years. Studies
have modeled the total fuel cycle costs of a reprocessing-recycling system based on one-time recycling of plutonium in existing thermal reactor
Thermal reactor
A thermal reactor is a nuclear reactor that uses slow or thermal neutrons. Most power reactors are of this type. These type of reactors use a neutron moderator to slow neutrons until they approach the average kinetic energy of the surrounding particles, that is, to reduce the speed of the neutrons...
s (as opposed to the proposed breeder reactor
Breeder reactor
A breeder reactor is a nuclear reactor capable of generating more fissile material than it consumes because its neutron economy is high enough to breed fissile from fertile material like uranium-238 or thorium-232. Breeders were at first considered superior because of their superior fuel economy...
cycle) and compare this to the total costs of an open fuel cycle with direct disposal. The range of results produced by these studies is very wide, but all are agreed that under current (2005) economic conditions the reprocessing-recycle option is the more costly.
If reprocessing is undertaken only to reduce the radioactivity level of spent fuel it should be taken into account that spent nuclear fuel becomes less radioactive over time. After 40 years its radioactivity drops by 99.9%, though it still takes over a thousand years for the level of radioactivity to approach that of natural uranium. However the level of transuranic elements,
including plutonium-239
Plutonium-239
Plutonium-239 is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 has also been used and is currently the secondary isotope. Plutonium-239 is also one of the three main isotopes demonstrated usable as fuel in...
, remains high for over 100,000 years, so if not reused as nuclear fuel, then those elements need secure disposal because of nuclear proliferation
Nuclear proliferation
Nuclear proliferation is a term now used to describe the spread of nuclear weapons, fissile material, and weapons-applicable nuclear technology and information, to nations which are not recognized as "Nuclear Weapon States" by the Treaty on the Nonproliferation of Nuclear Weapons, also known as the...
reasons as well as radiation hazard.
On 25 October 2011 a commission of the Japanese Atomic Energy Commission revealed during a meeting calculations about the costs of recycling nuclear fuel for power generation. These costs could be twice the costs of direct geological disposal of spent fuel: the cost of extracting plutonium and handling spent fuel was estimated at 1.98 to 2.14 yen per kilowatt-hour of electricity generated. Discarding the spent fuel as waste would cost only 1 to 1.35 yen per kilowatt-hour.
List of sites
| Country | Reprocessing site | Fuel type | Procedure | Reprocessing capacity tU/yr |
Commissioning or operating period |
|---|---|---|---|---|---|
 Belgium Belgium |
Mol | LWR, MTR (Material test reactor) | 80 | 1966-1974 | |
 Mainland China Mainland China |
intermediate pilot plant | 60-100 | 1968-early 1970s | ||
 Mainland China Mainland China |
Plant 404 | 50 | 2004 | ||
 Germany Germany |
Karlsruhe, WAK | LWR | 35 | 1971-1990 | |
 Early Modern France Early Modern France |
Marcoule, UP 1 | Military | 1,200 | 1958-1997 | |
 Early Modern France Early Modern France |
Marcoule, CEA APM | FBR | PUREX DIAMEX SANEX | 6 | 1988–present |
 Early Modern France Early Modern France |
La Hague, UP 2 | LWR | PUREX | 900 | 1967-1974 |
 Early Modern France Early Modern France |
La Hague, UP 2-400 | LWR | PUREX | 400 | 1976-1990 |
 Early Modern France Early Modern France |
La Hague COGEMA La Hague site The AREVA NC La Hague site is a nuclear fuel reprocessing plant of AREVA in La Hague on the French Cotentin Peninsula that currently has nearly half of the world's light water reactor spent nuclear fuel reprocessing capacity. It has been in operation since 1976, and has a capacity of about 1700... , UP 2-800 |
LWR | PUREX | 800 | 1990 |
 Early Modern France Early Modern France |
La Hague COGEMA La Hague site The AREVA NC La Hague site is a nuclear fuel reprocessing plant of AREVA in La Hague on the French Cotentin Peninsula that currently has nearly half of the world's light water reactor spent nuclear fuel reprocessing capacity. It has been in operation since 1976, and has a capacity of about 1700... , UP 3 |
LWR | PUREX | 800 | 1990 |
| Windscale | Magnox Magnox Magnox is a now obsolete type of nuclear power reactor which was designed and is still in use in the United Kingdom, and was exported to other countries, both as a power plant, and, when operated accordingly, as a producer of plutonium for nuclear weapons... |
1,000 | 1956-1962 | ||
| Sellafield Sellafield Sellafield is a nuclear reprocessing site, close to the village of Seascale on the coast of the Irish Sea in Cumbria, England. The site is served by Sellafield railway station. Sellafield is an off-shoot from the original nuclear reactor site at Windscale which is currently undergoing... , B205 B205 B205 is the name of the Magnox nuclear reprocessing plant at Sellafield in northern England. This plant uses PUREX chemistry to extract plutonium and uranium from used nuclear fuel.... |
Magnox | PUREX | 1,500 | 1964 | |
| Dounreay Dounreay Dounreay is the site of several nuclear research establishments located on the north coast of Caithness, in the Highland area of Scotland... |
FBR | 8 | 1980 | ||
| THORP Thorp nuclear fuel reprocessing plant The Thermal Oxide Reprocessing Plant, or THORP, is a nuclear fuel reprocessing plant at Sellafield in Cumbria, England. THORP is owned by the Nuclear Decommissioning Authority and operated by Sellafield Ltd... |
LWR | PUREX | 1,200 | 1990 | |
 Italy Italy |
Rotondella | Thorium Thorium fuel cycle The thorium fuel cycle is a nuclear fuel cycle that uses the naturally abundant isotope of thorium, , as the fertile material. In the reactor, is transmuted into the fissile artificial uranium isotope which is the nuclear fuel. Unlike natural uranium, natural thorium contains only trace amounts... |
5 | 1968 (shutdown) | |
 India India |
Trombay Bhabha Atomic Research Centre The Bhabha Atomic Research Centre is India's primary nuclear research facility based in Mumbai. It has a number of nuclear reactors, all of which are used for India's nuclear power and research programme.- History :... |
Military | PUREX | 60 | 1965 |
 India India |
Tarapur | PHWR | 100 | 1982 | |
 India India |
Kalpakkam Kalpakkam Kalpakkam is a small town in Tamil Nadu, India, situated on the Coromandel Coast 70 kilometres south of Chennai. A conglomerate of two villages and a DAE township, it is about 55 km from Thiruvanmiyur.... |
PHWR and FBTR | 100 | 1998 | |
 India India |
Tarapur | PHWR | 100 | 2011 | |
 Japan Japan |
Tokaimura Tokaimura nuclear accident The Tokaimura nuclear accident , which occurred on 30 September 1999, resulted in two deaths. At that time, it was Japan's worst civilian nuclear radiation accident. The criticality accident occurred in a uranium reprocessing facility operated by JCO , a subsidiary of Sumitomo Metal Mining Co... |
LWR | 210 | 1977 | |
 Japan Japan |
Rokkasho Rokkasho Reprocessing Plant The is a nuclear reprocessing plant with an annual capacity of 800 tons of uranium or 8 tons of plutonium, owned by Japan Nuclear Fuel Limited located in the village of Rokkasho in northeast Aomori Prefecture, Japan approximately 17 miles north of the US Air Force's Misawa Air Base... |
LWR | 800 | 2005 | |
 Pakistan Pakistan |
New Labs, Rawalpindi Rawalpindi Rawalpindi , locally known as Pindi, is a city in the Pothohar region of Pakistan near Pakistan's capital city of Islamabad, in the province of Punjab. Rawalpindi is the fourth largest city in Pakistan after Karachi, Lahore and Faisalabad... |
Military/Plutonium/Thorium Thorium fuel cycle The thorium fuel cycle is a nuclear fuel cycle that uses the naturally abundant isotope of thorium, , as the fertile material. In the reactor, is transmuted into the fissile artificial uranium isotope which is the nuclear fuel. Unlike natural uranium, natural thorium contains only trace amounts... |
80 | 1982–Present | |
 Pakistan Pakistan |
Khushab Nuclear Complex, Atomic City of Pakistan | HWR Heavy water reactor A pressurised heavy water reactor is a nuclear power reactor, commonly using unenriched natural uranium as its fuel, that uses heavy water as its coolant and moderator. The heavy water coolant is kept under pressure in order to raise its boiling point, allowing it to be heated to higher... /Military/Tritium Tritium Tritium is a radioactive isotope of hydrogen. The nucleus of tritium contains one proton and two neutrons, whereas the nucleus of protium contains one proton and no neutrons... |
22 kg | 1986–Present | |
 Russia Russia |
Mayak Mayak Mayak Production Association refers to an industrial complex that is one of the biggest nuclear facilities in the Russian Federation. It housed plutonium production reactors and a reprocessing plant... Plant B |
Military | 400 | 1948-196? | |
 Russia Russia |
Mayak Mayak Mayak Production Association refers to an industrial complex that is one of the biggest nuclear facilities in the Russian Federation. It housed plutonium production reactors and a reprocessing plant... Plant BB, RT-1 |
LWR | PUREX + Np separation | 400 | 1978 |
 Russia Russia |
Zheleznogorsk Zheleznogorsk, Krasnoyarsk Krai Zheleznogorsk is a closed town in Krasnoyarsk Krai, Russia, with a developed nuclear industry. It was formerly known as Krasnoyarsk-26. Population: -History:... (Krasnoyarsk-26), RT-2 |
VVER VVER The VVER, or WWER, is a series of pressurised water reactors originally developed by the Soviet Union, and now Russia, by OKB Gidropress. Power output ranges from 440 MWe to 1200 MWe with the latest Russian development of the design... |
1,500 | under construction | |
 United States, WA United States, WA |
Hanford Site Hanford Site The Hanford Site is a mostly decommissioned nuclear production complex on the Columbia River in the U.S. state of Washington, operated by the United States federal government. The site has been known by many names, including Hanford Works, Hanford Engineer Works or HEW, Hanford Nuclear Reservation... |
Military | bismuth phospate, REDOX, PUREX | 1944–present | |
 United States, SC United States, SCSouth Carolina South Carolina is a state in the Deep South of the United States that borders Georgia to the south, North Carolina to the north, and the Atlantic Ocean to the east. Originally part of the Province of Carolina, the Province of South Carolina was one of the 13 colonies that declared independence... |
Savannah River Site Savannah River Site The Savannah River Site is a nuclear reservation in the United States in the state of South Carolina, located on land in Aiken, Allendale and Barnwell Counties adjacent to the Savannah River, southeast of Augusta, Georgia. The site was built during the 1950s to refine nuclear materials for... |
Military/LWR/HWR/Tritium | PUREX, REDOX, THOREX, Np separation | 5000 | 1952–2002 |
 United States, NY United States, NY |
West Valley West Valley Reprocessing Plant West Valley Reprocessing Plant was a formerly operational plant for the reprocessing of used nuclear fuel at West Valley, New York, USA. It was operated from 1966-72. During this time period, 600,000 gallons of highly radioactive waste accumulated in an underground waste tank... |
LWR | PUREX | 300 | 1966-1972 |
See also
- Nuclear fuel cycleNuclear fuel cycleThe nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the front end, which are the preparation of the fuel, steps in the service period in which the fuel is used during reactor operation, and steps in...
- Breeder reactorBreeder reactorA breeder reactor is a nuclear reactor capable of generating more fissile material than it consumes because its neutron economy is high enough to breed fissile from fertile material like uranium-238 or thorium-232. Breeders were at first considered superior because of their superior fuel economy...
- Spent nuclear fuel shipping caskSpent nuclear fuel shipping caskSpent nuclear fuel shipping casks are used to transport spent nuclear fuel used in nuclear power plants and research reactors to disposal sites such as the nuclear reprocessing center at COGEMA La Hague site...
- Global Nuclear Energy PartnershipGlobal Nuclear Energy PartnershipThe International Framework for Nuclear Energy Cooperation formerly the Global Nuclear Energy Partnership began as a U.S. proposal, announced by United States Secretary of Energy Samuel Bodman on February 6, 2006, to form an international partnership to promote the use of nuclear power and close...
announced February, 2006
External links
- Processing of Used Nuclear Fuel, World Nuclear Association
- PUREX Process, European Nuclear Society
- Mixed Oxide Fuel (MOX) - World Nuclear Association
- Disposal Options for Surplus Weapons-Usable Plutonium - Congressional Research Service Report for Congress
- http://alsos.wlu.edu/qsearch.aspx?browse=science/Reprocessing+Spent+Fuel, Annotated bibliography on nuclear reprocessing from the Alsos Digital Library for Nuclear Issues
- Brief History of Fuel Reprocessing
- Annotated bibliography for reprocessing spent nuclear fuel from the Alsos Digital Library for Nuclear Issues

