Uranium trioxide
Overview
Uranyl
The uranyl ion is an oxycation of uranium in the oxidation state +6, with the chemical formula [UO2]2+. It has a linear structure with short U-O bonds, indicative of the presence of multiple bonds between uranium and oxygen. Four or more ligands are bound to the uranyl ion in an equatorial plane...
oxide, uranium(VI) oxide, and uranic oxide, is the hexavalent oxide
Oxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
of uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
. The solid may be obtained by heating uranyl nitrate
Uranyl nitrate
Uranyl nitrate is a water soluble yellow uranium salt. The yellow-green crystals of uranium nitrate hexahydrate are triboluminescent.Uranyl nitrate can be prepared by reaction of uranium salts with nitric acid...
to 400 °C. Its most commonly encountered polymorph
Polymorphism (materials science)
Polymorphism in materials science is the ability of a solid material to exist in more than one form or crystal structure. Polymorphism can potentially be found in any crystalline material including polymers, minerals, and metals, and is related to allotropy, which refers to chemical elements...
, γ-UO3, is a yellow-orange powder.
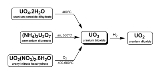
There are three methods to generate uranium trioxide. As noted below, two are used industrially in the reprocessing of nuclear fuel and uranium enrichment.
- U3O8 can be oxidized at 500°C with oxygen.

