
Rhodocene
Encyclopedia
Rhodocene, formally known as bis(η5-cyclopentadienyl)rhodium(II), is a chemical compound
with the formula [Rh(C5H5)2]. Each molecule contains an atom of rhodium
bound between two planar
systems of five carbon
atoms known as cyclopentadienyl
rings in a sandwich
arrangement. It is an organometallic compound
as it has (haptic
) covalent
rhodium–carbon bonds. The [Rh(C5H5)2] radical
is found above 150 °C or when trapped by cooling to liquid nitrogen
temperatures (−196 °C). At room temperature, pairs of these radicals combine to form a dimer, a yellow solid in which two of these cyclopentadienyl rings are joined.
The history of organometallic chemistry
includes the 19th century discoveries of Zeise's salt
and Ludwig Mond
's discovery of nickel tetracarbonyl. These compounds posed a challenge to chemists as the compounds did not fit with chemical bonding models as they were then understood. A further challenge arose with the discovery of ferrocene
, the iron analogue of rhodocene and the first of the class of compounds now known as metallocene
s. Ferrocene was found to be unusually chemically stable
, as were analogous chemical structures including rhodocenium, the unipositive cation of rhodocene and its cobalt
and iridium
counterparts. The study of organometallic species including these ultimately led to the development of new bonding models that explained both their formation and their stability. Work on sandwich compounds, including the rhodocenium / rhodocene system, earned Geoffrey Wilkinson
and Ernst Otto Fischer
the 1973 Nobel Prize for Chemistry
.
Owing to their stability and relative ease of preparation, rhodocenium salts are the usual starting material for preparing rhodocene and substituted rhodocenes, all of which are unstable. The original synthesis used a cyclopentadienyl anion and tris(acetylacetonato)rhodium(III); numerous other approaches have since been reported, including gas-phase redox transmetalation
and using half-sandwich precursors. Octaphenylrhodocene (a derivative with eight phenyl group
s attached) was the first substituted rhodocene to be isolated at room temperature, though even it decomposes rapidly in air. X-ray crystallography
confirmed that octaphenylrhodocene has a sandwich structure with a staggered conformation. Unlike cobaltocene, which has become a useful one-electron reducing agent
in the research laboratory setting, no rhodocene derivative yet discovered has sufficient stability for such applications.
Biomedical research
ers have examined the applications of rhodium compounds and their derivatives in medicine and reported one potential application for a rhodocene derivative as a radiopharmaceutical to treat small cancer
s. Rhodocene derivatives are also used to synthesise linked metallocenes so that metal–metal interactions can be studied; potential applications of these derivatives include molecular electronics
and research into the mechanisms of catalysis
. The value of rhodocenes tends to be in the insights they provide into the bonding and dynamics of novel chemical systems, rather than their direct use in applications.
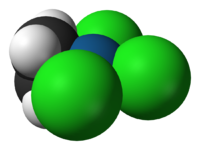 Discoveries in organometallic chemistry
Discoveries in organometallic chemistry
have led to important insights into chemical bonding. Zeise's salt
, K[PtCl3(C2H4)]·H2O, was reported in 1831 and Mond's
discovery of Ni(CO)4 occurred in 1888. Each contained a bond between a metal centre and small molecule, ethylene
in the case of Zeise's salt and carbon monoxide
in the case of nickel tetracarbonyl. The space-filling model
of the anion of Zeise's salt (image at left) shows direct bonding between the platinum
metal centre (shown in blue) and the carbon atoms (shown in black) of the ethylene ligand
; such metal–carbon bonds are the defining characteristic of organometallic species. However, bonding models were unable to explain the nature of such metal–alkene bonds until the Dewar-Chatt-Duncanson model
was proposed in the 1950s. The original formulation covered only metal–alkene bonds but the model was expanded over time to cover systems like metal carbonyl
s (including [Ni(CO)4]) where π backbonding is important.
Ferrocene
, [Fe(C5H5)2], was first synthesised in 1951 during an attempt to prepare the fulvalene bicyclopentadienylidene (C10H8) by oxidative coupling of cyclopentadiene
; the resultant product was found to have molecular formula C10H10Fe and reported to exhibit "remarkable stability". The discovery sparked substantial interest in the field of organometallic chemistry, in part because the structure proposed by Pauson and Kealy (shown at right) was inconsistent with existing bonding models and did not explain its unexpected stability. Consequently, the initial challenge was to definitively determine the structure of ferrocene in the hope that its bonding and properties would then be understood. The sandwich structure was deduced and reported independently by three groups in 1952: Robert Burns Woodward
and Geoffrey Wilkinson
investigated the reactivity in order to determine the structure and demonstrated that ferrocene undergoes similar reactions to a typical aromatic molecule (such as benzene
), Ernst Otto Fischer
not only deduced the sandwich structure but also began synthesising other metallocene
s including cobaltocene
, whilst Eiland and Pepinsky provided X-ray crystallographic
confirmation of the sandwich structure. Applying valence bond theory
to ferrocene by considering an Fe2+ centre and two cyclopentadienide anions (C5H5−), which are known to be aromatic
according to Hückel's rule
and hence highly stable, allowed correct prediction of the geometry of the molecule; however, it was only once molecular orbital theory
was successfully applied that the reasons for ferrocene's remarkable stability became clear.
The properties of cobaltocene reported by Wilkinson and Fischer demonstrated that the unipositive cobalticinium cation [Co(C5H5)2]+ exhibited stability similar to that of ferrocene itself. This observation is not unexpected given that the cobalticinium cation and ferrocene are isoelectronic, although the bonding was not understood at the time. Nevertheless, the observation led Wilkinson and F. Albert Cotton
to attempt the synthesis of rhodocenium and iridocenium salts. They reported the synthesis of numerous rhodocenium salts, including those containing the tribromide
([Rh(C5H5)2]Br3), perchlorate
([Rh(C5H5)2]ClO4), and reineckate ([Rh(C5H5)2] [Cr(NCS)4(NH3)2]·H2O) anions, and found that the addition of dipicrylamine produced a compound of composition [Rh(C5H5)2] [N(C6H2N3O6)2]. In each case, the rhodocenium cation was found to possess high stability. Wilkinson and Fischer went on to share the 1973 Nobel Prize
for Chemistry for their "for their pioneering work, performed independently, on the chemistry of the organometallic, so called sandwich compound
s".
The stability of metallocenes can be directly compared by looking at the reduction potential
s of the one-electron reduction of the unipositive cation. The following data are presented relative to the saturated calomel electrode
(SCE) in acetonitrile
:
These data clearly indicate the stability of neutral ferrocene and the cobaltocenium and rhodocenium cations. Rhodocene is ca. 500 mV more reducing than cobaltocene, indicating that it is more readily oxidised and hence less stable. An earlier polarographic
investigation of rhodocenium perchlorate at neutral pH
showed a cathodic wave peak at −1.53 V (versus SCE) at the dropping mercury electrode
, corresponding to the formation rhodocene in solution; however, the researchers were unable to isolate the neutral product from solution. In the same study, attempts to detect iridocene by exposing iridocenium salts to oxidising conditions were unsuccessful even at elevated pH. These data are consistent with rhodocene being highly unstable and may indicate that iridocene is even more unstable still.
is the equivalent of the octet rule
in main group chemistry and provides a useful guide for predicting the stability of organometallic compounds. It predicts that organometallic species "in which the sum of the metal valence electrons plus the electrons donated by the ligand groups total 18 are likely to be stable." This helps to explain the unusually high stability observed for ferrocene and for the cobalticinium and rhodocenium cations – all three species have analogous
geometries and are isoelectronic 18-valence electron structures. The instability of rhodocene and cobaltocene are also understandable in terms of the 18-electron rule, in that both are 19-valence electron structures; this explains early difficulties in isolating rhodocene from rhodocenium solutions. In fact, the chemistry of rhodocene is dominated by the drive to attain an 18-electron configuration.
Rhodocene exists as [Rh(C5H5)2], a paramagnetic 19-valence electron radical
monomer
only at or below −196 °C (liquid nitrogen
temperatures) or above 150 °C in the gas phase. It is this monomeric form that displays the typical staggered metallocene
sandwich structure. However, at room temperature (25 °C), the lifetime of the monomeric form in acetonitrile
is less than two seconds; instead, rhodocene forms [Rh(C5H5)2]2, a diamagnetic 18-valence electron bridged dimeric ansa-metallocene structure. Electron spin resonance (ESR), nuclear magnetic resonance
(NMR) and infrared spectroscopic
(IR) measurements point to the presence of an equilibrium
interconverting the monomeric and dimeric forms. ESR evidence confirms that the monomer possesses a high order axis of symmetry Cn, n > 2) with a mirror plane (σ) perpendicular to it as symmetry elements; this experimentally demonstrates that the monomer does possess the typical sandwich structure of a metallocene although the interpretation of the ESR data has been questioned. The decomposition pathway of the monomer has also been studied by mass spectrometry
. The dimerisation is a redox
process, the dimer being a rhodium(I) species whilst the monomer has a rhodium(II) metal centre. Rhodium
typically occupies oxidation state
s +I or +III in its stable compounds.
This dimerisation process has the overall effect of decreasing the electron count
around the rhodium centre from 19 to 18. This occurs because the oxidative coupling of the two cyclopentadienyl ligands produces a new ligand with lower hapticity
and which donates fewer electrons to the metal centre. The term hapticity is used to indicate the "number of carbon (or other) atoms through which [a ligand] binds (n)" to a metal centre and is symbolised as ηn. For example, the ethylene ligand in Zeise's salt is bound to the platinum centre through both carbon atoms, and it hence formally has the formula K[PtCl3(η2-C2H4)]·H2O. The carbonyl ligands in nickel tetracarbonyl are each bound through only a carbon atom and are hence described as monohapto ligands, but η1-notations are typically omitted in formulae. The cyclopentadienyl ligands in many metallocene
and half-sandwich compounds are pentahapto ligands, hence the formula [Rh(η5-C5H5)2] for the rhodocene monomer. In the rhodocene dimer, the coupled cyclopentadienyl ligands are 4-electron tetrahapto donors to each rhodium(I) metal centre, in contrast to the 6-electron pentahapto cyclopentadienyl donors. The increased stability of the 18-valence electron rhodium(I) dimer species as compared to the 19-valence electron rhodium(II) monomer likely explains why the monomer is only detected under extreme conditions.
Cotton and Wilkinson demonstrated that the 18-valence electron rhodium(III) rhodocenium cation [Rh(η5-C5H5)2]+ can be reduced in aqueous solution to the monomeric form; however, they were unable to isolate the neutral product as not only can it dimerise, the rhodium(II) radical monomer can also spontaneously form the mixed-hapticity stable rhodium(I) species [(η5-C5H5)Rh(η4-C5H6)]. The differences between rhodocene and this derivative are found in two areas: (1) One of the bound cyclopentadienyl ligands has formally gained a hydrogen atom to become cyclopentadiene, which remains bound to the metal centre but now as a 4-electron η4- donor. (2) The rhodium(II) metal centre has been reduced to rhodium(I). These two changes make the derivative an 18-valence electron species. Fischer and colleagues hypothesised that the formation of this rhodocene derivative might occur in separate protonation and reduction steps, but published no evidence to support this suggestion. (η4-Cyclopentadiene)(η5-cyclopentadienyl)rhodium(I), the resulting compound, is an unusual organometallic complex in that it has both a cyclopentadienyl anion and cyclopentadiene itself as ligands. It has been shown that this compound can also be prepared by sodium borohydride
reduction of a rhodocenium solution in aqueous ethanol
; the researchers who made this discovery characterised the product as biscyclopentadienylrhodium hydride.
Fischer and co-workers also studied the chemistry of iridocene, the third transition series analogue of rhodocene and cobaltocene, finding the chemistry of rhodocene and iridocene are generally similar. The synthesis of numerous iridocenium salts including the tribromide and hexafluorophosphate
have been described. Just as with rhodocene, iridocene dimerises at room temperature but a monomer form can be detected at low temperatures and in gas phase and IR, NMR, and ESR measurements indicate a chemical equilibrium is present and confirm the sandwich structure of the iridocene monomer. The complex [(η5-C5H5)Ir(η4-C5H6)], the analogue of rhodocene derivative reported by Fischer, has also been studied and demonstrates properties consistent with a greater degree of π-backbonding in iridium(I) systems than is found in the analogous cobalt(I) or rhodium(I) cases.
Grignard reagent
cyclopentadienylmagnesium bromide (C5H5MgBr) with tris(acetylacetonato)rhodium(III) (Rh(acac)3). More recently, gas-phase rhodocenium cations have been generated by a redox
transmetalation
reaction of rhodium(I) ions with ferrocene or nickelocene
.
Modern microwave synthetic methods
have also been reported. Rhodocenium hexafluorophosphate forms after reaction of cyclopentadiene and rhodium(III) chloride hydrate
in methanol
following work-up with methanolic ammonium hexafluorophosphate
; the reaction yield
exceeds 60% with only 30 seconds of exposure to microwave radiation.
Rhodocene itself is then formed by reduction of rhodocenium salts with molten sodium
. If a rhodocenium containing melt is treated with sodium or potassium metals and then sublimed onto a liquid nitrogen-cooled cold finger, a black polycrystalline material results. Warming this material to room temperature produces a yellow solid which has been confirmed as the rhodocene dimer. A similar method can be used to prepare the iridocene dimer.
reactions to produce cyclopentenes are well known and serve as precedent for vinylcyclopropenes rearranging
to cyclopentadienes. The [(η5-C5tBu3H2)Rh(η5-C5H5)]+ cation has been generated by a reaction sequence beginning with the addition the chlorobisethylenerhodium(I) dimer, [(η2-C2H4)2Rh(μ-Cl)]2, to 1,2,3-tri-tert-butyl-3-vinyl-1-cyclopropene followed by reaction with thallium cyclopentadienide:
The 18-valence electron rhodium(III) pentadienediyl species generated by this reaction demonstrates again the instability of the rhodocene moiety, in that it can be refluxed in toluene for months without 1,2,3-tri-tert-butylrhodocene forming but in oxidising conditions the 1,2,3-tri-tert-butylrhodocenium cation forms rapidly. Cyclic voltammetry has been used to investigate this and similar processes in detail. The mechanism of the reaction has been shown to involve a loss of one electron from the pentadienediyl ligand followed by a fast rearrangement (with loss of a hydrogen atom) to form the 1,2,3-tri-tert-butylrhodocenium cation. Both the tetrafluoroborate
and hexafluorophosphate salts of this cation have been structurally characterised by X-ray crystallography.
[(η5-C5tBu3H2)Rh(η5-C5H5)]BF4 forms a colourless centrosymmetric monoclinic
crystal belonging to the P21/c space group
, and with a density
of 1.486 g cm−3. Looking at the ORTEP
diagram of the structure of the cation (at right), it is evident that it possesses the typical geometry expected of a rhodocene or rhodocenium cation. The two cyclopentadienyl rings are close to parallel (the centroid
–Rh–centroid angle is 177.2°) and the rhodium centre is slightly closer to the substituted cyclopentadienyl ring (Rh–centroid distances are 1.819 Å
and 1.795 Å), an observation attributed to the greater inductive effect of the tert-butyl groups on the substituted ligand. The ORTEP diagram shows that the cation adopts an eclipsed conformation in the solid state. However, the crystal structure of the hexafluorophosphate salt shows three crystallographically independent cations, one eclipsed, one staggered, and one which is rotationally disordered. This suggests that the conformation adopted is dependent on the anion present and also illustrates that the energy barrier to rotation is low – in ferrocene, the rotational energy barrier is known to be ~5 kJ mol−1 in both solution and gas phase.
The diagram above shows the rhodium–carbon ( inside pentagons on the left) and carbon–carbon ( outside pentagons on the left) bond distances for both ligands, along with the bond angles ( inside pentagons on the right) within each cyclopentadienyl ring. The atom labels used are the same as those shown in the crystal structure above. Within the unsubstituted cyclopentadienyl ligand, the carbon–carbon bond lengths vary between 1.35 Å and 1.40 Å and the internal bond angles vary between 107° and 109°. For comparison, the internal angle at each vertex of a regular pentagon is 108°. The rhodium–carbon bond lengths vary between 2.16 Å and 2.18 Å. These results are consistent with η5-coordination of the ligand to the metal centre. In the case of the substituted cyclopentadienyl ligand, there is somewhat greater variation: carbon–carbon bond lengths vary between 1.39 Å and 1.48 Å, the internal bond angles vary between 106° and 111°, and the rhodium–carbon bond lengths vary between 2.14 Å and 2.20 Å. The greater variation in the substituted ligand is attributed to the distortions necessary to relieve the steric strain imposed by neighbouring tert-butyl substituents; despite these variations, the data demonstrate that the substituted cyclopentadienyl is also η5-coordinated.
The stability of metallocenes changes with ring substitution. Comparing the reduction potentials of the cobaltocenium and decamethylcobaltocenium cations shows that the decamethyl species is ca. 600 mV more reducing than its parent metallocene, a situation also observed in the ferrocene and rhodocene systems. The following data are presented relative to the ferrocenium / ferrocene redox couple:
The differences in reduction potentials is attributed in the cobaltocenium system to the inductive effect of the alkyl groups, further stabilising the 18-valence electron species. A similar effect is seen in the rhodocenium data shown above, again consistent with inductive effects. In the substituted iridocenium system, cyclic voltammetry investigations shows irreversible reductions at temperatures as low as −60 °C; by comparison, the reduction of the corresponding rhodocenes is quasi-reversible at room temperature and fully reversible at −35 °C. The irreversibility of the substituted iridocenium reductions is attributed to the extremely rapid dimerisation of the resulting 19-valence electron species, which further illustrates that iridocenes are less stable than their corresponding rhodocenes.
of the pentamethylcyclopentadienyl and pentaphenylcyclopentadienyl ligands being well-known. Substitutions on the cyclopentadienyl rings of rhodocenes and rhodocenium salts produce compounds of higher stability as they allow for the increased delocalisation of positive charge or electron density
whilst also providing steric hindrance against other species approaching the metal centre. Various mono- and di-substituted rhodocenium species are known, but substantial stabilisation is not achieved without greater substitutions. Known highly substituted rhodocenium salts include decamethylrhodocenium hexafluorophosphate [(η5-C5Me5)2Rh]PF6, decaisopropylrhodocenium hexafluorophosphate [(η5-C5iPr
5)2Rh]PF6, and octaphenylrhodocenium hexafluorophosphate [(η5-C5Ph4H)2Rh]PF6. Decamethylrhodocenium tetrafluoroborate can be synthesised from the tris(acetone) complex [(η5-C5Me5)Rh(Me2CO)3](BF4)2 by reaction with pentamethylcyclopentadiene
, and the analogous iridium synthesis is also known. Decaisopropylrhodicnium hexafluorophosphate was synthesised in 1,2-dimethoxyethane (solvent
) in an unusual one-pot synthesis
that involves the formation of 20 carbon–carbon bonds:
In a similar reaction, pentaisopropylrhodocenium hexafluorophosphate [(η5-C5iPr5)Rh(η5-C5H5)]PF6 can be synthesised from pentamethylrhodocenium hexafluorophosphate [(η5-C5Me5)Rh(η5-C5H5)]PF6 in 80% yield. These reactions demonstrate that the acidity of the methyl hydrogens in a pentamethylcyclopentadienyl complex can be considerably increased by the presence of the metal centre. Mechanistically, the reaction proceeds with potassium hydroxide
deprotonating a methyl group and the resulting carbanion undergoing nucleophilic substitution
with methyl iodide to form a new carbon–carbon bond.
The compounds pentaphenylrhodocenium tetrafluoroborate
[(η5-C5Ph5)Rh(η5-C5H5)]BF4, and pentamethylpentaphenylrhodocenium tetrafluoroborate [(η5-C5Ph5)Rh(η5-C5Me5)]BF4 have also been reported. They demonstrate that rhodium sandwich compounds can be prepared from half-sandwich precursors. For example, in an approach broadly similar to the tris(acetone) synthesis of decamethylrhodocenium tetrafluoroborate, pentaphenylrhodocenium tetrafluoroborate has been synthesised from the tris(acetonitrile
) salt [(η5-C5Ph5)Rh(CH3CN)3](BF4)2 by reaction with sodium cyclopentadienide
:
Octaphenylrhodocene, [(η5-C5Ph4H)2Rh], is the first rhodocene derivative to be isolated at room temperature. Its olive green crystals decompose rapidly in solution, and within minutes in air, demonstrating a dramatically greater air sensitivity than the analogous cobalt
complex, although it is significantly more stable than rhodocene itself. This difference is attributed to the relatively lower stability of the rhodium(II) state as compared to the cobalt(II) state. The reduction potential for the [(η5-C5Ph4H)2Rh]+ cation (measured in dimethylformamide
relative the ferrocenium / ferrocene couple) is −1.44 V, consistent with the greater thermodynamic stabilisation of the rhodocene by the C5HPh4 ligand compared with the C5H5 or C5Me5 ligands. Cobaltocene is a useful one-electron reducing agent
in the research laboratory as it is soluble in non-polar organic solvents, and its redox couple is sufficiently well behaved that it may be used as an internal standard
in cyclic voltammetry
. No substituted rhodocene yet prepared has demonstrated sufficient stability to be used in a similar way.
The synthesis of octaphenylrhodocene proceeds in three steps, with a diglyme
reflux followed by workup with hexafluorophosphoric acid
, then a sodium amalgam
reduction in tetrahydrofuran
:
The crystal structure
of octaphenylrhodocene shows a staggered conformation (similar to that of ferrocene, and in contrast to the eclipsed conformation of ruthenocene
). The rhodium–centroid distance is 1.904 Å and the rhodium–carbon bond lengths average 2.26 Å, whilst the carbon–carbon bond lengths average 1.44 Å. These distances are all similar to those found in the 1,2,3-tri-tert-butylrhodocenium cation described above, with the one difference that the effective size of the rhodium centre appears larger, an observation consistent with the expanded ionic radius of rhodium(II) compared with rhodium(III).
 There has been extensive research into metallopharmaceutical
There has been extensive research into metallopharmaceutical
s, including discussion of rhodium compounds in medicine. A substantial body of research has examined using metallocene derivatives of ruthenium
and iron as metallopharmaceuticals. One area of such research has utilised metallocenes in place of the fluorophenyl group in haloperidol
, which is a pharmaceutical classified as a typical antipsychotic
. The ferrocenyl–haloperidol compound investigated has structure (C5H5)Fe(C5H4)–C(=O)–(CH2)3–N(CH2CH2)2C(OH)–C6H4Cl and can be converted to the ruthenium analog via a transmetalation reaction. Using the radioactive isotope
103Ru produces a ruthenocenyl–haloperidol radiopharmaceutical with a high affinity for lung
but not brain
tissue
in mice
and rat
s. Beta-decay of 103Ru produces the metastable isotope 103mRh in a rhodocenyl–haloperidol compound. This compound, like other rhodocene derivatives, has an unstable 19-valence electron configuration and rapidly oxidises to the expected cationic rhodocenium–haloperidol species. The separation of the ruthenocenyl–haloperidol and the rhodocenium–haloperidol species and the distributions of each amongst bodily organs has been studied. 103mRh has a half-life
of 56 min and emits a gamma ray
of energy 39.8 keV
, so the gamma-decay of the rhodium isotope should follow soon after the beta-decay of the ruthenium isotope. Beta- and gamma-emitting radionuclide
s used medically include 131I
, 59Fe, and 47Ca, and 103mRh has been proposed for use in radiotherapy for small tumours.
, semi-conducting (and possibly ferromagnetic) metallocene polymers (an example of a molecular wire), and exploring the threshold between heterogeneous
and homogeneous catalysis
. Examples of known bimetallocenes and termetallocenes that possess the rhodocenyl moiety include the hexafluorophosphate salts of rhodocenylferrocene, 1,1'-dirhodocenylferrocene, and 1-cobaltocenyl-1'-rhodocenylferrocene, each shown at right. Linked metallocenes can also be formed by introducing several metallocenyl substituents onto a single cyclopentadienyl ligand.
Structural studies of termetallocene systems have shown they typically adopt an "eclipsed double transoid" "crankshaft" geometry. Taking as an example the 1-cobaltocenyl-1'-rhodocenylferrocene cation shown above, this means that the cobaltocenyl and rhodocenyl moieties are eclipsed, and thus carbon atoms 1 and 1' on the central ferrocene core are as close to vertically aligned as is possible given the staggered conformation of the cyclopentadienyl rings within each metallocene unit. Viewed from side-on, this means termetallocenes resemble the down–up–down pattern of a crankshaft
. The synthesis of this termetallocene involves the combining of rhodocenium and cobaltocenium solutions with 1,1'-dilithioferrocene. This produces an uncharged intermediate with linked cyclopentadienyl–cyclopentadiene ligands whose bonding resembles that found in the rhodocene dimer. These ligands then react with the triphenylmethyl carbocation
to generate the termetallocene salt, [(η5-C5H5)Rh(μ-η5:η5-C5H4–C5H4)Fe(μ-η5:η5-C5H4–C5H4)Co(η5-C5H5)](PF6)2. This synthetic pathway is illustrated below:
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
with the formula [Rh(C5H5)2]. Each molecule contains an atom of rhodium
Rhodium
Rhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
bound between two planar
Coplanarity
In geometry, a set of points in space is coplanar if all the points lie in the same geometric plane. For example, three distinct points are always coplanar; but a fourth point or more added in space can exist in another plane, incoplanarly....
systems of five carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atoms known as cyclopentadienyl
Cyclopentadienyl
In organic chemistry, cyclopentadienyl is a cyclic group of atoms with the formula C5H5. Cyclopentadienyl are closely related to cyclopentadiene. Cyclopentadienyl have five carbon atoms bonded together in a pentagonal planar ring, all five of which are bonded to individual hydrogen atoms...
rings in a sandwich
Sandwich compound
In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic covalent bonds to two arene ligands. The arenes have the formula CnHn, substituted derivatives and heterocyclic derivatives...
arrangement. It is an organometallic compound
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
as it has (haptic
Hapticity
The term hapticity is used to describe how a group of contiguous atoms of a ligand are coordinated to a central atom. Hapticity of a ligand is indicated by the Greek character 'eta', η. A superscripted number following the η denotes the number of contiguous atoms of the ligand that are bound to...
) covalent
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
rhodium–carbon bonds. The [Rh(C5H5)2] radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
is found above 150 °C or when trapped by cooling to liquid nitrogen
Liquid nitrogen
Liquid nitrogen is nitrogen in a liquid state at a very low temperature. It is produced industrially by fractional distillation of liquid air. Liquid nitrogen is a colourless clear liquid with density of 0.807 g/mL at its boiling point and a dielectric constant of 1.4...
temperatures (−196 °C). At room temperature, pairs of these radicals combine to form a dimer, a yellow solid in which two of these cyclopentadienyl rings are joined.
The history of organometallic chemistry
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
includes the 19th century discoveries of Zeise's salt
Zeise's salt
Zeise's salt, potassium trichloroplatinate, is the chemical compound with the formula KPtCl3]·H2O. The anion of this air-stable, yellow, coordination complex contains an η2-ethylene ligand. The anion features a platinum atom with a square planar geometry.-Preparation:This compound is commercially...
and Ludwig Mond
Ludwig Mond
Dr Ludwig Mond , was a German-born chemist and industrialist who took British nationality.-Education and career:...
's discovery of nickel tetracarbonyl. These compounds posed a challenge to chemists as the compounds did not fit with chemical bonding models as they were then understood. A further challenge arose with the discovery of ferrocene
Ferrocene
Ferrocene is an organometallic compound with the formula Fe2. It is the prototypical metallocene, a type of organometallic chemical compound consisting of two cyclopentadienyl rings bound on opposite sides of a central metal atom. Such organometallic compounds are also known as sandwich compounds...
, the iron analogue of rhodocene and the first of the class of compounds now known as metallocene
Metallocene
A metallocene is a compound typically consisting of two cyclopentadienyl anions bound to a metal center in the oxidation state II, with the resulting general formula 2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride, vanadocene dichloride...
s. Ferrocene was found to be unusually chemically stable
Chemical stability
Chemical stability when used in the technical sense in chemistry, means thermodynamic stability of a chemical system.Thermodynamic stability occurs when a system is in its lowest energy state, or chemical equilibrium with its environment. This may be a dynamic equilibrium, where individual atoms...
, as were analogous chemical structures including rhodocenium, the unipositive cation of rhodocene and its cobalt
Cobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
and iridium
Iridium
Iridium is the chemical element with atomic number 77, and is represented by the symbol Ir. A very hard, brittle, silvery-white transition metal of the platinum family, iridium is the second-densest element and is the most corrosion-resistant metal, even at temperatures as high as 2000 °C...
counterparts. The study of organometallic species including these ultimately led to the development of new bonding models that explained both their formation and their stability. Work on sandwich compounds, including the rhodocenium / rhodocene system, earned Geoffrey Wilkinson
Geoffrey Wilkinson
Sir Geoffrey Wilkinson FRS was a Nobel laureate English chemist who pioneered inorganic chemistry and homogeneous transition metal catalysis.-Biography:...
and Ernst Otto Fischer
Ernst Otto Fischer
Ernst Otto Fischer was a German chemist who won the Nobel Prize for pioneering work in the area of organometallic chemistry.-Early life:...
the 1973 Nobel Prize for Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
.
Owing to their stability and relative ease of preparation, rhodocenium salts are the usual starting material for preparing rhodocene and substituted rhodocenes, all of which are unstable. The original synthesis used a cyclopentadienyl anion and tris(acetylacetonato)rhodium(III); numerous other approaches have since been reported, including gas-phase redox transmetalation
Transmetalation
Transmetalation is a general chemical reaction type in organometallic chemistry describing the exchange of ligands between two metal centers....
and using half-sandwich precursors. Octaphenylrhodocene (a derivative with eight phenyl group
Phenyl group
In organic chemistry, the phenyl group or phenyl ring is a cyclic group of atoms with the formula C6H5. Phenyl groups are closely related to benzene. Phenyl groups have six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the...
s attached) was the first substituted rhodocene to be isolated at room temperature, though even it decomposes rapidly in air. X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
confirmed that octaphenylrhodocene has a sandwich structure with a staggered conformation. Unlike cobaltocene, which has become a useful one-electron reducing agent
Reducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
in the research laboratory setting, no rhodocene derivative yet discovered has sufficient stability for such applications.
Biomedical research
Biomedical research
Biomedical research , in general simply known as medical research, is the basic research, applied research, or translational research conducted to aid and support the body of knowledge in the field of medicine...
ers have examined the applications of rhodium compounds and their derivatives in medicine and reported one potential application for a rhodocene derivative as a radiopharmaceutical to treat small cancer
Cancer
Cancer , known medically as a malignant neoplasm, is a large group of different diseases, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invade nearby parts of the body. The cancer may also spread to more distant parts of the...
s. Rhodocene derivatives are also used to synthesise linked metallocenes so that metal–metal interactions can be studied; potential applications of these derivatives include molecular electronics
Molecular electronics
Molecular electronics, sometimes called moletronics, involves the study and application of molecular building blocks for the fabrication of electronic components...
and research into the mechanisms of catalysis
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
. The value of rhodocenes tends to be in the insights they provide into the bonding and dynamics of novel chemical systems, rather than their direct use in applications.
History

Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
have led to important insights into chemical bonding. Zeise's salt
Zeise's salt
Zeise's salt, potassium trichloroplatinate, is the chemical compound with the formula KPtCl3]·H2O. The anion of this air-stable, yellow, coordination complex contains an η2-ethylene ligand. The anion features a platinum atom with a square planar geometry.-Preparation:This compound is commercially...
, K[PtCl3(C2H4)]·H2O, was reported in 1831 and Mond's
Ludwig Mond
Dr Ludwig Mond , was a German-born chemist and industrialist who took British nationality.-Education and career:...
discovery of Ni(CO)4 occurred in 1888. Each contained a bond between a metal centre and small molecule, ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
in the case of Zeise's salt and carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
in the case of nickel tetracarbonyl. The space-filling model
Space-filling model
In chemistry a space-filling model, also known as calotte model, is a type of three-dimensional molecular model where the atoms are represented by spheres whose radii are proportional to the radii of the atoms and whose center-to-center distances are proportional to the distances between the atomic...
of the anion of Zeise's salt (image at left) shows direct bonding between the platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
metal centre (shown in blue) and the carbon atoms (shown in black) of the ethylene ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
; such metal–carbon bonds are the defining characteristic of organometallic species. However, bonding models were unable to explain the nature of such metal–alkene bonds until the Dewar-Chatt-Duncanson model
Dewar-Chatt-Duncanson model
The Dewar–Chatt–Duncanson model is a model in organometallic chemistry which explains the type of chemical bonding between an alkene and a metal in certain organometallic compounds. The model is named after Michael J. S. Dewar, Joseph Chatt and L. A...
was proposed in the 1950s. The original formulation covered only metal–alkene bonds but the model was expanded over time to cover systems like metal carbonyl
Metal carbonyl
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. These complexes may be homoleptic, that is containing only CO ligands, such as nickel carbonyl , but more commonly metal carbonyls contain a mix of ligands, such as Re3Cl...
s (including [Ni(CO)4]) where π backbonding is important.
Ferrocene
Ferrocene
Ferrocene is an organometallic compound with the formula Fe2. It is the prototypical metallocene, a type of organometallic chemical compound consisting of two cyclopentadienyl rings bound on opposite sides of a central metal atom. Such organometallic compounds are also known as sandwich compounds...
, [Fe(C5H5)2], was first synthesised in 1951 during an attempt to prepare the fulvalene bicyclopentadienylidene (C10H8) by oxidative coupling of cyclopentadiene
Cyclopentadiene
Cyclopentadiene is an organic compound with the formula C5H6. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction...
; the resultant product was found to have molecular formula C10H10Fe and reported to exhibit "remarkable stability". The discovery sparked substantial interest in the field of organometallic chemistry, in part because the structure proposed by Pauson and Kealy (shown at right) was inconsistent with existing bonding models and did not explain its unexpected stability. Consequently, the initial challenge was to definitively determine the structure of ferrocene in the hope that its bonding and properties would then be understood. The sandwich structure was deduced and reported independently by three groups in 1952: Robert Burns Woodward
Robert Burns Woodward
Robert Burns Woodward was an American organic chemist, considered by many to be the preeminent organic chemist of the twentieth century...
and Geoffrey Wilkinson
Geoffrey Wilkinson
Sir Geoffrey Wilkinson FRS was a Nobel laureate English chemist who pioneered inorganic chemistry and homogeneous transition metal catalysis.-Biography:...
investigated the reactivity in order to determine the structure and demonstrated that ferrocene undergoes similar reactions to a typical aromatic molecule (such as benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
), Ernst Otto Fischer
Ernst Otto Fischer
Ernst Otto Fischer was a German chemist who won the Nobel Prize for pioneering work in the area of organometallic chemistry.-Early life:...
not only deduced the sandwich structure but also began synthesising other metallocene
Metallocene
A metallocene is a compound typically consisting of two cyclopentadienyl anions bound to a metal center in the oxidation state II, with the resulting general formula 2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride, vanadocene dichloride...
s including cobaltocene
Cobaltocene
Cobaltocene, known also as biscobalt or even "bis Cp cobalt", is an organocobalt compound with the formula Co2. It is a dark purple solid that sublimes readily slightly above room temperature. Cobaltocene was discovered shortly after ferrocene, the first metallocene...
, whilst Eiland and Pepinsky provided X-ray crystallographic
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
confirmation of the sandwich structure. Applying valence bond theory
Valence bond theory
In chemistry, valence bond theory is one of two basic theories, along with molecular orbital theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds...
to ferrocene by considering an Fe2+ centre and two cyclopentadienide anions (C5H5−), which are known to be aromatic
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
according to Hückel's rule
Hückel's rule
In organic chemistry, Hückel's rule estimates whether a planar ring molecule will have aromatic properties. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931...
and hence highly stable, allowed correct prediction of the geometry of the molecule; however, it was only once molecular orbital theory
Molecular orbital theory
In chemistry, molecular orbital theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule...
was successfully applied that the reasons for ferrocene's remarkable stability became clear.
The properties of cobaltocene reported by Wilkinson and Fischer demonstrated that the unipositive cobalticinium cation [Co(C5H5)2]+ exhibited stability similar to that of ferrocene itself. This observation is not unexpected given that the cobalticinium cation and ferrocene are isoelectronic, although the bonding was not understood at the time. Nevertheless, the observation led Wilkinson and F. Albert Cotton
F. Albert Cotton
Frank Albert Cotton was the W.T. Doherty-Welch Foundation Chair and Distinguished Professor of Chemistry at Texas A&M University. He authored over 1700 scientific articles. Cotton was recognized for his research on the chemistry of the transition metals.-Education:Frank Albert Cotton was born on...
to attempt the synthesis of rhodocenium and iridocenium salts. They reported the synthesis of numerous rhodocenium salts, including those containing the tribromide
Bromide
A bromide is a chemical compound containing bromide ion, that is bromine atom with effective charge of −1. The class name can include ionic compounds such as caesium bromide or covalent compounds such as sulfur dibromide.-Natural occurrence:...
([Rh(C5H5)2]Br3), perchlorate
Perchlorate
Perchlorates are the salts derived from perchloric acid . They occur both naturally and through manufacturing. They have been used as a medicine for more than 50 years to treat thyroid gland disorders. They are used extensively within the pyrotechnics industry, and ammonium perchlorate is also a...
([Rh(C5H5)2]ClO4), and reineckate ([Rh(C5H5)2] [Cr(NCS)4(NH3)2]·H2O) anions, and found that the addition of dipicrylamine produced a compound of composition [Rh(C5H5)2] [N(C6H2N3O6)2]. In each case, the rhodocenium cation was found to possess high stability. Wilkinson and Fischer went on to share the 1973 Nobel Prize
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
for Chemistry for their "for their pioneering work, performed independently, on the chemistry of the organometallic, so called sandwich compound
Sandwich compound
In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic covalent bonds to two arene ligands. The arenes have the formula CnHn, substituted derivatives and heterocyclic derivatives...
s".
The stability of metallocenes can be directly compared by looking at the reduction potential
Reduction potential
Reduction potential is a measure of the tendency of a chemical species to acquire electrons and thereby be reduced. Reduction potential is measured in volts , or millivolts...
s of the one-electron reduction of the unipositive cation. The following data are presented relative to the saturated calomel electrode
Saturated calomel electrode
The Saturated calomel electrode is a reference electrode based on the reaction between elemental mercury and mercury chloride. The aqueous phase in contact with the mercury and the mercury chloride is a saturated solution of potassium chloride in water...
(SCE) in acetonitrile
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
:
- [Fe(C5H5)2]+ / [Fe(C5H5)2] +0.38 V
- [Co(C5H5)2]+ / [Co(C5H5)2] −0.94 V
- [Rh(C5H5)2]+ / [Rh(C5H5)2] −1.41 V
These data clearly indicate the stability of neutral ferrocene and the cobaltocenium and rhodocenium cations. Rhodocene is ca. 500 mV more reducing than cobaltocene, indicating that it is more readily oxidised and hence less stable. An earlier polarographic
Polarography
Polarography is a subclass of voltammetry where the working electrode is a dropping mercury electrode or a static mercury drop electrode ., useful for its wide cathodic range and renewable surface...
investigation of rhodocenium perchlorate at neutral pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
showed a cathodic wave peak at −1.53 V (versus SCE) at the dropping mercury electrode
Dropping mercury electrode
The dropping mercury electrode is a working electrode made of mercury and used in polarography. Experiments run with mercury electrodes are referred to as forms of polarography even if the experiments are identical or very similar to a corresponding voltammetry experiment which use solid working...
, corresponding to the formation rhodocene in solution; however, the researchers were unable to isolate the neutral product from solution. In the same study, attempts to detect iridocene by exposing iridocenium salts to oxidising conditions were unsuccessful even at elevated pH. These data are consistent with rhodocene being highly unstable and may indicate that iridocene is even more unstable still.
Speciation
The 18-electron rule18-Electron rule
The 18-electron rule is a rule of thumb used primarily for predicting formulas for stable metal complexes. The rule rests on the fact that valence shells of a transition metal consists of nine valence orbitals, which collectively can accommodate 18 electrons either as nonbinding electron pairs or...
is the equivalent of the octet rule
Octet rule
The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low (The octet rule is a chemical rule of thumb that states that atoms of low (...
in main group chemistry and provides a useful guide for predicting the stability of organometallic compounds. It predicts that organometallic species "in which the sum of the metal valence electrons plus the electrons donated by the ligand groups total 18 are likely to be stable." This helps to explain the unusually high stability observed for ferrocene and for the cobalticinium and rhodocenium cations – all three species have analogous
Analog (chemistry)
In chemistry, a structural analog , also known as chemical analog or simply analog, is a compound having a structure similar to that of another one, but differing from it in respect of a certain component. It can differ in one or more atoms, functional groups, or substructures, which are replaced...
geometries and are isoelectronic 18-valence electron structures. The instability of rhodocene and cobaltocene are also understandable in terms of the 18-electron rule, in that both are 19-valence electron structures; this explains early difficulties in isolating rhodocene from rhodocenium solutions. In fact, the chemistry of rhodocene is dominated by the drive to attain an 18-electron configuration.
Rhodocene exists as [Rh(C5H5)2], a paramagnetic 19-valence electron radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
only at or below −196 °C (liquid nitrogen
Liquid nitrogen
Liquid nitrogen is nitrogen in a liquid state at a very low temperature. It is produced industrially by fractional distillation of liquid air. Liquid nitrogen is a colourless clear liquid with density of 0.807 g/mL at its boiling point and a dielectric constant of 1.4...
temperatures) or above 150 °C in the gas phase. It is this monomeric form that displays the typical staggered metallocene
Metallocene
A metallocene is a compound typically consisting of two cyclopentadienyl anions bound to a metal center in the oxidation state II, with the resulting general formula 2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride, vanadocene dichloride...
sandwich structure. However, at room temperature (25 °C), the lifetime of the monomeric form in acetonitrile
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
is less than two seconds; instead, rhodocene forms [Rh(C5H5)2]2, a diamagnetic 18-valence electron bridged dimeric ansa-metallocene structure. Electron spin resonance (ESR), nuclear magnetic resonance
Nuclear magnetic resonance
Nuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation...
(NMR) and infrared spectroscopic
Infrared spectroscopy
Infrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
(IR) measurements point to the presence of an equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
interconverting the monomeric and dimeric forms. ESR evidence confirms that the monomer possesses a high order axis of symmetry Cn, n > 2) with a mirror plane (σ) perpendicular to it as symmetry elements; this experimentally demonstrates that the monomer does possess the typical sandwich structure of a metallocene although the interpretation of the ESR data has been questioned. The decomposition pathway of the monomer has also been studied by mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
. The dimerisation is a redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
process, the dimer being a rhodium(I) species whilst the monomer has a rhodium(II) metal centre. Rhodium
Rhodium
Rhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
typically occupies oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
s +I or +III in its stable compounds.
This dimerisation process has the overall effect of decreasing the electron count
Electron counting
Electron counting is a formalism used for classifying compounds and for explaining or predicting electronic structure and bonding. Many rules in chemistry rely on electron-counting:...
around the rhodium centre from 19 to 18. This occurs because the oxidative coupling of the two cyclopentadienyl ligands produces a new ligand with lower hapticity
Hapticity
The term hapticity is used to describe how a group of contiguous atoms of a ligand are coordinated to a central atom. Hapticity of a ligand is indicated by the Greek character 'eta', η. A superscripted number following the η denotes the number of contiguous atoms of the ligand that are bound to...
and which donates fewer electrons to the metal centre. The term hapticity is used to indicate the "number of carbon (or other) atoms through which [a ligand] binds (n)" to a metal centre and is symbolised as ηn. For example, the ethylene ligand in Zeise's salt is bound to the platinum centre through both carbon atoms, and it hence formally has the formula K[PtCl3(η2-C2H4)]·H2O. The carbonyl ligands in nickel tetracarbonyl are each bound through only a carbon atom and are hence described as monohapto ligands, but η1-notations are typically omitted in formulae. The cyclopentadienyl ligands in many metallocene
Metallocene
A metallocene is a compound typically consisting of two cyclopentadienyl anions bound to a metal center in the oxidation state II, with the resulting general formula 2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride, vanadocene dichloride...
and half-sandwich compounds are pentahapto ligands, hence the formula [Rh(η5-C5H5)2] for the rhodocene monomer. In the rhodocene dimer, the coupled cyclopentadienyl ligands are 4-electron tetrahapto donors to each rhodium(I) metal centre, in contrast to the 6-electron pentahapto cyclopentadienyl donors. The increased stability of the 18-valence electron rhodium(I) dimer species as compared to the 19-valence electron rhodium(II) monomer likely explains why the monomer is only detected under extreme conditions.
Cotton and Wilkinson demonstrated that the 18-valence electron rhodium(III) rhodocenium cation [Rh(η5-C5H5)2]+ can be reduced in aqueous solution to the monomeric form; however, they were unable to isolate the neutral product as not only can it dimerise, the rhodium(II) radical monomer can also spontaneously form the mixed-hapticity stable rhodium(I) species [(η5-C5H5)Rh(η4-C5H6)]. The differences between rhodocene and this derivative are found in two areas: (1) One of the bound cyclopentadienyl ligands has formally gained a hydrogen atom to become cyclopentadiene, which remains bound to the metal centre but now as a 4-electron η4- donor. (2) The rhodium(II) metal centre has been reduced to rhodium(I). These two changes make the derivative an 18-valence electron species. Fischer and colleagues hypothesised that the formation of this rhodocene derivative might occur in separate protonation and reduction steps, but published no evidence to support this suggestion. (η4-Cyclopentadiene)(η5-cyclopentadienyl)rhodium(I), the resulting compound, is an unusual organometallic complex in that it has both a cyclopentadienyl anion and cyclopentadiene itself as ligands. It has been shown that this compound can also be prepared by sodium borohydride
Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
reduction of a rhodocenium solution in aqueous ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
; the researchers who made this discovery characterised the product as biscyclopentadienylrhodium hydride.
Fischer and co-workers also studied the chemistry of iridocene, the third transition series analogue of rhodocene and cobaltocene, finding the chemistry of rhodocene and iridocene are generally similar. The synthesis of numerous iridocenium salts including the tribromide and hexafluorophosphate
Hexafluorophosphate
Hexafluorophosphate is an anion with chemical formula of . This octahedral species is isoelectronic with sulfur hexafluoride, SF6, and is valence isoelectronic with the highly stable superacid anion fluoroantimonate . As a non-coordinating anion, it is a poor nucleophile...
have been described. Just as with rhodocene, iridocene dimerises at room temperature but a monomer form can be detected at low temperatures and in gas phase and IR, NMR, and ESR measurements indicate a chemical equilibrium is present and confirm the sandwich structure of the iridocene monomer. The complex [(η5-C5H5)Ir(η4-C5H6)], the analogue of rhodocene derivative reported by Fischer, has also been studied and demonstrates properties consistent with a greater degree of π-backbonding in iridium(I) systems than is found in the analogous cobalt(I) or rhodium(I) cases.
Synthesis
Rhodocenium salts were first reported within two years of the discovery of ferrocene. These salts were prepared by reacting the carbanionCarbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
Grignard reagent
Grignard reaction
The Grignard reaction is an organometallic chemical reaction in which alkyl- or aryl-magnesium halides add to a carbonyl group in an aldehyde or ketone. This reaction is an important tool for the formation of carbon–carbon bonds...
cyclopentadienylmagnesium bromide (C5H5MgBr) with tris(acetylacetonato)rhodium(III) (Rh(acac)3). More recently, gas-phase rhodocenium cations have been generated by a redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
transmetalation
Transmetalation
Transmetalation is a general chemical reaction type in organometallic chemistry describing the exchange of ligands between two metal centers....
reaction of rhodium(I) ions with ferrocene or nickelocene
Nickelocene
Nickelocene is the organonickel compound with the formula Ni2. Also known as bisnickel or NiCp2, this bright green paramagnetic solid is of enduring academic interest, although it yet has no practical applications....
.
- Rh+ + [(η5-C5H5)2M] → M + [(η5-C5H5)2Rh]+ M = Ni or Fe
Modern microwave synthetic methods
Microwave chemistry
Microwave chemistry is the science of applying microwave irradiation to chemical reactions. Microwaves act as high frequency electric fields and will generally heat any material containing mobile electric charges, such as polar molecules in a solvent or conducting ions in a solid...
have also been reported. Rhodocenium hexafluorophosphate forms after reaction of cyclopentadiene and rhodium(III) chloride hydrate
Rhodium(III) chloride
Rhodium chloride refers to inorganic compounds with the formula RhCl3n, where n varies from 0 to 3. These are diamagnetic solids featuring octahedral Rh centres. Depending on the value of n, the material is either a dense brown solid or a soluble reddish salt...
in methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
following work-up with methanolic ammonium hexafluorophosphate
Ammonium hexafluorophosphate
Ammonium hexafluorophosphate is a commonly available form of the hexafluorophosphate anion....
; the reaction yield
Yield (chemistry)
In chemistry, yield, also referred to as chemical yield and reaction yield, is the amount of product obtained in a chemical reaction. The absolute yield can be given as the weight in grams or in moles...
exceeds 60% with only 30 seconds of exposure to microwave radiation.
- RhCl3.xH2O + 2 C5H6 + NH4PF6 → [(η5-C5H5)2Rh]PF6 + 2 HCl + NH4Cl + xH2O
Rhodocene itself is then formed by reduction of rhodocenium salts with molten sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
. If a rhodocenium containing melt is treated with sodium or potassium metals and then sublimed onto a liquid nitrogen-cooled cold finger, a black polycrystalline material results. Warming this material to room temperature produces a yellow solid which has been confirmed as the rhodocene dimer. A similar method can be used to prepare the iridocene dimer.
The [(η5-C5tBu3H2)Rh(η5-C5H5)]+ cation
Novel approaches to synthesising substituted cyclopentadienyl complexes have been developed using substituted vinylcyclopropene starting materials. Ring-enlarging vinylcyclopropane rearrangementVinylcyclopropane rearrangement
The vinylcyclopropane rearrangement or vinylcyclopropane-cyclopentene rearrangement is a ring expansion reaction, converting a vinyl-substituted cyclopropane ring into a cyclopentene ring. The general scope is extended by introducing heteroatoms in any of the 5 framework positions or by replacing...
reactions to produce cyclopentenes are well known and serve as precedent for vinylcyclopropenes rearranging
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
to cyclopentadienes. The [(η5-C5tBu3H2)Rh(η5-C5H5)]+ cation has been generated by a reaction sequence beginning with the addition the chlorobisethylenerhodium(I) dimer, [(η2-C2H4)2Rh(μ-Cl)]2, to 1,2,3-tri-tert-butyl-3-vinyl-1-cyclopropene followed by reaction with thallium cyclopentadienide:
The 18-valence electron rhodium(III) pentadienediyl species generated by this reaction demonstrates again the instability of the rhodocene moiety, in that it can be refluxed in toluene for months without 1,2,3-tri-tert-butylrhodocene forming but in oxidising conditions the 1,2,3-tri-tert-butylrhodocenium cation forms rapidly. Cyclic voltammetry has been used to investigate this and similar processes in detail. The mechanism of the reaction has been shown to involve a loss of one electron from the pentadienediyl ligand followed by a fast rearrangement (with loss of a hydrogen atom) to form the 1,2,3-tri-tert-butylrhodocenium cation. Both the tetrafluoroborate
Tetrafluoroborate
Tetrafluoroborate is the anion BF4−. This tetrahedral species is isoelectronic with tetrafluoromethane, CF4 and tetrafluoroammonium NF4+, and is valence isoelectronic with many stable and important species including the closely related anion perchlorate, ClO4−...
and hexafluorophosphate salts of this cation have been structurally characterised by X-ray crystallography.
[(η5-C5tBu3H2)Rh(η5-C5H5)]BF4 forms a colourless centrosymmetric monoclinic
Monoclinic crystal system
In crystallography, the monoclinic crystal system is one of the 7 lattice point groups. A crystal system is described by three vectors. In the monoclinic system, the crystal is described by vectors of unequal length, as in the orthorhombic system. They form a rectangular prism with a...
crystal belonging to the P21/c space group
Space group
In mathematics and geometry, a space group is a symmetry group, usually for three dimensions, that divides space into discrete repeatable domains.In three dimensions, there are 219 unique types, or counted as 230 if chiral copies are considered distinct...
, and with a density
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
of 1.486 g cm−3. Looking at the ORTEP
Molecular graphics
Molecular graphics is the discipline and philosophy of studying molecules and their properties through graphical representation. IUPAC limits the definition to representations on a "graphical display device"...
diagram of the structure of the cation (at right), it is evident that it possesses the typical geometry expected of a rhodocene or rhodocenium cation. The two cyclopentadienyl rings are close to parallel (the centroid
Centroid
In geometry, the centroid, geometric center, or barycenter of a plane figure or two-dimensional shape X is the intersection of all straight lines that divide X into two parts of equal moment about the line. Informally, it is the "average" of all points of X...
–Rh–centroid angle is 177.2°) and the rhodium centre is slightly closer to the substituted cyclopentadienyl ring (Rh–centroid distances are 1.819 Å
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
and 1.795 Å), an observation attributed to the greater inductive effect of the tert-butyl groups on the substituted ligand. The ORTEP diagram shows that the cation adopts an eclipsed conformation in the solid state. However, the crystal structure of the hexafluorophosphate salt shows three crystallographically independent cations, one eclipsed, one staggered, and one which is rotationally disordered. This suggests that the conformation adopted is dependent on the anion present and also illustrates that the energy barrier to rotation is low – in ferrocene, the rotational energy barrier is known to be ~5 kJ mol−1 in both solution and gas phase.
The diagram above shows the rhodium–carbon ( inside pentagons on the left) and carbon–carbon ( outside pentagons on the left) bond distances for both ligands, along with the bond angles ( inside pentagons on the right) within each cyclopentadienyl ring. The atom labels used are the same as those shown in the crystal structure above. Within the unsubstituted cyclopentadienyl ligand, the carbon–carbon bond lengths vary between 1.35 Å and 1.40 Å and the internal bond angles vary between 107° and 109°. For comparison, the internal angle at each vertex of a regular pentagon is 108°. The rhodium–carbon bond lengths vary between 2.16 Å and 2.18 Å. These results are consistent with η5-coordination of the ligand to the metal centre. In the case of the substituted cyclopentadienyl ligand, there is somewhat greater variation: carbon–carbon bond lengths vary between 1.39 Å and 1.48 Å, the internal bond angles vary between 106° and 111°, and the rhodium–carbon bond lengths vary between 2.14 Å and 2.20 Å. The greater variation in the substituted ligand is attributed to the distortions necessary to relieve the steric strain imposed by neighbouring tert-butyl substituents; despite these variations, the data demonstrate that the substituted cyclopentadienyl is also η5-coordinated.
The stability of metallocenes changes with ring substitution. Comparing the reduction potentials of the cobaltocenium and decamethylcobaltocenium cations shows that the decamethyl species is ca. 600 mV more reducing than its parent metallocene, a situation also observed in the ferrocene and rhodocene systems. The following data are presented relative to the ferrocenium / ferrocene redox couple:
- [Fe(C5H5)2]+ / [Fe(C5H5)2] 0 V (by definition)
- [Fe(C5Me5)2]+ / [Fe(C5Me5)2] −0.59 V
- [Co(C5H5)2]+ / [Co(C5H5)2] −1.33 V
- [Co(C5Me5)2]+ / [Co(C5Me5)2] −1.94 V
- [Rh(C5H5)2]+ / [Rh(C5H5)2] −1.79 V (after correcting by 0.38 V for the different standard)
- [Rh(C5Me5)2]+ / [Rh(C5Me5)2] −2.38 V
- [(C5tBu3H2)Rh(C5H5)]+ / [(C5tBu3H2)Rh(C5H5)] −1.83 V
- [(C5tBu3H2)Rh(C5Me5)]+ / [(C5tBu3H2)Rh(C5Me5)] −2.03 V
- [(C5H5Ir(C5Me5)]+ / [(C5H5Ir(C5Me5)] −2.41 V (after correcting by 0.38 V for the different standard)
- [Ir(C5Me5)2]+ / [Ir(C5Me5)2] −2.65 V (after correcting by 0.38 V for the different standard)
The differences in reduction potentials is attributed in the cobaltocenium system to the inductive effect of the alkyl groups, further stabilising the 18-valence electron species. A similar effect is seen in the rhodocenium data shown above, again consistent with inductive effects. In the substituted iridocenium system, cyclic voltammetry investigations shows irreversible reductions at temperatures as low as −60 °C; by comparison, the reduction of the corresponding rhodocenes is quasi-reversible at room temperature and fully reversible at −35 °C. The irreversibility of the substituted iridocenium reductions is attributed to the extremely rapid dimerisation of the resulting 19-valence electron species, which further illustrates that iridocenes are less stable than their corresponding rhodocenes.
Penta-substituted cyclopentadienyl ligands
The body of knowledge concerning compounds with penta-substituted cyclopentadienyl ligands is extensive, with organometallic complexesComplex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
of the pentamethylcyclopentadienyl and pentaphenylcyclopentadienyl ligands being well-known. Substitutions on the cyclopentadienyl rings of rhodocenes and rhodocenium salts produce compounds of higher stability as they allow for the increased delocalisation of positive charge or electron density
Electron density
Electron density is the measure of the probability of an electron being present at a specific location.In molecules, regions of electron density are usually found around the atom, and its bonds...
whilst also providing steric hindrance against other species approaching the metal centre. Various mono- and di-substituted rhodocenium species are known, but substantial stabilisation is not achieved without greater substitutions. Known highly substituted rhodocenium salts include decamethylrhodocenium hexafluorophosphate [(η5-C5Me5)2Rh]PF6, decaisopropylrhodocenium hexafluorophosphate [(η5-C5iPr
Isopropyl
In organic chemistry, isopropyl is a propyl with a group attached to the secondary carbon. If viewed as a functional group an isopropyl is an organic compound with a propyl group attached at its secondary carbon.The bond is therefore on the middle carbon....
5)2Rh]PF6, and octaphenylrhodocenium hexafluorophosphate [(η5-C5Ph4H)2Rh]PF6. Decamethylrhodocenium tetrafluoroborate can be synthesised from the tris(acetone) complex [(η5-C5Me5)Rh(Me2CO)3](BF4)2 by reaction with pentamethylcyclopentadiene
Pentamethylcyclopentadiene
1,2,3,4,5-Pentamethylcyclopentadiene is a cyclic diolefin with the formula C5Me5H . 1,2,3,4,5-Pentamethylcyclopentadiene is the precursor to the ligand 1,2,3,4,5-pentamethylcyclopentadienyl, which is often denoted as Cp*...
, and the analogous iridium synthesis is also known. Decaisopropylrhodicnium hexafluorophosphate was synthesised in 1,2-dimethoxyethane (solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
) in an unusual one-pot synthesis
One-pot synthesis
In chemistry a one-pot synthesis is a strategy to improve the efficiency of a chemical reaction whereby a reactant is subjected to successive chemical reactions in just one reactor...
that involves the formation of 20 carbon–carbon bonds:
In a similar reaction, pentaisopropylrhodocenium hexafluorophosphate [(η5-C5iPr5)Rh(η5-C5H5)]PF6 can be synthesised from pentamethylrhodocenium hexafluorophosphate [(η5-C5Me5)Rh(η5-C5H5)]PF6 in 80% yield. These reactions demonstrate that the acidity of the methyl hydrogens in a pentamethylcyclopentadienyl complex can be considerably increased by the presence of the metal centre. Mechanistically, the reaction proceeds with potassium hydroxide
Potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
deprotonating a methyl group and the resulting carbanion undergoing nucleophilic substitution
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
with methyl iodide to form a new carbon–carbon bond.
The compounds pentaphenylrhodocenium tetrafluoroborate
Tetrafluoroborate
Tetrafluoroborate is the anion BF4−. This tetrahedral species is isoelectronic with tetrafluoromethane, CF4 and tetrafluoroammonium NF4+, and is valence isoelectronic with many stable and important species including the closely related anion perchlorate, ClO4−...
[(η5-C5Ph5)Rh(η5-C5H5)]BF4, and pentamethylpentaphenylrhodocenium tetrafluoroborate [(η5-C5Ph5)Rh(η5-C5Me5)]BF4 have also been reported. They demonstrate that rhodium sandwich compounds can be prepared from half-sandwich precursors. For example, in an approach broadly similar to the tris(acetone) synthesis of decamethylrhodocenium tetrafluoroborate, pentaphenylrhodocenium tetrafluoroborate has been synthesised from the tris(acetonitrile
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
) salt [(η5-C5Ph5)Rh(CH3CN)3](BF4)2 by reaction with sodium cyclopentadienide
Sodium cyclopentadienide
Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp or CpNa, where Cp− is the cyclopentadienide anion. Cp is also used as an abbreviation for the cyclopentadienyl ligand in coordination chemistry.-Preparation:Sodium...
:
- [(η5-C5Ph5)Rh(MeCN)3](BF4)2 + NaC5H5 → [(η5-C5Ph5)Rh(η5-C5H5)]BF4 + NaBF4 + 3 MeCN
Octaphenylrhodocene, [(η5-C5Ph4H)2Rh], is the first rhodocene derivative to be isolated at room temperature. Its olive green crystals decompose rapidly in solution, and within minutes in air, demonstrating a dramatically greater air sensitivity than the analogous cobalt
Cobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
complex, although it is significantly more stable than rhodocene itself. This difference is attributed to the relatively lower stability of the rhodium(II) state as compared to the cobalt(II) state. The reduction potential for the [(η5-C5Ph4H)2Rh]+ cation (measured in dimethylformamide
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
relative the ferrocenium / ferrocene couple) is −1.44 V, consistent with the greater thermodynamic stabilisation of the rhodocene by the C5HPh4 ligand compared with the C5H5 or C5Me5 ligands. Cobaltocene is a useful one-electron reducing agent
Reducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
in the research laboratory as it is soluble in non-polar organic solvents, and its redox couple is sufficiently well behaved that it may be used as an internal standard
Internal standard
An internal standard in analytical chemistry is a chemical substance that is added in a constant amount to samples, the blank and calibration standards in a chemical analysis. This substance can then be used for calibration by plotting the ratio of the analyte signal to the internal standard signal...
in cyclic voltammetry
Cyclic voltammetry
Cyclic voltammetry or CV is a type of potentiodynamic electrochemical measurement. In a cyclic voltammetry experiment the working electrode potential is ramped linearly versus time like linear sweep voltammetry. Cyclic voltammetry takes the experiment a step further than linear sweep voltammetry...
. No substituted rhodocene yet prepared has demonstrated sufficient stability to be used in a similar way.
The synthesis of octaphenylrhodocene proceeds in three steps, with a diglyme
Diglyme
Diglyme, or bis ether, is a solvent with a high boiling point. It is an organic compound which is the dimethyl ether of diethylene glycol. It is a clear, colorless liquid with a slight ether-like odor...
reflux followed by workup with hexafluorophosphoric acid
Hexafluorophosphoric acid
Hexafluorophosphoric acid is the chemical compound with the chemical formula HPF6. This strong Brønsted acid features a non-coordinating anion, hexafluorophosphate . It is formed from the reaction of hydrogen fluoride with phosphorus pentafluoride....
, then a sodium amalgam
Sodium amalgam
Sodium amalgam, commonly denoted Na, is an alloy of mercury and sodium. The term amalgam is used for alloys, intermetallic compounds, and solutions involving mercury as a major component. Sodium amalgam is often used in reactions as strong reducing agents with better handling properties compared...
reduction in tetrahydrofuran
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
:
- Rh(acac)3 + 2 KC5Ph4H → [(η5-C5Ph4H)2Rh]+ + 2 K+ + 3 acac−
- [(η5-C5Ph4H)2Rh]+ + 3 acac− + 3 HPF6 → [(η5-C5Ph4H)2Rh]PF6 + 3 HacacAcetylacetoneAcetylacetone is an organic compound that famously exists in two tautomeric forms that rapidly interconvert. The less stable tautomer is a diketone formally named pentane-2,4-dione. The more common tautomer is the enol form. The pair of tautomers rapidly interconvert and are treated as a single...
+ 2 PF6−
- [(η5-C5Ph4H)2Rh]PF6 + Na/Hg → [(η5-C5Ph4H)2Rh] + NaPF6
The crystal structure
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
of octaphenylrhodocene shows a staggered conformation (similar to that of ferrocene, and in contrast to the eclipsed conformation of ruthenocene
Ruthenocene
Ruthenocene is an organoruthenium compound with the formula 2Ru. This pale yellow, volatile solid is classified as a sandwich compound and more specifically, as a metallocene.-Structure and bonding:...
). The rhodium–centroid distance is 1.904 Å and the rhodium–carbon bond lengths average 2.26 Å, whilst the carbon–carbon bond lengths average 1.44 Å. These distances are all similar to those found in the 1,2,3-tri-tert-butylrhodocenium cation described above, with the one difference that the effective size of the rhodium centre appears larger, an observation consistent with the expanded ionic radius of rhodium(II) compared with rhodium(III).
Biomedical use of a derivative

Metallopharmaceutical
A metallopharmaceutical is a drug that contains a metal as an active ingredient. Most commonly metallopharmaceuticals are used as anticancer or antimicrobial agents.Examples of metallopharmaceuticals include:...
s, including discussion of rhodium compounds in medicine. A substantial body of research has examined using metallocene derivatives of ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
and iron as metallopharmaceuticals. One area of such research has utilised metallocenes in place of the fluorophenyl group in haloperidol
Haloperidol
Haloperidol is a typical antipsychotic. It is in the butyrophenone class of antipsychotic medications and has pharmacological effects similar to the phenothiazines....
, which is a pharmaceutical classified as a typical antipsychotic
Typical antipsychotic
Typical antipsychotics are a class of antipsychotic drugs first developed in the 1950s and used to treat psychosis...
. The ferrocenyl–haloperidol compound investigated has structure (C5H5)Fe(C5H4)–C(=O)–(CH2)3–N(CH2CH2)2C(OH)–C6H4Cl and can be converted to the ruthenium analog via a transmetalation reaction. Using the radioactive isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
103Ru produces a ruthenocenyl–haloperidol radiopharmaceutical with a high affinity for lung
Lung
The lung is the essential respiration organ in many air-breathing animals, including most tetrapods, a few fish and a few snails. In mammals and the more complex life forms, the two lungs are located near the backbone on either side of the heart...
but not brain
Brain
The brain is the center of the nervous system in all vertebrate and most invertebrate animals—only a few primitive invertebrates such as sponges, jellyfish, sea squirts and starfishes do not have one. It is located in the head, usually close to primary sensory apparatus such as vision, hearing,...
tissue
Tissue (biology)
Tissue is a cellular organizational level intermediate between cells and a complete organism. A tissue is an ensemble of cells, not necessarily identical, but from the same origin, that together carry out a specific function. These are called tissues because of their identical functioning...
in mice
MICE
-Fiction:*Mice , alien species in The Hitchhiker's Guide to the Galaxy*The Mice -Acronyms:* "Meetings, Incentives, Conferencing, Exhibitions", facilities terminology for events...
and rat
Rat
Rats are various medium-sized, long-tailed rodents of the superfamily Muroidea. "True rats" are members of the genus Rattus, the most important of which to humans are the black rat, Rattus rattus, and the brown rat, Rattus norvegicus...
s. Beta-decay of 103Ru produces the metastable isotope 103mRh in a rhodocenyl–haloperidol compound. This compound, like other rhodocene derivatives, has an unstable 19-valence electron configuration and rapidly oxidises to the expected cationic rhodocenium–haloperidol species. The separation of the ruthenocenyl–haloperidol and the rhodocenium–haloperidol species and the distributions of each amongst bodily organs has been studied. 103mRh has a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 56 min and emits a gamma ray
Gamma ray
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency . Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei...
of energy 39.8 keV
Kev
Kev can refer to:*Kev Hawkins, a fictional character.*Kevin, a given name occasionally shortened to "Kev".*Kiloelectronvolt, a unit of energy who symbol is "KeV".* Krefelder Eislauf-VereinKEV can refer to:...
, so the gamma-decay of the rhodium isotope should follow soon after the beta-decay of the ruthenium isotope. Beta- and gamma-emitting radionuclide
Radionuclide
A radionuclide is an atom with an unstable nucleus, which is a nucleus characterized by excess energy available to be imparted either to a newly created radiation particle within the nucleus or to an atomic electron. The radionuclide, in this process, undergoes radioactive decay, and emits gamma...
s used medically include 131I
Iodine-131
Iodine-131 , also called radioiodine , is an important radioisotope of iodine. It has a radioactive decay half-life of about eight days. Its uses are mostly medical and pharmaceutical...
, 59Fe, and 47Ca, and 103mRh has been proposed for use in radiotherapy for small tumours.
Metal–metal interactions in linked metallocenes
The original motivation for research investigations of the rhodocene system was to understand the nature of and bonding within the metallocene class of compounds. In more recent times, interest has been rekindled by the desire to explore and apply the metal–metal interactions that occur when metallocene systems are linked. Potential applications for such systems include molecular electronicsMolecular electronics
Molecular electronics, sometimes called moletronics, involves the study and application of molecular building blocks for the fabrication of electronic components...
, semi-conducting (and possibly ferromagnetic) metallocene polymers (an example of a molecular wire), and exploring the threshold between heterogeneous
Heterogeneous catalysis
In chemistry, heterogeneous catalysis refers to the form of catalysis where the phase of the catalyst differs from that of the reactants. Phase here refers not only to solid, liquid, vs gas, but also immiscible liquids, e.g. oil and water. The great majority of practical heterogeneous catalysts...
and homogeneous catalysis
Homogeneous catalysis
In chemistry, homogeneous catalysis is a sequence of reactions that involve a catalyst in the same phase as the reactants. Most commonly, a homogeneous catalyst is codissolved in a solvent with the reactants.-Acid catalysis:...
. Examples of known bimetallocenes and termetallocenes that possess the rhodocenyl moiety include the hexafluorophosphate salts of rhodocenylferrocene, 1,1'-dirhodocenylferrocene, and 1-cobaltocenyl-1'-rhodocenylferrocene, each shown at right. Linked metallocenes can also be formed by introducing several metallocenyl substituents onto a single cyclopentadienyl ligand.
Structural studies of termetallocene systems have shown they typically adopt an "eclipsed double transoid" "crankshaft" geometry. Taking as an example the 1-cobaltocenyl-1'-rhodocenylferrocene cation shown above, this means that the cobaltocenyl and rhodocenyl moieties are eclipsed, and thus carbon atoms 1 and 1' on the central ferrocene core are as close to vertically aligned as is possible given the staggered conformation of the cyclopentadienyl rings within each metallocene unit. Viewed from side-on, this means termetallocenes resemble the down–up–down pattern of a crankshaft
Crankshaft
The crankshaft, sometimes casually abbreviated to crank, is the part of an engine which translates reciprocating linear piston motion into rotation...
. The synthesis of this termetallocene involves the combining of rhodocenium and cobaltocenium solutions with 1,1'-dilithioferrocene. This produces an uncharged intermediate with linked cyclopentadienyl–cyclopentadiene ligands whose bonding resembles that found in the rhodocene dimer. These ligands then react with the triphenylmethyl carbocation
Triphenylmethyl hexafluorophosphate
Triphenylmethyl hexafluorophosphate is an organic salt with the formula C3PF6. This compound is a brown powder that is air sensitive and changes colors when exposed to light. Triphenylmethyl hexafluorophosphate is used as a catalyst and reagent in organic syntheses.-Preparation:Triphenylmethyl...
to generate the termetallocene salt, [(η5-C5H5)Rh(μ-η5:η5-C5H4–C5H4)Fe(μ-η5:η5-C5H4–C5H4)Co(η5-C5H5)](PF6)2. This synthetic pathway is illustrated below:

