
Cyclic voltammetry
Encyclopedia
Voltammetry
Voltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes. In voltammetry, information about an analyte is obtained by measuring the current as the potential is varied.- Three electrode system :...
electrochemical
Electrochemistry
Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
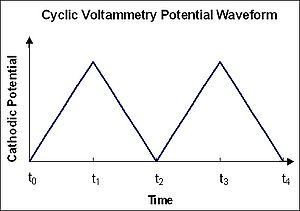
measurement. In a cyclic voltammetry experiment the working electrode potential is ramped linearly versus time like linear sweep voltammetry
Linear sweep voltammetry
Linear sweep voltammetry is a voltammetric method where the current at a working electrode is measured while the potential between the working electrode and a reference electrode is swept linearly in time...
. Cyclic voltammetry takes the experiment a step further than linear sweep voltammetry which ends when it reaches a set potential. When cyclic voltammetry reaches a set potential, the working electrode's potential ramp is inverted. This inversion can happen multiple times during a single experiment. The current at the working electrode is plotted versus the applied voltage to give the cyclic voltammogram trace. Cyclic voltammetry is generally used to study the electrochemical properties of an analyte
Analyte
An analyte, or component , is a substance or chemical constituent that is of interest in an analytical procedure. Grammatically, it is important to note that experiments always seek to measure properties of analytes—and that analytes themselves can never be measured. For instance, one cannot...
in solution.
Experimental method
For instance if the electronic transfer at the surface is fast and the current is limited by the diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
of species to the electrode surface, then the current peak will be proportional to the square root
Square root
In mathematics, a square root of a number x is a number r such that r2 = x, or, in other words, a number r whose square is x...
of the scan rate. This relationship is described by the Cottrell equation
Cottrell equation
In electrochemistry, the Cottrell equation describes the change in electric current with respect to time in a controlled potential experiment, such as chronoamperometry. Specifically it describes the current response when the potential is a step function. It was derived by Frederick Gardner...
.
Characterization
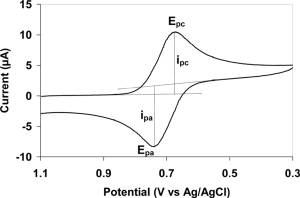
The utility of cyclic voltammetry is highly dependent on the analyte being studied. The analyte has to be redox active within the experimental potential window. It is also highly desirable for the analyte to display a reversible wave. A reversible wave is when an analyte is reduced or oxidized on a forward scan and is then reoxidized or rereduced in a predictable way on the return scan as shown in the first figure.Even reversible couples contain polarization overpotential
Overpotential
Overpotential is an electrochemical term which refers to the potential difference between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly related to a cell's voltage efficiency...
and thus display a hysteresis between absolute potential between the reduction (Epc) and oxidation peak (Epa). This overpotential emerges from a combination of analyte diffusion rates and the intrinsic activation barrier of transferring electrons from an electrode to analyte. A theoretical description of polarization overpotential is in part described by the Butler-Volmer equation and Cottrell equation
Cottrell equation
In electrochemistry, the Cottrell equation describes the change in electric current with respect to time in a controlled potential experiment, such as chronoamperometry. Specifically it describes the current response when the potential is a step function. It was derived by Frederick Gardner...
. Conveniently in an ideal system the relationships reduces to,
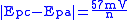 , for an n electron process.
, for an n electron process.Reversible couples will display a ratio of the peak currents passed at reduction (ipc) and oxidation (ipa) that is near unity (1 = ipa/ipc). This ratio can be perturbed for reversible couples in the presence of a following chemical reaction
Electrochemical reaction mechanism
In chemistry, an electrochemical reaction mechanism is the step by step sequence of elementary steps, involving at least one outer sphere electron transfer, by which an overall chemical change occurs .- Overview :...
, stripping wave, or nucleation event.
When such reversible peaks are observed thermodynamic information
Bordwell thermodynamic cycle
A Bordwell thermodynamic cycle use experimentally determined and reasonable estimates of Gibbs free energy values to determine unknown and experimentally inaccessible values.- Overview :...
in the form of half cell potential E01/2 can be determined. When waves are semi-reversible such as when ipa/ipc is less than or greater than 1, it can be possible to determine even more information especially kinetic processes like following chemical reaction
Electrochemical reaction mechanism
In chemistry, an electrochemical reaction mechanism is the step by step sequence of elementary steps, involving at least one outer sphere electron transfer, by which an overall chemical change occurs .- Overview :...
.
When waves are non-reversible it is impossible to determine what their thermodynamic E01/2 is with cyclic voltammetry. This E01/2 can be determined, however it often requires equal quantities of the analyte in both oxidation states. When a wave is non-reversible cyclic voltammetry can not determine if the wave is at its thermodynamic potential or shifted to a more extreme potential by some form of overpotential
Overpotential
Overpotential is an electrochemical term which refers to the potential difference between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly related to a cell's voltage efficiency...
. The couple could be irreversible because of a following chemical process, a common example for transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
s is a shift in the geometry of the coordination
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
sphere. If this is the case, then higher scan rates may show a reversible wave. It is also possible that the wave is irreversible due to a physical process most commonly some form of precipitation
Precipitation (chemistry)
Precipitation is the formation of a solid in a solution or inside anothersolid during a chemical reaction or by diffusion in a solid. When the reaction occurs in a liquid, the solid formed is called the precipitate, or when compacted by a centrifuge, a pellet. The liquid remaining above the solid...
as discussed below. Some speculation can be made in regards to irreversible waves however they are generally outside the scope of cyclic voltammetry.
Experimental setup
The method uses a reference electrodeReference electrode
A reference electrode is an electrode which has a stable and well-known electrode potential. The high stability of the electrode potential is usually reached by employing a redox system with constant concentrations of each participants of the redox reaction.There are many ways reference...
, working electrode
Working electrode
The working electrode is the electrode in an electrochemical system on which the reaction of interest is occurring. The working electrode is often used in conjunction with an auxiliary electrode, and a reference electrode in a three electrode system...
, and counter electrode which in combination are sometimes referred to as a three-electrode setup
Voltammetry
Voltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes. In voltammetry, information about an analyte is obtained by measuring the current as the potential is varied.- Three electrode system :...
. Electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
is usually added to the test solution to ensure sufficient conductivity. The combination of the solvent, electrolyte and specific working electrode material determines the range of the potential.
Electrodes are static and sit in unstirred solutions during cyclic voltammetry. This "still" solution method results in cyclic voltammetry's characteristic diffusion controlled peaks. This method also allows a portion of the analyte
Analyte
An analyte, or component , is a substance or chemical constituent that is of interest in an analytical procedure. Grammatically, it is important to note that experiments always seek to measure properties of analytes—and that analytes themselves can never be measured. For instance, one cannot...
to remain after reduction or oxidation where it may display further redox activity. Stirring the solution between cyclic voltammetry traces is important as to supply the electrode surface with fresh analyte for each new experiment. The solubility of an analyte can change drastically with its overall charge. Since cyclic voltammetry usually alters the charge of the analyte it is common for reduced or oxidized analyte to precipitate out onto the electrode. This layering of analyte can insulate the electrode surface, display its own redox activity in subsequent scans, or at the very least alter the electrode surface. For this and other reasons it is often necessary to clean electrodes between scans.
Common materials for working electrode
Working electrode
The working electrode is the electrode in an electrochemical system on which the reaction of interest is occurring. The working electrode is often used in conjunction with an auxiliary electrode, and a reference electrode in a three electrode system...
s include glassy carbon
Glassy carbon
Glassy carbon, also called vitreous carbon, is a non-graphitizing carbon which combines glassy and ceramic properties with those of graphite. The most important properties are high temperature resistance, hardness , low density, low electrical resistance, low friction, low thermal resistance,...
, platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
, and gold
Gold
Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a...
. These electrodes are generally encased in a rod of inert insulator with a disk exposed at one end. A regular working electrode has a radius within an order of magnitude of 1 mm. Having a controlled surface area with a defined shape is important for interpreting cyclic voltammetry results.
To run cyclic voltammetry experiments at high scan rates a regular working electrode is insufficient. High scan rates create peaks with large currents and increased resistances which result in distortions. Ultramicroelectrode
Ultramicroelectrode
An Ultramicroelectrode is a working electrode used in a three electrode system. The small size of UME give them relatively large diffusion layers and small overall currents. These features allow UME to achieve useful steady-state conditions and very high scan rates with limited distortion...
s can be used to minimize the current and resistance.
The counter electrode, also known as the auxiliary or second electrode, can be any material which conducts easily and won't react with the bulk solution. Reactions occurring at the counter electrode surface are unimportant as long as it continues to conduct current well. To maintain the observed current the counter electrode will often oxidize or reduce the solvent or bulk electrolyte.
Reference electrode
Reference electrode
A reference electrode is an electrode which has a stable and well-known electrode potential. The high stability of the electrode potential is usually reached by employing a redox system with constant concentrations of each participants of the redox reaction.There are many ways reference...
s are a complex subject and worth investigating elsewhere.
Variations
In some experiments an electroactive species is fixed to the surface of the electrode, for instance in microparticle voltammetry.Potentiodynamic techniques also exist that add low-amplitude ac perturbation to a potential ramp and measure variable response in a single frequency (ac voltammetry) or in many frequencies simultaneously (potentiodynamic electrochemical impedance spectroscopy). The response in alternating current is two-dimensional – it is characterised by amplitude
Amplitude
Amplitude is the magnitude of change in the oscillating variable with each oscillation within an oscillating system. For example, sound waves in air are oscillations in atmospheric pressure and their amplitudes are proportional to the change in pressure during one oscillation...
and phase
Phase (waves)
Phase in waves is the fraction of a wave cycle which has elapsed relative to an arbitrary point.-Formula:The phase of an oscillation or wave refers to a sinusoidal function such as the following:...
. The amplitude and phase depend differently on frequency for constituents of ac response attributed to different processes (charge transfer, diffusion, double layer charging, etc.). Frequency response
Frequency response
Frequency response is the quantitative measure of the output spectrum of a system or device in response to a stimulus, and is used to characterize the dynamics of the system. It is a measure of magnitude and phase of the output as a function of frequency, in comparison to the input...
analysis enables simultaneous monitoring of the various processes that contribute to the potentiodynamic ac response of electrochemical system.
Distinctions
Cyclic voltammetry is not a hydrodynamic techniqueHydrodynamic technique
Hydrodynamic technique is a subcategory of electroanalytical methods in which the analyte solution flows relative to a working electrode. In many voltammetry techniques, the solution is intentionally left still to allow diffusion controlled mass transfer...
. In a hydrodynamic technique flow is achieved at the electrode surface by stirring the solution, pumping the solution, or rotating the electrode as is the case with rotating disk electrode
Rotating disk electrode
A rotating disk electrode is a hydrodynamic working electrode used in a three electrode system. The electrode rotates during experiments inducing a flux of analyte to the electrode. These working electrodes are used in electrochemical studies when investigating reaction mechanisms related to...
s and rotating ring-disk electrode
Rotating ring-disk electrode
A rotating ring-disk electrode is double working electrode used in hydrodynamic voltammetry, very similar to a rotating disk electrode . The electrode actually rotates during experiments inducing a flux of analyte to the electrode...
s. These techniques target steady state conditions which appear the same scanned from the positive or the negative, thus limiting them to linear sweep voltammetry
Linear sweep voltammetry
Linear sweep voltammetry is a voltammetric method where the current at a working electrode is measured while the potential between the working electrode and a reference electrode is swept linearly in time...
.

