
Saturated calomel electrode
Encyclopedia

Reference electrode
A reference electrode is an electrode which has a stable and well-known electrode potential. The high stability of the electrode potential is usually reached by employing a redox system with constant concentrations of each participants of the redox reaction.There are many ways reference...
based on the reaction between elemental mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
and mercury(I) chloride
Mercury(I) chloride
Mercury chloride is the chemical compound with the formula Hg2Cl2. Also known as calomel or mercurous chloride, this dense white or yellowish-white, odorless solid is the principal example of a mercury compound...
. The aqueous phase in contact with the mercury and the mercury(I) chloride (Hg2Cl2, "calomel
Mercury(I) chloride
Mercury chloride is the chemical compound with the formula Hg2Cl2. Also known as calomel or mercurous chloride, this dense white or yellowish-white, odorless solid is the principal example of a mercury compound...
") is a saturated solution of potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
chloride in water. The electrode is normally linked via a porous frit to the solution in which the other electrode is immersed. This porous frit is a salt bridge
Salt bridge
A salt bridge, in chemistry, is a laboratory device used to connect the oxidation and reduction half-cells of a galvanic cell , a type of electrochemical cell...
.
In cell notation
Cell notation
Cell notation in chemistry is a shorthand way of expressing a certain reaction in an electrochemical cell. The cell anode and cathode are separated by two bars or slashes representing a salt bridge, with the anode on the left and cathode on the right. Individual solid, liquid or aqueous phases...
the electrode is written as:
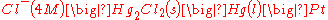
Theory of operation
The electrode is based on the redox reaction
The Nernst equation
Nernst equation
In electrochemistry, the Nernst equation is an equation that can be used to determine the equilibrium reduction potential of a half-cell in an electrochemical cell. It can also be used to determine the total voltage for a full electrochemical cell...
for this reaction is

where E0 is the standard electrode potential
Standard electrode potential
In electrochemistry, the standard electrode potential, abbreviated E° or E , is the measure of individual potential of a reversible electrode at standard state, which is with solutes at an effective concentration of 1 mol dm−3, and gases at a pressure of 1 atm...
for the reaction and aHg is the activity
Activity (chemistry)
In chemical thermodynamics, activity is a measure of the “effective concentration” of a species in a mixture, meaning that the species' chemical potential depends on the activity of a real solution in the same way that it would depend on concentration for an ideal solution.By convention, activity...
for the mercury cation (the activity for a liquid of 1 Molar is 1).
This activity can be found from the solubility product
Solubility equilibrium
Solubility equilibrium is a type of dynamic equilibrium. It exists when a chemical compound in the solid state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation or with chemical reaction with another constituent of the solvent, such as...
of the reaction

By replacing the activity in the Nernst equation with the value in the solubility equation, we get
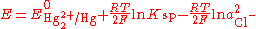
The only variable in this equation is the activity (or concentration) of the chloride anion. But since the inner solution is saturated with potassium chloride, this activity is fixed by the solubility of potassium chloride. When saturated the redox potential of the calomel electrode is +0.2444 V vs. SHE
Standard hydrogen electrode
The standard hydrogen electrode , is a redox electrode which forms the basis of the thermodynamic scale of oxidation-reduction potentials...
at 25 °C, but slightly higher when the chloride solution is less than saturated. For example, a 3.5M KCl electrolyte solution increases the reference potential to +0.250 V vs. SHE at 25 °C, and a 0.1 M solution to +0.3356 V at the same temperature.
Application
The SCE is used in pHPH meter
A pH meter is an electronic instrument used for measuring the pH of a liquid...
measurement, cyclic voltammetry
Cyclic voltammetry
Cyclic voltammetry or CV is a type of potentiodynamic electrochemical measurement. In a cyclic voltammetry experiment the working electrode potential is ramped linearly versus time like linear sweep voltammetry. Cyclic voltammetry takes the experiment a step further than linear sweep voltammetry...
and general aqueous electrochemistry
Electrochemistry
Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
.
This electrode and the silver/silver chloride reference electrode
Silver chloride electrode
A silver chloride electrode is a type of reference electrode, commonly used in electrochemical measurements. For example, it is usually the internal reference electrode in pH meters...
work in the same way. In both electrodes, the activity of the metal ion is fixed by the solubility of the metal salt.
The calomel electrode contains mercury, which poses much greater health hazards than the silver metal used in the Ag/AgCl electrode.
See also
- Cyclic voltammetryCyclic voltammetryCyclic voltammetry or CV is a type of potentiodynamic electrochemical measurement. In a cyclic voltammetry experiment the working electrode potential is ramped linearly versus time like linear sweep voltammetry. Cyclic voltammetry takes the experiment a step further than linear sweep voltammetry...
- Standard Hydrogen ElectrodeStandard hydrogen electrodeThe standard hydrogen electrode , is a redox electrode which forms the basis of the thermodynamic scale of oxidation-reduction potentials...
- Table of standard electrode potentials
- Reference electrodeReference electrodeA reference electrode is an electrode which has a stable and well-known electrode potential. The high stability of the electrode potential is usually reached by employing a redox system with constant concentrations of each participants of the redox reaction.There are many ways reference...

