Metal carbonyl
Encyclopedia
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
s with carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
ligands. These complexes may be homoleptic
Homoleptic
In inorganic chemistry, a homoleptic chemical compound is a metal compound with all ligands identical. The term uses a homo prefix to indicate that something is the same for all....
, that is containing only CO ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s, such as nickel carbonyl
Nickel carbonyl
Nickel carbonyl is the organonickel compound with the formula Ni4. This pale-yellow liquid is the principal carbonyl of nickel. It is an intermediate in the Mond process for the purification of nickel and a reagent in organometallic chemistry...
(Ni(CO)4), but more commonly metal carbonyls contain a mix of ligands, such as Re(CO)3(2,2'-bipyridine
2,2'-Bipyridine
2,2'-Bipyridine is a organic compound with the formula . This colorless solid, commonly abbreviated bipy or bpy , is an important isomer of the bipyridine family. It is a bidentate chelating ligand, forming complexes with many transition metals...
)Cl. Carbon monoxide is an important building block for the synthesis of many compounds, for example hydroformylation
Hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group and a hydrogen atom to a carbon-carbon double bond...
, and metal carbonyl catalysts are central to its utilization. Metal carbonyls are toxic, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin
Carboxyhemoglobin
Carboxyhemoglobin is a stable complex of carbon monoxide and hemoglobin that forms in red blood cells when carbon monoxide is inhaled or produced in normal metabolism. Large quantities of it hinder delivery of oxygen to the body...
, which will not bind O2.
Structure and properties

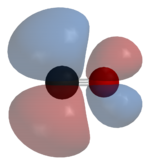
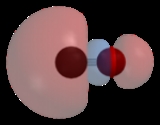
Carbon monoxide bonds to transition metals using "synergic π* back-bonding." The bonding has three components, giving rise to a partial triple bond. A sigma bond
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
arises from overlap of nonbonding electron pair on carbon with a blend of d-, s-, and p-orbitals on the metal. A pair of π bonds arises from overlap of filled d-orbitals on the metal with a pair of π-antibonding orbitals projecting from the carbon of the CO. The latter kind of binding requires that the metal have d-electrons, and that the metal is in a relatively low oxidation state (<+2). The π-bonding has the effect of weakening the carbon-oxygen bond
Carbon-oxygen bond
A carbon–oxygen bond is a covalent bond between carbon and oxygen and one of the most abundant in organic chemistry and biochemistry. Oxygen has 6 valence electrons and prefers to share two electrons in bonding with carbon, leaving the remaining 4 nonbonding electrons in 2 lone pairs...
compared with free carbon monoxide. Because of the multiple bond character of the M-CO linkage, the distance between the metal and carbon is relatively short, often < 1.8 Â, about 0.2 Â shorter than a metal-alkyl bond.
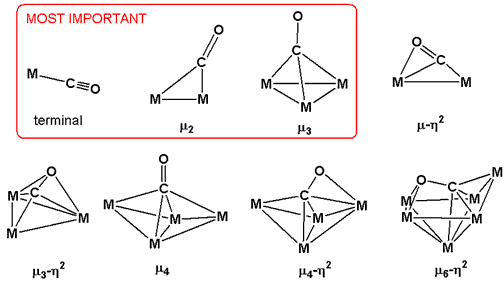
Bonding modes in clusters
The carbonyl ligand engages in a range of bonding modes in metal carbonyl cluster chemistry. Most frequently CO binds in the familiar terminal mode (see above), but CO often bridges between two (μ2) or three (μ3) metals. Much less common are bonding modes in which both C and O bond to the metal, e.g. μ3-η2.The increased π-bonding due to back-donation from multiple metal centers results in further weakening of the C-O bond.
Characterization
| Compound | νCO (cm-1) |
|---|---|
| CO | 2143 |
| Ti(CO)6-2 | 1748 |
| V(CO)6-1 | 1859 |
| Cr(CO)6 | 2000 |
| Mn(CO)6+ | 2100 |
| Fe(CO)62+ | 2204 |
| Fe(CO)5 | 2022, 2000 |
The most important technique for characterizing metal carbonyls is infra-red spectroscopy. The C-O vibration, typically called νCO, occurs at 2143 cm-1 for CO gas. The positions of the νCO band(s) for the metal carbonyls is inversely correlated with the strength of the pi-bonding between the metal and the carbon:
In addition to their frequency, the number of the νCO bands is diagnostic of structure of the complex. Octahedral complexes, e.g. Cr(CO)6, exhibits only a single νCO band in its IR spectrum. Spectra for complexes of lower symmetry are more complex. For example, the IR spectrum of Fe2(CO)9 displays CO bands at 2082, 2019, 1829 cm-1.
In cluster carbonyls, νCO is a sensitive probe for the CO coordination geometry. For bridging (μ2) ligands νCO is usually shifted by 100-200 cm-1 to lower wavenumbers compared to the signatures of μ1-CO. Bands for face capping (μ3) CO ligands appear at even lower energies. Typical values for rhodium cluster carbonyls are:
| carbonyl | νCO, µ1 (cm-1) | νCO, µ2 (cm-1) | νCO, µ3 (cm-1) |
|---|---|---|---|
| Rh2(CO)8 | 2060, 2084 | 1846, 1862 | |
| Rh4(CO)12 | 2044, 2070, 2074 | 1886 | |
| Rh6(CO)16 | 2045, 2075 | 1819 |
Synthesis
Although nickel carbonylNickel carbonyl
Nickel carbonyl is the organonickel compound with the formula Ni4. This pale-yellow liquid is the principal carbonyl of nickel. It is an intermediate in the Mond process for the purification of nickel and a reagent in organometallic chemistry...
and iron pentacarbonyl
Iron pentacarbonyl
Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula 5. Under standard conditions Fe5 is a free-flowing, straw-colored liquid with a pungent odour. This compound is a common precursor to diverse iron compounds, including many that are useful in organic synthesis. Fe5 is...
form upon treatment of the metals with carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
, most metal carbonyls are prepared less directly. The other homoleptic carbonyls are usually made by "reductive carbonylation" of metal salts or metal oxides under a high pressure of carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
in autoclave
Autoclave
An autoclave is an instrument used to sterilize equipment and supplies by subjecting them to high pressure saturated steam at 121 °C for around 15–20 minutes depending on the size of the load and the contents. It was invented by Charles Chamberland in 1879, although a precursor known as the...
:
- Re2O7 + 17 CO → Re2(CO)10 + 7 CO2
Once prepared, these homoleptic carbonyls undergo extensive substitution and redox reactions.
Mixed ligand carbonyls of ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
, osmium
Osmium
Osmium is a chemical element with the symbol Os and atomic number 76. Osmium is a hard, brittle, blue-gray or blue-blacktransition metal in the platinum family, and is the densest natural element. Osmium is twice as dense as lead. The density of osmium is , slightly greater than that of iridium,...
, rhodium
Rhodium
Rhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
, and iridium
Iridium
Iridium is the chemical element with atomic number 77, and is represented by the symbol Ir. A very hard, brittle, silvery-white transition metal of the platinum family, iridium is the second-densest element and is the most corrosion-resistant metal, even at temperatures as high as 2000 °C...
are often generated by abstraction of CO from solvents such as dimethylformamide
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
(DMF) and 2-methoxyethanol
2-Methoxyethanol
2-Methoxyethanol, or methyl cellosolve, is an organic compound that is used mainly as a solvent. It is a clear, colorless liquid with an ether-like odor. It is in a class of solvents known as glycol ethers which are notable for their ability to dissolve a variety of different types of chemical...
. Typical is the synthesis of IrCl(CO)(PPh3)2 from the reaction of iridium(III) chloride
Iridium(III) chloride
Iridium chloride is the inorganic compound with the formula IrCl3. This material is relatively rare, but the related hydrate is useful for preparing other iridium compounds. The anhydrous salt is a dark green crystalline solid...
and triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
in boiling DMF solution.
Occurrence in nature
The hydrogenaseHydrogenase
A hydrogenase is an enzyme that catalyses the reversible oxidation of molecular hydrogen . Hydrogenases play a vital role in anaerobic metabolism....
enzymes contain CO bound to iron, apparently the CO stabilizes low oxidation states which facilitates the binding of hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
. Certain metal carbonyls have been observed in trace amounts in landfills, where the reducing environment is compatible with their formation.
Compounds
Most metal carbonyl complexes contain a mixture of ligands. Examples include the historically important IrCl(CO)(P(C6H5)3)2Vaska's complex
Vaska's complex is the trivial name for the chemical compound trans-chlorocarbonylbisiridium, which has the formula IrCl[P3]2. This square planar diamagnetic organometallic complex consists of a central iridium atom bound to two mutually trans triphenylphosphine ligands, carbon monoxide, and a...
and the anti-knock agent (CH3C5H4)Mn(CO)3
Methylcyclopentadienyl manganese tricarbonyl
Methylcyclopentadienyl manganese tricarbonyl is an organomanganese compound with the formula Mn3. Marketed initially in 1958 as a supplement to the gasoline additive tetraethyl lead to increase the fuel's octane rating, MMT was later used in unleaded gasoline...
. The parent compounds for many of these mixed ligand complexes are the binary carbonyls, i.e. species of the formula [M(CO)n]z, many of which are commercially available. The formula of many metal carbonyls can be inferred from the 18 electron rule.
Charge-neutral binary metal carbonyls
- Group 4 elementGroup 4 elementThe Group 4 elements are a group of chemical elements in the periodic table. In the modern IUPAC nomenclature, Group 4 of the periodic table contains titanium , zirconium , hafnium and rutherfordium . This group lies in the d-block of the periodic table...
s with 4 valence electrons are rare, but substituted derivatives of Ti(CO)7 are known. - Group 5 elementGroup 5 elementA Group 5 element is a chemical element in the fifth group in the periodic table. In the modern IUPAC nomenclature, Group 5 of the periodic table contains vanadium , niobium , tantalum and dubnium . This group lies in the d-block of the periodic table...
s with 5 valence electrons, again are subject to steric effects that prevent the formation of M-M bonded species such as V2(CO)12, which is unknown. The 17 VE V(CO)6Vanadium carbonylVanadium carbonyl, also known as vanadium hexacarbonyl, is the inorganic compound with the formula V6. This highly reactive species is noteworthy from theoretical and scholarly perspectives. It is a rare isolable homoleptic metal carbonyl that is paramagnetic...
is however well known. - Group 6 elementGroup 6 elementA Group 6 element is one in the series of elements in group 6 in the periodic table, which consists of the transition metals chromium , molybdenum , tungsten , and seaborgium ....
s with 6 valence electrons form metal carbonyls Cr(CO)6Chromium carbonylChromium carbonyl, also known as chromium hexacarbonyl, is the chemical compound with the formula Cr6. At room temperature the solid is stable to air, although it does have a high vapor pressure and sublimes readily. Cr6 is zerovalent, meaning that Cr has a formal charge of zero, and it is called...
, Mo(CO)6Molybdenum hexacarbonylMolybdenum hexacarbonyl is the chemical compound with the formula Mo6. This colorless solid, like its chromium and tungsten analogues, is noteworthy as a volatile, air-stable derivative of a metal in its zero oxidation state.-Structure and properties:Mo6 adopts an octahedral geometry consisting...
, and W(CO)6Tungsten hexacarbonylTungsten hexacarbonyl is the chemical compound with the formula W6. This complex gave rise to the first example of a dihydrogen complex....
(6 + 6x2 = 18 electrons). - Group 7 elementGroup 7 elementA Group 7 element is one in the series of elements in group 7 in the periodic table, which consists of manganese , technetium , rhenium , and bohrium...
s with 7 valence electrons form metal carbonyl dimers Mn2(CO)10Dimanganese decacarbonylDimanganese decacarbonyl is the chemical compound with the formula Mn210. This metal carbonyl is an important reagent in the organometallic chemistry of manganese.-Synthesis:...
, Tc2(CO)10, and Re2(CO)10 (7 + 1 + 5x2 = 18 electrons). - Group 8 elementGroup 8 elementA Group 8 element is one in the series of elements in group 8 in the periodic table, which consists of the transition metals iron , ruthenium , osmium and hassium ....
s with 8 valence electrons form metal carbonyls Fe(CO)5Iron pentacarbonylIron pentacarbonyl, also known as iron carbonyl, is the compound with formula 5. Under standard conditions Fe5 is a free-flowing, straw-colored liquid with a pungent odour. This compound is a common precursor to diverse iron compounds, including many that are useful in organic synthesis. Fe5 is...
,Ru(CO)5 and Os(CO)5 (8 + 5x2 = 18 electrons). The heavier two members are unstable, tending to decarbonylate to give Ru3(CO)12Triruthenium dodecacarbonylTriruthenium dodecacarbonyl is the chemical compound with the formula Ru312. This orange-colored metal carbonyl cluster is a precursor to other organoruthenium compounds.-Structure and synthesis:...
, and Os3(CO)12Triosmium dodecacarbonylTriosmium dodecacarbonyl is a chemical compound with the formula Os312. This yellow-colored metal carbonyl cluster is an important precursor to organo-osmium compounds...
. The two other principal iron carbonyls are Fe3(CO)12Triiron dodecacarbonylTriiron dodecarbonyl is the chemical compound with the formula Fe312. It was one of the first metal carbonyl clusters synthesized. It is a more reactive source of iron than is iron pentacarbonyl.-General properties:...
and Fe2(CO)9Diiron nonacarbonylDiiron nonacarbonyl is an inorganic compound with the formula Fe29. This metal carbonyl is an important reagent in organometallic chemistry and of occasional use in organic synthesis. It is a more reactive source of Fe than Fe5 and less dangerous to handle because it is nonvolatile...
. - Group 9 elementGroup 9 elementIn modern IUPAC nomenclature, Group 9 of the periodic table contains the elements cobalt , rhodium , iridium , and meitnerium . These are all d-block transition metals...
s with 9 valence electrons and are expected to form metal carbonyl dimers M2(CO)8. In fact the cobalt derivative of this octacarbonyl is the only stable member, but all three tetramers are well known: Co4(CO)12, Rh4(CO)12Tetrarhodium dodecacarbonylTetrarhodium dodecacarbonyl is the chemical compound with the formula Rh412. This dark-red crystalline solid is the smallest stable binary rhodium carbonyl. It is used as a catalyst in organic synthesis.-Structure, synthesis, reactions:...
, Rh6(CO)16HexadecacarbonylhexarhodiumHexadecacarbonylhexarhodium is a metal carbonyl cluster with the formula Rh616. It exists as black crystals that are soluble in organic solvents.-Discovery and synthesis:...
, and Ir4(CO)12Tetrairidium dodecacarbonylTetrairidium dodecacarbonyl is the chemical compound with the formula Ir412. This tetrahedral cluster is the most common and most stable "binary" carbonyl of iridium. This air-stable species is only poorly soluble in organic solvents. It has been used to prepare bimetallic clusters and catalysts,...
(9 + 3 + 3x2 = 18 electrons). Co2(CO)8Dicobalt octacarbonylDicobalt octacarbonyl is the inorganic compound Co28. This metal carbonyl is a reagent and catalyst in organometallic chemistry and organic synthesis. It is used as a catalyst for hydroformylation, the conversion of alkenes to aldehydes....
unlike the majority of the other 18 VE transition metal carbonyls is sensitive to oxygen. - Group 10 elementGroup 10 elementA Group 10 element is one in the series of elements in group 10 in the periodic table, which consists of the transition metals nickel , palladium , platinum , and darmstadtium ....
s with 10 valence electrons form metal carbonyls Ni(CO)4Nickel carbonylNickel carbonyl is the organonickel compound with the formula Ni4. This pale-yellow liquid is the principal carbonyl of nickel. It is an intermediate in the Mond process for the purification of nickel and a reagent in organometallic chemistry...
(10 + 4x2 = 18 electrons). Curiously Pd(CO)4 and Pt(CO)4 are not stable.
Anionic binary metal carbonyls
- Group 4 elementGroup 4 elementThe Group 4 elements are a group of chemical elements in the periodic table. In the modern IUPAC nomenclature, Group 4 of the periodic table contains titanium , zirconium , hafnium and rutherfordium . This group lies in the d-block of the periodic table...
s as dianions resemble neutral group 6 derivatives: [Ti(CO)6]2-. - Group 5 elementGroup 5 elementA Group 5 element is a chemical element in the fifth group in the periodic table. In the modern IUPAC nomenclature, Group 5 of the periodic table contains vanadium , niobium , tantalum and dubnium . This group lies in the d-block of the periodic table...
s as monoanions resemble again neutral group 6 derivatives: [V(CO)6]-. - Group 7 elementGroup 7 elementA Group 7 element is one in the series of elements in group 7 in the periodic table, which consists of manganese , technetium , rhenium , and bohrium...
s as monoanions resemble neutral group 8 derivatives: [M(CO)5]- (M = Mn, Tc, Re). - Group 8 elementGroup 8 elementA Group 8 element is one in the series of elements in group 8 in the periodic table, which consists of the transition metals iron , ruthenium , osmium and hassium ....
s as dianaions resemble neutral group 10 derivatives: [M(CO)4]2- (M = Fe, Ru, Os)Disodium tetracarbonylferrateDisodium tetracarbonylferrate is the chemical compound with the formula Na2[Fe4]. This oxygen-sensitive colourless solid is employed in organic synthesis", mainly to synthesise aldehydes. It is commonly used with dioxane complexed to the sodium cation, this dioxane solvate being known as Collman's...
. Condensed derivatives are also known. - Group 9 elementGroup 9 elementIn modern IUPAC nomenclature, Group 9 of the periodic table contains the elements cobalt , rhodium , iridium , and meitnerium . These are all d-block transition metals...
s as monoanions resemble neutral group 10 metal carbonyl. [Co(CO)4]- is the best studied member.
Large anionic clusters of Ni, Pd, and Pt are also well known.
Cationic binary metal carbonyls
- Group 7 elementGroup 7 elementA Group 7 element is one in the series of elements in group 7 in the periodic table, which consists of manganese , technetium , rhenium , and bohrium...
s as monocations resemble neutral group 6 derivative [M(CO)6]+ (M = Mn, Tc, Re). - Group 8 elementGroup 8 elementA Group 8 element is one in the series of elements in group 8 in the periodic table, which consists of the transition metals iron , ruthenium , osmium and hassium ....
s as dications also resemble neutral group 6 derivatives [M(CO)6]2+ (M = Fe, Ru, Os).
Metal carbonyl hydrides
| Metal Carbonyl hydride | pKa |
|---|---|
| HCo(CO)4 Tetracarbonylhydrocobalt Cobalt tetracarbonyl hydride is the organometallic compound with the formula HCo4. It is a yellow liquid that forms a colorless vapor and has an intolerable odor. Its main use is as a catalyst in hydroformylation.-Structure and properties:... |
"strong" |
| HCo(CO)3(P(OPh)3) | 5.0 |
| HCo(CO)3(PPh3) | 7.0 |
| HMn(CO)5 Pentacarbonylhydridomanganese Pentacarbonylhydridomanganese is an organometallic compound with formula HMn5. This compound is one of the most stable “first-row” transition metal hydrides.-Preparation:... |
7.1 |
| H2Fe(CO)4 Iron tetracarbonyl hydride Iron tetracarbonyl hydride is the organometallic compound with the formula H2Fe4. Also known as tetracarbonyldihydridoiron, or iron tetracarbonyldihydride, this compound was the first metal hydride discovered... |
4.4, 14 |
| [HCo(dmgH Dimethylglyoxime Dimethylglyoxime is a chemical compound described by the formula CH3CCCH3. This colourless solid is the dioxime derivative of the diketone diacetyl . DmgH2 is used in the analysis of palladium or nickel. Its coordination complexes are of theoretical interest as models for enzymes and as catalysts... )2PBu3 |
10.5 |
Metal carbonyls are relatively distinctive in forming complexes with negative oxidation states. Examples include the anions discussed above. These anions can be protonated to give the corresponding metal carbonyl hydrides. The neutral metal carbonyl hydrides are often volatile and can be quite acidic.
Related compounds
Many ligands are known to form homoleptic and mixed ligand complexes that are analogous to the metal carbonyls.Complexes of nitrosyls
Metal nitrosylMetal nitrosyl
Metal nitrosyl complexes are complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand.-Bonding and structure:...
s, featuring NO as a ligand are numerous, although homoleptic derivatives are not. Relative to CO, NO is a stronger acceptor and isocyanides are better donors. Well known nitrosyl carbonyls include CoNO(CO)3 and Fe(NO)2(CO)2.
Complexes of thiocarbonyls
Complexes containing CS are known but are uncommon. The rarity of such complexes is attributable in part to the fact that the obvious source material, carbon monosulfide, is unstable. Thus, the synthesis of thiocarbonyl complexes requires more elaborate routes, such as the reaction of disodium tetracarbonylferrateDisodium tetracarbonylferrate
Disodium tetracarbonylferrate is the chemical compound with the formula Na2[Fe4]. This oxygen-sensitive colourless solid is employed in organic synthesis", mainly to synthesise aldehydes. It is commonly used with dioxane complexed to the sodium cation, this dioxane solvate being known as Collman's...
with thiophosgene
Thiophosgene
Thiophosgene is a red liquid with the formula CSCl2. It is a molecule with trigonal planar geometry. There are two reactive C–Cl bonds that allow it to be used in diverse organic syntheses.-Synthesis of CSCl2:...
:
- Na2Fe(CO)4 + CSCl2 → Fe(CO)4CS + 2 NaCl
Complexes of CSe and CTe are very rare.
Complexes of phosphines
All metal carbonyls undergo substitution by organophosphorus ligands. For example, the series Fe(CO)5-x(PR3)x is well known for various phosphine ligands for x = 1, 2, and 3. PF3Phosphorus trifluoride
Phosphorus trifluoride , is a colorless and odorless gas. It is highly toxic and it reacts slowly with water. Its main use is as a ligand in metal complexes...
behaves similarly but is remarkable because it readily forms homoleptic analogues of the binary metal carbonyls. For example the volatile, stable complexes Fe(PF3)5 and Co2(PF3)8 represent CO-free analogues of Fe(CO)5 and Co2(CO)8 (unbridged isomer).
Complexes of isocyanides
IsocyanideIsocyanide
An isocyanide is an organic compound with the functional group -N≡C. It is the isomer of the related cyanide , hence the prefix iso....
s also form extensive families of complexes that are related to the metal carbonyls. Typical isocyanide ligands are MeNC
Methyl isocyanide
Methyl isocyanide or Isocyanomethane is an organic compound and a member of the isocyanide family. This colorless liquid is structurally similar to the isomeric methyl cyanide , but its reactivity is very different...
and t-butylisocyanide
Tert-Butyl isocyanide
tert-Butyl isocyanide is an organic compound with the formula Me3CNC . It is an isocyanide, commonly called isonitrile or carbylamine, as defined by the functional group C≡N-R. tert-Butyl isocyanide, like most alkyl isocyanides, is a reactive colorless liquid with an extremely unpleasant odor...
((Me3CNC). A special case is CF3NC
Trifluoromethylisocyanide
Trifluoromethylisocyanide is the chemical compound with the formula CF3NC. It is an isocyanide and a fluorocarbon. Polymerisation occurs even at temperatures below its boiling point of -80°C. As a ligand in coordination chemistry, this species behaves similarly to carbon monoxide.The compound...
, an unstable molecule that forms stable complexes whose behavior closely parallels that of the metal carbonyls.
History
Initial experiments on the reaction of carbon monoxide with metals were carried out by Justus von LiebigJustus von Liebig
Justus von Liebig was a German chemist who made major contributions to agricultural and biological chemistry, and worked on the organization of organic chemistry. As a professor, he devised the modern laboratory-oriented teaching method, and for such innovations, he is regarded as one of the...
in 1834. By passing carbon monoxide over molten potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
he prepared a substance having the empirical formula KCO, which he called Kohlenoxidkalium. As demonstrated later, the compound was not a metal carbonyl, but the potassium salt of ´hexahydroxy benzene and the potassium salt of dihydroxy acetylene
The synthesis of the first true, however, heteroleptic metal carbonyl complex was performed by Paul Schützenberger in 1868 by passing chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
and carbon monoxide over platinum black
Platinum black
Platinum black is a fine powder of platinum with good catalytic properties. The name of platinum black is due to its black color....
, where Dicarbonyl dichloro platinum (Pt(CO)2Cl2) was formed.
Ludwig Mond
Ludwig Mond
Dr Ludwig Mond , was a German-born chemist and industrialist who took British nationality.-Education and career:...
, one of the founders of Imperial Chemical Industries
Imperial Chemical Industries
Imperial Chemical Industries was a British chemical company, taken over by AkzoNobel, a Dutch conglomerate, one of the largest chemical producers in the world. In its heyday, ICI was the largest manufacturing company in the British Empire, and commonly regarded as a "bellwether of the British...
, investigated in the 1890s with Carl Langer and Friedrich Quincke various processes for the recovery of chlorine which was lost in the Solvay process
Solvay process
The Solvay process, also referred to as the ammonia-soda process, is the major industrial process for the production of soda ash . The ammonia-soda process was developed into its modern form by Ernest Solvay during the 1860s...
by nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
metals, oxides and salts. As part of their experiments the group treated nickel with carbon monoxide. They found that the resulting gas colored the gas flame of a burner
Burner
Burner may refer to:* Gas burner or oil burner, a mechanical device that burns a gas or liquid fuel into a flame in a controlled manner* Hot-air balloon device, a device to inflate a hot air balloon* Burner * Burner, West Virginia...
in a greenish-yellowish color; when heated in a glass tube it formed a nickel mirror. The gas could be condensed to a colorless, water-clear liquid with a boiling point of 43 °C. Thus, Mond and his coworker had discovered the first pure, homoleptic metal carbonyl, nickel tetracarbonyl (Ni(CO)4). The unusual high volatility of the metal compound nickel tetracarbonyl led Kelvin
William Thomson, 1st Baron Kelvin
William Thomson, 1st Baron Kelvin OM, GCVO, PC, PRS, PRSE, was a mathematical physicist and engineer. At the University of Glasgow he did important work in the mathematical analysis of electricity and formulation of the first and second laws of thermodynamics, and did much to unify the emerging...
with the statement that Mond had "given wings to the metals' .
The following year, Mond and Marcellin Berthelot
Marcellin Berthelot
Marcelin Pierre Eugène Berthelot was a French chemist and politician noted for the Thomsen-Berthelot principle of thermochemistry. He synthesized many organic compounds from inorganic substances and disproved the theory of vitalism. He is considered as one of the greatest chemists of all time.He...
independently discovered iron pentacarbonyl
Iron pentacarbonyl
Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula 5. Under standard conditions Fe5 is a free-flowing, straw-colored liquid with a pungent odour. This compound is a common precursor to diverse iron compounds, including many that are useful in organic synthesis. Fe5 is...
, which is produced by a similar procedure as nickel tetracarbonyl. Mond recognized the economic potential of this class of compounds, which he commercially used in the Mond process
Mond process
The Mond process, sometimes known as the carbonyl process is a technique created by Ludwig Mond in 1890 to extract and purify nickel. The process was used commercially before the end of the 19th century...
and financed more research on related compounds. Heinrich Hirtz and his colleague M. Dalton Cowap synthesized metal carbonyls of cobalt
Cobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
, molybdenum
Molybdenum
Molybdenum , is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek , meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores...
, ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
, and diiron nonacarbonyl
Diiron nonacarbonyl
Diiron nonacarbonyl is an inorganic compound with the formula Fe29. This metal carbonyl is an important reagent in organometallic chemistry and of occasional use in organic synthesis. It is a more reactive source of Fe than Fe5 and less dangerous to handle because it is nonvolatile...
.
The economic benefits of metal-catalysed carbonylation
Carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry.-Organic chemistry:...
s, e.g. Reppe chemistry and hydroformylation
Hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group and a hydrogen atom to a carbon-carbon double bond...
, led to growth of the area. Metal carbonyl compounds were discovered in the active sites of three naturally occurring enzymes.