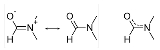
Dimethylformamide
Overview
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
with the formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
(CH3)2NC(O)H. Commonly abbreviated as DMF (though this acronym is sometimes used for dimethylfuran
2,5-Dimethylfuran
2,5-Dimethylfuran is a heterocyclic compound with the formula 2C4H2O. Although often abbreviated DMF, it should not be confused with dimethylformamide. A derivative of furan, this simple compound is a potential biofuel, being derivable from cellulose.-Production:Fructose can be converted into...
), this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
for chemical reactions. Pure dimethylformamide is odorless whereas technical grade or degraded dimethylformamide often has a fishy smell due to impurity of dimethylamine
Dimethylamine
Dimethylamine is an organic compound with the formula 2NH. This secondary amine is a colorless, flammable liquified gas with an ammonia-like odor. Dimethylamine is generally encountered as a solution in water at concentrations up to around 40%...
.
Unanswered Questions

