Vinylcyclopropane rearrangement
Encyclopedia
The vinylcyclopropane rearrangement or vinylcyclopropane-cyclopentene rearrangement is a ring expansion reaction
, converting a vinyl-substituted cyclopropane
ring into a cyclopentene
ring. The general scope is extended by introducing heteroatom
s in any of the 5 framework positions or by replacing the vinyl
group by an allene
or an alkyne
. A closely related reaction is the divinylcyclopropane-cycloheptadiene rearrangement
.
(400 °C) of 1,1-dichloro-2-vinylcyclopropane to 2-chlorocyclopentadiene. In 1960, Overberger and Borchert worked with 1-cyclopropylethyl acetate, a precursor to vinylcyclopropane, and also in 1960, Vogel worked with vinylcyclopropane itself. Vinyl substituted oxirane was first successfully converted to 2,3-dihydrofuran
by Paladini and Chuche in 1971. The conversion of imine
substituted cyclopropane to a pyrroline
was first reported by Cloke in 1929 and that of a vinyl aziridine
by Atkinson and Rees in 1967.
process. Due to the high reactivity and partial pi character of the cyclopropane ring, the reaction often proceeds readily at lower temperatures. Vinylcyclopropane rearrangements may be effected thermally or photochemically.
Ring expansion reaction
In chemistry, ring expansion and ring contraction are chemical reactions that increase or decrease, respectively, the number of atoms in a ring of a cyclic compound.- Ring expansion reactions :Examples of ring expansion reactions are:...
, converting a vinyl-substituted cyclopropane
Cyclopropane
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms...
ring into a cyclopentene
Cyclopentene
Cyclopentene is a chemical compound with the formula 58. It is a colorless liquid with a petrol-like odor. It is one of the cycloalkenes.Cyclopentene is produced industrially in large amounts...
ring. The general scope is extended by introducing heteroatom
Heteroatom
In organic chemistry, a heteroatom is any atom that is not carbon or hydrogen. Usually, the term is used to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular structure...
s in any of the 5 framework positions or by replacing the vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
group by an allene
Allene
An allene is a compound in which one carbon atom has double bonds with each of its two adjacent carbon centres. Allenes are classified as polyenes with cumulated dienes. The parent compound of allene is propadiene. Compounds with an allene-type structure but with more than three carbon atoms are...
or an alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
. A closely related reaction is the divinylcyclopropane-cycloheptadiene rearrangement
Divinylcyclopropane-cycloheptadiene rearrangement
The divinylcyclopropane-cycloheptadiene rearrangement is an organic chemical transformation that involves the isomerization of a 1,2-divinylcyclopropane into a cycloheptadiene or -triene...
.
History
The reaction was discovered in 1959 by Neureiter who studied the pyrolysisPyrolysis
Pyrolysis is a thermochemical decomposition of organic material at elevated temperatures without the participation of oxygen. It involves the simultaneous change of chemical composition and physical phase, and is irreversible...
(400 °C) of 1,1-dichloro-2-vinylcyclopropane to 2-chlorocyclopentadiene. In 1960, Overberger and Borchert worked with 1-cyclopropylethyl acetate, a precursor to vinylcyclopropane, and also in 1960, Vogel worked with vinylcyclopropane itself. Vinyl substituted oxirane was first successfully converted to 2,3-dihydrofuran
2,3-Dihydrofuran
2,3-Dihydrofuran is a heterocyclic compound. It is one of the simplest enol ethers....
by Paladini and Chuche in 1971. The conversion of imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
substituted cyclopropane to a pyrroline
Pyrroline
Pyrrolines, also known under the name dihydropyrroles, are three different heterocyclic organic chemical compounds that differ in the position of the double bond. Pyrrolines are formally derived from the aromate pyrrole by hydrogenation...
was first reported by Cloke in 1929 and that of a vinyl aziridine
Aziridine
Aziridines are organic compounds containing the aziridine functional group, a three-membered heterocycle with one amine group and two methylene groups...
by Atkinson and Rees in 1967.
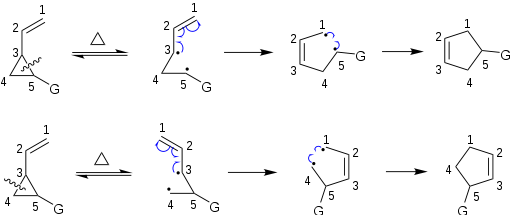
Mechanism
The vinylcyclopropane rearrangement is often classified as a sigmatropic rearrangement; however, evidence suggest that the mechanism of the reaction is not concerted, and that it instead likely proceeds through a multistep biradicalRadical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
process. Due to the high reactivity and partial pi character of the cyclopropane ring, the reaction often proceeds readily at lower temperatures. Vinylcyclopropane rearrangements may be effected thermally or photochemically.


