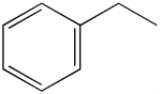
Styrene
Encyclopedia
Styrene, also known as vinyl benzene, is an organic compound
with the chemical formula
C6H5CH=CH2. This derivative of benzene
is a colorless oily liquid
that evaporates easily and has a sweet smell, although high concentrations confer a less pleasant odor. Styrene is the precursor to polystyrene
and several copolymers. Approximately 15 billion pounds are produced annually. On 10 June 2011, the US National Toxicology Program
has described styrene as "reasonably anticipated to be a human carcinogen".
However, an academic panel, funded by the styrene industry, recently reviewed the relevant scientific literature and concluded that "The available epidemiologic evidence does not support a causal relationship between styrene exposure and any type of human cancer."[15]
tree, the Oriental sweetgum (Liquidambar orientalis)
, from which it was first isolated, and not for the tropical Styrax
trees from which benzoin resin
is produced. Low levels of styrene occur naturally in many kinds of plants, as well as a variety of foods such as fruits, vegetables, nuts, beverages, and meats.
The production of styrene in the United States increased dramatically during the 1940s, when it was popularized as a feedstock for synthetic rubber
.
The presence of the vinyl group allows styrene to polymerize. Commercially significant products include polystyrene
, ABS
, styrene-butadiene
(SBR) rubber
, styrene-butadiene latex, SIS (styrene-isoprene-styrene), S-EB-S (styrene-ethylene/butylene-styrene), styrene-divinylbenzene
(S-DVB), styrene-acrylonitrile resin
(SAN) and unsaturated polyesters
. These materials are used in rubber, plastic, insulation, fiberglass
, pipes, automobile
and boat parts, food containers, and carpet backing.
, which is in turn prepared on a large scale by alkylation
of benzene
with ethylene
.
of ethylbenzene
. Ethylbenzene is mixed in the gas phase with 10–15 times its volume in high-temperature steam
, and passed over a solid catalyst bed. Most ethylbenzene dehydrogenation catalysts are based on iron(III) oxide
, promoted by several percent potassium oxide
or potassium carbonate
.
Steam serves several roles in this reaction. It is the source of heat for powering the endothermic reaction, and it removes coke that tends to form on the iron oxide catalyst through the water gas shift reaction
. The potassium promoter enhances this decoking reaction. The steam also dilutes the reactant and products, shifting the position of chemical equilibrium
towards products. A typical styrene plant consists of two or three reactors in series, which operate under vacuum to enhance the conversion and selectivity. Typical per-pass conversions are ca. 65% for two reactors and 70-75% for three reactors. Selectivity to styrene is 93-97%. The main byproducts are benzene and toluene
. Because styrene and ethylbenzene have similar boiling points (145 and 136 °C, respectively), their separation requires tall distillation towers and high return/reflux ratios. At its distillation temperatures, styrene tends to polymerize. To minimize this problem, early styrene plants added elemental sulfur to inhibit the polymerization. During the 1970s, new free radical inhibitors consisting of nitrated phenol
-based retarders were developed. More recently, a number of additives have been developed that exhibit superior inhibition against polymerization. However, the nitrated phenols are still widely used because of their relatively low cost. These reagents are added prior to the distillation.
Improving conversion and so reducing the amount of ethylbenzene that must be separated is the chief impetus for researching alternative routes to styrene. Other than the POSM process, none of these routes like obtaining styrene from butadiene have been commercially demonstrated.
) or SM/PO (Shell
) for styrene monomer / propylene oxide. In this process ethylbenzene is treated with oxygen to form the ethylbenzene hydroperoxide. This hydroperoxide is then used to oxidize propylene
to propylene oxide. The resulting 2-phenylethanol is dehydrated to give styrene:
of cinnamic acid
. Styrene was first prepared by this method.
and methanol
, which are cheaper raw materials than those in the conventional process. Historically, however, this process has suffered from low selectivity due to competing decomposition of methanol. Exelus Inc. claims to have developed this process with commercially viable selectivities, at 400-425 °C and atmospheric pressure, by forcing these components through a proprietary zeolitic catalyst. It is reported that an approximately 9:1 mixture of styrene and ethylbenzene
is obtained, with a total styrene yield of over 60%.
Another developing route to styrene is via benzene
and ethane
. This process is being developed by Snamprogetti S.p.A. and Dow. Ethane, along with ethylbenzene
, is fed to a dehydrogenation reactor with a catalyst capable of simultaneously producing styrene and ethylene
. The dehydrogenation effluent is cooled and separated and the ethylene stream is recycled to the alkylation unit. The process attempts to overcome previous shortcomings in earlier attempts to develop production of styrene from ethane and benzene, such as inefficient recovery of aromatics, production of high levels of heavies and tars, and inefficient separation of hydrogen and ethane. Development of the process is ongoing.
has described styrene as "reasonably anticipated to be a human carcinogen". However, an academic panel, funded by the styrene industry, recently reviewed the relevant scientific literature and concluded that "The available epidemiologic evidence does not support a causal relationship between styrene exposure and any type of human cancer."
The U.S. EPA does not have a cancer classification for styrene, but currently is evaluating styrene's cancer-causing potential through its Integrated Risk Information System (IRIS) program. The U.S. National Toxicology Program of the U.S. Department of Health and Human Services also currently is evaluating styrene's potential toxicity To date, no regulatory body anywhere in the world has classified styrene as a known human carcinogen, although several refer to it in various contexts as a possible or potential human carcinogen. The International Agency for Research on Cancer considers styrene to be "possibly carcinogenic to humans.". Chronic exposure to styrene leads to tiredness/lethargy, memory deficits, headaches and vertigo
.
According to the Styrene Information and Research Center
(an organization representing nearly all of the "North American styrene industry"), polystyrene plastic neither contains, nor breaks down into bisphenol A
(BPA), a chemical used in plastic compounds that leads to developmental and reproductive problems in both adults and children.
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
with the chemical formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
C6H5CH=CH2. This derivative of benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
is a colorless oily liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
that evaporates easily and has a sweet smell, although high concentrations confer a less pleasant odor. Styrene is the precursor to polystyrene
Polystyrene
Polystyrene ) also known as Thermocole, abbreviated following ISO Standard PS, is an aromatic polymer made from the monomer styrene, a liquid hydrocarbon that is manufactured from petroleum by the chemical industry...
and several copolymers. Approximately 15 billion pounds are produced annually. On 10 June 2011, the US National Toxicology Program
National Toxicology Program
The National Toxicology Program is an inter-agency program run by the United States Department of Health and Human Services to coordinate, evaluate, and report on toxicology within public agencies....
has described styrene as "reasonably anticipated to be a human carcinogen".
However, an academic panel, funded by the styrene industry, recently reviewed the relevant scientific literature and concluded that "The available epidemiologic evidence does not support a causal relationship between styrene exposure and any type of human cancer."[15]
Occurrence, history, and use
Styrene is named for "styrax" (also called "storax Levant"), the resin from a TurkishTurkey
Turkey , known officially as the Republic of Turkey , is a Eurasian country located in Western Asia and in East Thrace in Southeastern Europe...
tree, the Oriental sweetgum (Liquidambar orientalis)
Liquidambar orientalis
Liquidambar orientalis, commonly known as oriental sweetgum or Turkish sweetgum, is a deciduous tree in the genus Liquidambar, native to the eastern Mediterranean region, that occurs as pure stands mainly in the flood plains of southwestern Turkey and on the Greek island of Rhodes.-Biotope:The...
, from which it was first isolated, and not for the tropical Styrax
Styrax
Styrax is a genus of about 130 species of large shrubs or small trees in the family Styracaceae, mostly native to warm temperate to tropical regions of the Northern Hemisphere, with the majority in eastern and southeastern Asia, but also crossing the equator in South America...
trees from which benzoin resin
Benzoin resin
Benzoin resin or styrax resin is a balsamic resin obtained from the bark of several species of trees in the genus Styrax. It is used in perfumes, some kinds of incense, as a flavoring, and medicine . Its principal component is benzoic acid...
is produced. Low levels of styrene occur naturally in many kinds of plants, as well as a variety of foods such as fruits, vegetables, nuts, beverages, and meats.
The production of styrene in the United States increased dramatically during the 1940s, when it was popularized as a feedstock for synthetic rubber
Synthetic rubber
Synthetic rubber is is any type of artificial elastomer, invariably a polymer. An elastomer is a material with the mechanical property that it can undergo much more elastic deformation under stress than most materials and still return to its previous size without permanent deformation...
.
The presence of the vinyl group allows styrene to polymerize. Commercially significant products include polystyrene
Polystyrene
Polystyrene ) also known as Thermocole, abbreviated following ISO Standard PS, is an aromatic polymer made from the monomer styrene, a liquid hydrocarbon that is manufactured from petroleum by the chemical industry...
, ABS
Acrylonitrile butadiene styrene
Acrylonitrile butadiene styrene is a common thermoplastic. Its melting point is approximately 105 °C ....
, styrene-butadiene
Styrene-butadiene
Styrene-Butadiene or Styrene-Butadiene-Rubber is a synthetic rubber copolymer consisting of styrene and butadiene. It has good abrasion resistance and good aging stability when protected by additives, and is widely used in car tires, where it may be blended with natural rubber...
(SBR) rubber
Rubber
Natural rubber, also called India rubber or caoutchouc, is an elastomer that was originally derived from latex, a milky colloid produced by some plants. The plants would be ‘tapped’, that is, an incision made into the bark of the tree and the sticky, milk colored latex sap collected and refined...
, styrene-butadiene latex, SIS (styrene-isoprene-styrene), S-EB-S (styrene-ethylene/butylene-styrene), styrene-divinylbenzene
Divinylbenzene
Divinylbenzene consists of a benzene ring bonded to two vinyl groups. It is related to styrene by the addition of a second vinyl group. Divinylbenzene, as it is usually encountered, is a 2:1 mixture of m- and p-divinylbenzene, containing also the corresponding ethylvinylbenzene isomers. It is...
(S-DVB), styrene-acrylonitrile resin
Styrene-acrylonitrile resin
Styrene acrylonitrile resin is a copolymer plastic consisting of styrene and acrylonitrile. It is also known as SAN. It is widely used in place of polystyrene owing to its greater thermal resistance...
(SAN) and unsaturated polyesters
Polyester resin
Polyester resins are unsaturated resins formed by the reaction of dibasic organic acids and polyhydric alcohols. Polyester resins are used in sheet moulding compound, bulk moulding compound and the toner of laser printers...
. These materials are used in rubber, plastic, insulation, fiberglass
Fiberglass
Glass fiber is a material consisting of numerous extremely fine fibers of glass.Glassmakers throughout history have experimented with glass fibers, but mass manufacture of glass fiber was only made possible with the invention of finer machine tooling...
, pipes, automobile
Automobile
An automobile, autocar, motor car or car is a wheeled motor vehicle used for transporting passengers, which also carries its own engine or motor...
and boat parts, food containers, and carpet backing.
Production
Styrene is produced in industrial quantities from ethylbenzeneEthylbenzene
Ethylbenzene is an organic compound with the formula C6H5CH2CH3. This aromatic hydrocarbon is important in the petrochemical industry as an intermediate in the production of styrene, which in turn is used for making polystyrene, a common plastic material....
, which is in turn prepared on a large scale by alkylation
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
of benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
with ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
.
Dehydrogenation of ethylbenzene
Styrene is most commonly produced by the catalytic dehydrogenationDehydrogenation
Dehydrogenation is a chemical reaction that involves the elimination of hydrogen . It is the reverse process of hydrogenation. Dehydrogenation reactions may be either large scale industrial processes or smaller scale laboratory procedures....
of ethylbenzene
Ethylbenzene
Ethylbenzene is an organic compound with the formula C6H5CH2CH3. This aromatic hydrocarbon is important in the petrochemical industry as an intermediate in the production of styrene, which in turn is used for making polystyrene, a common plastic material....
. Ethylbenzene is mixed in the gas phase with 10–15 times its volume in high-temperature steam
Steam
Steam is the technical term for water vapor, the gaseous phase of water, which is formed when water boils. In common language it is often used to refer to the visible mist of water droplets formed as this water vapor condenses in the presence of cooler air...
, and passed over a solid catalyst bed. Most ethylbenzene dehydrogenation catalysts are based on iron(III) oxide
Iron(III) oxide
Iron oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron oxide , which is rare, and iron oxide , which also occurs naturally as the mineral magnetite. As the mineral known as hematite, Fe2O3 is the main...
, promoted by several percent potassium oxide
Potassium oxide
Potassium oxide is an ionic compound of potassium and oxygen. This pale yellow solid, the simplest oxide of potassium, is a rarely encountered, highly reactive compound...
or potassium carbonate
Potassium carbonate
Potassium carbonate is a white salt, soluble in water , which forms a strongly alkaline solution. It can be made as the product of potassium hydroxide's absorbent reaction with carbon dioxide. It is deliquescent, often appearing a damp or wet solid...
.
Steam serves several roles in this reaction. It is the source of heat for powering the endothermic reaction, and it removes coke that tends to form on the iron oxide catalyst through the water gas shift reaction
Water gas shift reaction
The water-gas shift reaction is a chemical reaction in which carbon monoxide reacts with water vapor to form carbon dioxide and hydrogen:The water-gas shift reaction is an important industrial reaction. It is often used in conjunction with steam reforming of methane or other hydrocarbons, which is...
. The potassium promoter enhances this decoking reaction. The steam also dilutes the reactant and products, shifting the position of chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
towards products. A typical styrene plant consists of two or three reactors in series, which operate under vacuum to enhance the conversion and selectivity. Typical per-pass conversions are ca. 65% for two reactors and 70-75% for three reactors. Selectivity to styrene is 93-97%. The main byproducts are benzene and toluene
Toluene
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
. Because styrene and ethylbenzene have similar boiling points (145 and 136 °C, respectively), their separation requires tall distillation towers and high return/reflux ratios. At its distillation temperatures, styrene tends to polymerize. To minimize this problem, early styrene plants added elemental sulfur to inhibit the polymerization. During the 1970s, new free radical inhibitors consisting of nitrated phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
-based retarders were developed. More recently, a number of additives have been developed that exhibit superior inhibition against polymerization. However, the nitrated phenols are still widely used because of their relatively low cost. These reagents are added prior to the distillation.
Improving conversion and so reducing the amount of ethylbenzene that must be separated is the chief impetus for researching alternative routes to styrene. Other than the POSM process, none of these routes like obtaining styrene from butadiene have been commercially demonstrated.
Via ethylbenzenehydroperoxide
Commercially styrene is also co-produced with propylene oxide in a process known as POSM (Lyondell Chemical CompanyLyondell Chemical Company
LyondellBasell Industries is a public multinational chemical company based in Rotterdam, Netherlands. It was formed in December 2007 by the acquisition of Lyondell Chemical Company by Basell Polyolefins for $12.7 billion. LyondellBasell was listed on the New York Stock Exchange on October 14, 2010...
) or SM/PO (Shell
Royal Dutch Shell
Royal Dutch Shell plc , commonly known as Shell, is a global oil and gas company headquartered in The Hague, Netherlands and with its registered office in London, United Kingdom. It is the fifth-largest company in the world according to a composite measure by Forbes magazine and one of the six...
) for styrene monomer / propylene oxide. In this process ethylbenzene is treated with oxygen to form the ethylbenzene hydroperoxide. This hydroperoxide is then used to oxidize propylene
Propylene
Propene, also known as propylene or methylethylene, is an unsaturated organic compound having the chemical formula C3H6. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons, and it is also second in natural abundance.-Properties:At room temperature and...
to propylene oxide. The resulting 2-phenylethanol is dehydrated to give styrene:
- C6H5CH2CH3 + O2 → C6H5CH2CH2O2H
- C6H5CH2CH2O2H + CH3CH=CH2 → C6H5CH2CH2OH + CH3CHCH2OPropylene oxidePropylene oxide is an organic compound with the molecular formula CH3CHCH2O. This colourless volatile liquid is produced on a large scale industrially, its major application being its use for the production of polyether polyols for use in making polyurethane plastics...
- C6H5CH2CH2OH → C6H5CH=CH2 + H2O
Laboratory synthesis
A laboratory synthesis of styrene entails the decarboxylationDecarboxylation
Decarboxylation is a chemical reaction that releases carbon dioxide . Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carbonation, the addition of CO2 to...
of cinnamic acid
Cinnamic acid
Cinnamic acid is a white crystalline organic acid, which is slightly soluble in water.It is obtained from oil of cinnamon, or from balsams such as storax. It is also found in shea butter and is the best indication of its environmental history and post-extraction conditions...
. Styrene was first prepared by this method.
Other methods
Styrene can be produced from tolueneToluene
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
and methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
, which are cheaper raw materials than those in the conventional process. Historically, however, this process has suffered from low selectivity due to competing decomposition of methanol. Exelus Inc. claims to have developed this process with commercially viable selectivities, at 400-425 °C and atmospheric pressure, by forcing these components through a proprietary zeolitic catalyst. It is reported that an approximately 9:1 mixture of styrene and ethylbenzene
Ethylbenzene
Ethylbenzene is an organic compound with the formula C6H5CH2CH3. This aromatic hydrocarbon is important in the petrochemical industry as an intermediate in the production of styrene, which in turn is used for making polystyrene, a common plastic material....
is obtained, with a total styrene yield of over 60%.
Another developing route to styrene is via benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
and ethane
Ethane
Ethane is a chemical compound with chemical formula C2H6. It is the only two-carbon alkane that is an aliphatic hydrocarbon. At standard temperature and pressure, ethane is a colorless, odorless gas....
. This process is being developed by Snamprogetti S.p.A. and Dow. Ethane, along with ethylbenzene
Ethylbenzene
Ethylbenzene is an organic compound with the formula C6H5CH2CH3. This aromatic hydrocarbon is important in the petrochemical industry as an intermediate in the production of styrene, which in turn is used for making polystyrene, a common plastic material....
, is fed to a dehydrogenation reactor with a catalyst capable of simultaneously producing styrene and ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
. The dehydrogenation effluent is cooled and separated and the ethylene stream is recycled to the alkylation unit. The process attempts to overcome previous shortcomings in earlier attempts to develop production of styrene from ethane and benzene, such as inefficient recovery of aromatics, production of high levels of heavies and tars, and inefficient separation of hydrogen and ethane. Development of the process is ongoing.
Health effects
Styrene is regarded as a "hazardous chemical", especially in case of eye contact, but also in case of skin contact, of ingestion and of inhalation, according to several sources. The US EPA has described styrene to be "a suspected toxin to the gastrointestinal tract, kidney, and respiratory system, among others." On 10 June 2011, the US National Toxicology ProgramNational Toxicology Program
The National Toxicology Program is an inter-agency program run by the United States Department of Health and Human Services to coordinate, evaluate, and report on toxicology within public agencies....
has described styrene as "reasonably anticipated to be a human carcinogen". However, an academic panel, funded by the styrene industry, recently reviewed the relevant scientific literature and concluded that "The available epidemiologic evidence does not support a causal relationship between styrene exposure and any type of human cancer."
The U.S. EPA does not have a cancer classification for styrene, but currently is evaluating styrene's cancer-causing potential through its Integrated Risk Information System (IRIS) program. The U.S. National Toxicology Program of the U.S. Department of Health and Human Services also currently is evaluating styrene's potential toxicity To date, no regulatory body anywhere in the world has classified styrene as a known human carcinogen, although several refer to it in various contexts as a possible or potential human carcinogen. The International Agency for Research on Cancer considers styrene to be "possibly carcinogenic to humans.". Chronic exposure to styrene leads to tiredness/lethargy, memory deficits, headaches and vertigo
Vertigo (medical)
Vertigo is a type of dizziness, where there is a feeling of motion when one is stationary. The symptoms are due to a dysfunction of the vestibular system in the inner ear...
.
According to the Styrene Information and Research Center
Styrene Information and Research Center
The Styrene Information and Research Center was formed in 1987 as the principal focal point for public information and research on styrene. SIRC is a non-profit organization consisting of 17 Voting member companies involved in the manufacturing or processing of styrene, and 11 Associate member...
(an organization representing nearly all of the "North American styrene industry"), polystyrene plastic neither contains, nor breaks down into bisphenol A
Bisphenol A
Bisphenol A is an organic compound with two phenol functional groups. It is used to make polycarbonate plastic and epoxy resins, along with other applications....
(BPA), a chemical used in plastic compounds that leads to developmental and reproductive problems in both adults and children.

