
Reaction mechanism
Encyclopedia
In chemistry
, a reaction mechanism is the step by step sequence
of elementary reaction
s by which overall chemical change occurs.
Although only the net chemical change is directly observable
for most chemical reaction
s, experiment
s can often be designed that suggest the possible sequence of steps in a reaction mechanism. Recently, electrospray ionization mass spectrometry has been used to corroborate the mechanism of several organic reaction proposals.
, activated complex
, and transition state
, and which bonds are broken (and in what order), and which bonds are formed (and in what order). A complete mechanism must also account for all reactants used, the function of a catalyst, stereochemistry
, all product
s formed and the amount of each, and what the relative rates of the steps are. Reaction intermediates are chemical species, often unstable and short-lived, which are not reactants or products of the overall chemical reaction, but are temporary products and reactants in the mechanism's reaction steps. Reaction intermediates are often free radicals or ion
s. Transition states can be unstable intermediate molecular states even in the elementary reactions. Transition states are commonly molecular entities involving an unstable number of bonds and/or unstable geometry which may be at chemical potential
maxima.
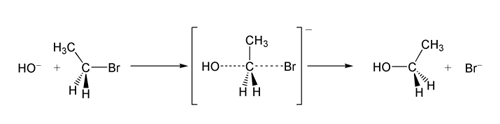 The electron or arrow pushing
The electron or arrow pushing
method is often used in illustrating a reaction mechanism; for example, see the illustration of the mechanism for benzoin condensation
in the following examples section.
A reaction mechanism must also account for the order in which molecules react. Often what appears to be a single step conversion is in fact a multistep reaction.
In this case, it has been experimentally determined that this reaction takes place according to the rate law . Therefore, a possible mechanism by which this reaction takes place is:
. Therefore, a possible mechanism by which this reaction takes place is:
Each step is called an elementary step, and each has its own rate law and molecularity
. The elementary steps should add up to the original reaction.
When determining the overall rate law for a reaction, the slowest step is the step that determines the reaction rate. Because the first step (in the above reaction) is the slowest step, it is the rate-determining step
. Because it involves the collision of two NO2 molecules, it is a bimolecular reaction with a rate law of . If we were to cancel out all the molecules that appear on both sides of the reaction, we would be left with the original reaction.
. If we were to cancel out all the molecules that appear on both sides of the reaction, we would be left with the original reaction.
In organic chemistry
, one of the first reaction mechanisms proposed was that for the benzoin condensation
, put forward in 1903 by A. J. Lapworth.
. For many combustion and plasma systems, detailed mechanisms are not available or require development.
Even when information is available, identifying and assembling the relevant data from a variety of sources, reconciling discrepant values and extrapolating to different conditions can be a difficult process without expert help. Rate constants or thermochemical data are often not available in the literature, so computational chemistry techniques or group-additivity methods must be used to obtain the required parameters.
At the different stages of a reaction mechanism's elaboration, appropriate methods must be used.
is the number of colliding molecular entities
that are involved in a single reaction step
.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, a reaction mechanism is the step by step sequence
Sequence
In mathematics, a sequence is an ordered list of objects . Like a set, it contains members , and the number of terms is called the length of the sequence. Unlike a set, order matters, and exactly the same elements can appear multiple times at different positions in the sequence...
of elementary reaction
Elementary reaction
An elementary reaction is a chemical reaction in which one or more of the chemical species react directly to form products in a single reaction step and with a single transition state....
s by which overall chemical change occurs.
Although only the net chemical change is directly observable
Observation
Observation is either an activity of a living being, such as a human, consisting of receiving knowledge of the outside world through the senses, or the recording of data using scientific instruments. The term may also refer to any data collected during this activity...
for most chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s, experiment
Experiment
An experiment is a methodical procedure carried out with the goal of verifying, falsifying, or establishing the validity of a hypothesis. Experiments vary greatly in their goal and scale, but always rely on repeatable procedure and logical analysis of the results...
s can often be designed that suggest the possible sequence of steps in a reaction mechanism. Recently, electrospray ionization mass spectrometry has been used to corroborate the mechanism of several organic reaction proposals.
Description
A chemical mechanism describes in detail exactly what takes place at each stage of an overall chemical reaction (transformation). It also describes each reaction intermediateReaction intermediate
A reaction intermediate or an intermediate is a molecular entity that is formed from the reactants and reacts further to give the directly observed products of a chemical reaction. Most chemical reactions are stepwise, that is they take more than one elementary step to complete...
, activated complex
Activated complex
In chemistry an activated complex is defined by the International Union of Pure and Applied Chemistry as "that assembly of atoms which corresponds to an arbitrary infinitesimally small region at or near the col of a potential energy surface"...
, and transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
, and which bonds are broken (and in what order), and which bonds are formed (and in what order). A complete mechanism must also account for all reactants used, the function of a catalyst, stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
, all product
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
s formed and the amount of each, and what the relative rates of the steps are. Reaction intermediates are chemical species, often unstable and short-lived, which are not reactants or products of the overall chemical reaction, but are temporary products and reactants in the mechanism's reaction steps. Reaction intermediates are often free radicals or ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s. Transition states can be unstable intermediate molecular states even in the elementary reactions. Transition states are commonly molecular entities involving an unstable number of bonds and/or unstable geometry which may be at chemical potential
Chemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
maxima.

Arrow pushing
Arrow pushing or electron pushing is a technique used to describe the progression of organic chemistry reaction mechanisms. In using arrow pushing, "curved arrows" or "curly arrows" are superimposed over the structural formulae of reactants in a chemical equation to show the reaction mechanism...
method is often used in illustrating a reaction mechanism; for example, see the illustration of the mechanism for benzoin condensation
Benzoin condensation
The benzoin condensation is a reaction between two aromatic aldehydes, particularly benzaldehyde. The reaction is catalyzed by a nucleophile such as the cyanide anion or an N-heterocyclic carbene. The reaction product is an aromatic acyloin with benzoin as the parent compound...
in the following examples section.
A reaction mechanism must also account for the order in which molecules react. Often what appears to be a single step conversion is in fact a multistep reaction.
Examples
Consider the following reaction:- CO + NO2 → CO2 + NO
In this case, it has been experimentally determined that this reaction takes place according to the rate law
 . Therefore, a possible mechanism by which this reaction takes place is:
. Therefore, a possible mechanism by which this reaction takes place is:- 2 NO2 → NO3 + NO (slow)
- NO3 + CO → NO2 + CO2 (fast)
Each step is called an elementary step, and each has its own rate law and molecularity
Molecularity
Molecularity in chemistry is the number of colliding molecular entities that are involved in a single reaction step. While the order of a reaction is derived experimentally, the molecularity is a theoretical concept and can only be applied to elementary reactions...
. The elementary steps should add up to the original reaction.
When determining the overall rate law for a reaction, the slowest step is the step that determines the reaction rate. Because the first step (in the above reaction) is the slowest step, it is the rate-determining step
Rate-determining step
The rate-determining step is a chemistry term for the slowest step in a chemical reaction. The rate-determining step is often compared to the neck of a funnel; the rate at which water flows through the funnel is determined by the width of the neck, not by the speed at which water is poured in. In...
. Because it involves the collision of two NO2 molecules, it is a bimolecular reaction with a rate law of
 . If we were to cancel out all the molecules that appear on both sides of the reaction, we would be left with the original reaction.
. If we were to cancel out all the molecules that appear on both sides of the reaction, we would be left with the original reaction.In organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, one of the first reaction mechanisms proposed was that for the benzoin condensation
Benzoin condensation
The benzoin condensation is a reaction between two aromatic aldehydes, particularly benzaldehyde. The reaction is catalyzed by a nucleophile such as the cyanide anion or an N-heterocyclic carbene. The reaction product is an aromatic acyloin with benzoin as the parent compound...
, put forward in 1903 by A. J. Lapworth.
Modelling
A correct reaction mechanism is an important part of accurate predictive modellingPredictive modelling
Predictive modelling is the process by which a model is created or chosen to try to best predict the probability of an outcome. In many cases the model is chosen on the basis of detection theory to try to guess the probability of an outcome given a set amount of input data, for example given an...
. For many combustion and plasma systems, detailed mechanisms are not available or require development.
Even when information is available, identifying and assembling the relevant data from a variety of sources, reconciling discrepant values and extrapolating to different conditions can be a difficult process without expert help. Rate constants or thermochemical data are often not available in the literature, so computational chemistry techniques or group-additivity methods must be used to obtain the required parameters.
At the different stages of a reaction mechanism's elaboration, appropriate methods must be used.
Molecularity
Molecularity in chemistryChemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
is the number of colliding molecular entities
Molecular entity
According to the IUPAC Gold Book a molecular entity is "any constitutionally or isotopically distinct atom, molecule, ion, ion pair, radical, radical ion, complex, conformer, etc., identifiable as a separately distinguishable entity"....
that are involved in a single reaction step
Reaction step
A reaction step of a chemical reaction is defined as: "An elementary reaction, constituting one of the stages of a stepwise reaction in which a reaction intermediate is converted into the next reaction intermediate in the sequence of intermediates between reactants and products"....
.
- A reaction involving one molecular entity is called unimolecular.
- A reaction involving two molecular entities is called bimolecular.
- A reaction involving three molecular entities is called termolecular.
See also
- Organic reactions by mechanism under Organic reactionOrganic reactionOrganic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
- Nucleophilic acyl substitutionNucleophilic acyl substitutionNucleophilic acyl substitution describes the substitution reaction involving nucleophiles and acyl compounds. Acyl compounds are carboxylic acid derivatives including esters, amides and acid halides...
- Neighbouring group participationNeighbouring group participationNeighbouring group participation or NGP in organic chemistry has been defined by IUPAC as the interaction of a reaction centre with a lone pair of electrons in an atom or the electrons present in a sigma bond or pi bond . When NGP is in operation it is normal for the reaction rate to be increased...
- Finkelstein reactionFinkelstein reactionThe Finkelstein reaction, named for the German chemist Hans Finkelstein , is an SN2 reaction that involves the exchange of one halogen atom for another...
- Lindemann mechanismLindemann mechanismIn chemical kinetics, the Lindemann mechanism, sometimes called the Lindemann-Hinshelwood mechanism, is a schematic reaction mechanism. Frederick Lindemann discovered the concept in 1921 and Cyril Hinshelwood developed it....
- Electrochemical reaction mechanismElectrochemical reaction mechanismIn chemistry, an electrochemical reaction mechanism is the step by step sequence of elementary steps, involving at least one outer sphere electron transfer, by which an overall chemical change occurs .- Overview :...
- Arrow pushingArrow pushingArrow pushing or electron pushing is a technique used to describe the progression of organic chemistry reaction mechanisms. In using arrow pushing, "curved arrows" or "curly arrows" are superimposed over the structural formulae of reactants in a chemical equation to show the reaction mechanism...

