Purine
Encyclopedia
A purine is a heterocyclic
aromatic organic compound
, consisting of a pyrimidine
ring fused to an imidazole
ring. Purines, including substituted purines and their tautomer
s, are the most widely distributed kind of nitrogen-containing heterocycle in nature.
Purines and pyrimidines make up the two groups of nitrogenous bases, including the two groups of nucleotide bases. Two of the four deoxyribonucleotide
s and two of the four ribonucleotide
s, the respective building-blocks of DNA
and RNA
, are purines.
s, adenine
(2) and guanine
(3), are purines. In DNA
, these bases form hydrogen bond
s with their complementary
pyrimidine
s thymine
and cytosine
, respectively. This is called complementary base pairing. In RNA
, the complement of adenine is uracil
instead of thymine.
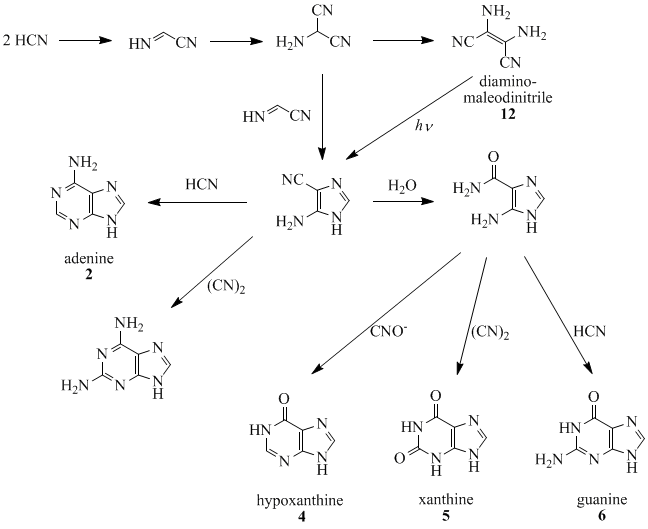
Other notable purines are hypoxanthine
(4), xanthine
(5), theobromine
(6), caffeine
(7), uric acid
(8) and isoguanine
(9).

, GTP
, cyclic AMP, NADH, and coenzyme A
. Purine (1) itself, has not been found in nature, but it can be produced by organic synthesis
.
They may also function directly as neurotransmitters, acting upon purinergic receptors. Adenosine activates adenosine receptor
s.
chemist
Emil Fischer
in 1884. He synthesized it for the first time in 1899. The starting material for the reaction sequence was uric acid
(8), which had been isolated from kidney stones by Scheele in 1776. Uric acid (8) was reacted with PCl5 to give 2,6,8-trichloropurine (10), which was converted with HI
and PH4I
to give 2,6-diiodopurine (11). The product was reduced to purine (1) using zinc-dust. Purines are also much larger than pyrimidines.

s to synthesize and break down purines.
Purines are biologically synthesized as nucleoside
s (bases attached to ribose
).
Examples of high-purine sources include: sweetbread
s, anchovies, sardine
s, liver, beef kidneys, brains, meat extract
s (e.g., Oxo, Bovril), herring, mackerel
, scallop
s, game meats, beer (from the yeast) and gravy.
A moderate amount of purine is also contained in beef, pork, poultry, other fish and seafood, asparagus, cauliflower, spinach, mushrooms, green peas, lentils, dried peas, beans, oatmeal, wheat bran, wheat germ, and hawthorn.
Higher levels of meat and seafood consumption are associated with an increased risk of gout
, whereas a higher level of consumption of dairy products is associated with a decreased risk. Moderate intake of purine-rich vegetables or protein is not associated with an increased risk of gout.
In August 2011, a report, based on NASA
studies with meteorites found on Earth
, was published suggesting purine and related organic molecules (including the DNA
and RNA
components, adenine
and guanine
) may have been formed extraterrestrially in outer space
.
synthesis of purines in purine metabolism
, purine can also be created artificially.
Purine (1) is obtained in good yield when formamide
is heated in an open vessel at 170 °C for 28 hours.

This remarkable reaction and others like it have been discussed in the context of the origin of life
.
Procedure:
Formamide (45 grams) was heated in an open vessel with a condenser for 28 hours in an oil bath at 170-190 °C. After removing excess formamide (32.1 grams) by vacuum distillation, the residue was refluxed with methanol. The methanol solvent was filtered, the solvent removed from the filtrate by vacuum distillation, and almost pure purine obtained; yield 4.93 grams (71% yield from formamide consumed). Crystallization from acetone afforded purine as colorless crystals; melting point 218 °C.
Oro, Orgel and co-workers have shown that four molecules of HCN tetramerize to form diaminomaleodinitrile (12), which can be converted into almost all natural-occurring purines.
The Traube purine synthesis (1900) is a classic reaction (named after Wilhelm Traube
) between an amine
-substituted pyrimidine
and formic acid
.

Heterocyclic compound
A heterocyclic compound is a cyclic compound which has atoms of at least two different elements as members of its ring. The counterparts of heterocyclic compounds are homocyclic compounds, the rings of which are made of a single element....
aromatic organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
, consisting of a pyrimidine
Pyrimidine
Pyrimidine is a heterocyclic aromatic organic compound similar to benzene and pyridine, containing two nitrogen atoms at positions 1 and 3 of the six-member ring...
ring fused to an imidazole
Imidazole
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
ring. Purines, including substituted purines and their tautomer
Tautomer
Tautomers are isomers of organic compounds that readily interconvert by a chemical reaction called tautomerization. This reaction commonly results in the formal migration of a hydrogen atom or proton, accompanied by a switch of a single bond and adjacent double bond...
s, are the most widely distributed kind of nitrogen-containing heterocycle in nature.
Purines and pyrimidines make up the two groups of nitrogenous bases, including the two groups of nucleotide bases. Two of the four deoxyribonucleotide
Deoxyribonucleotide
A deoxyribonucleotide is the monomer, or single unit, of DNA, or deoxyribonucleic acid. Each deoxyribonucleotide comprises three parts: a nitrogenous base, a deoxyribose sugar, and one phosphate group. The nitrogenous base is always bonded to the 1' carbon of the deoxyribose, which is distinguished...
s and two of the four ribonucleotide
Ribonucleotide
A ribonucleotide or ribotide is a nucleotide in which a purine or pyrimidine base is linked to a ribose molecule and exactly one phosphate group. In living organisms the most common bases for ribonucleotides are adenine , guanine , cytosine , or uracil ....
s, the respective building-blocks of DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
and RNA
RNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
, are purines.
Notable purines
The quantity of naturally occurring purines produced on earth is huge. Two of the four bases in nucleic acidNucleic acid
Nucleic acids are biological molecules essential for life, and include DNA and RNA . Together with proteins, nucleic acids make up the most important macromolecules; each is found in abundance in all living things, where they function in encoding, transmitting and expressing genetic information...
s, adenine
Adenine
Adenine is a nucleobase with a variety of roles in biochemistry including cellular respiration, in the form of both the energy-rich adenosine triphosphate and the cofactors nicotinamide adenine dinucleotide and flavin adenine dinucleotide , and protein synthesis, as a chemical component of DNA...
(2) and guanine
Guanine
Guanine is one of the four main nucleobases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine . In DNA, guanine is paired with cytosine. With the formula C5H5N5O, guanine is a derivative of purine, consisting of a fused pyrimidine-imidazole ring system with...
(3), are purines. In DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
, these bases form hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
s with their complementary
Complementarity (molecular biology)
In molecular biology, complementarity is a property of double-stranded nucleic acids such as DNA, as well as DNA:RNA duplexes. Each strand is complementary to the other in that the base pairs between them are non-covalently connected via two or three hydrogen bonds...
pyrimidine
Pyrimidine
Pyrimidine is a heterocyclic aromatic organic compound similar to benzene and pyridine, containing two nitrogen atoms at positions 1 and 3 of the six-member ring...
s thymine
Thymine
Thymine is one of the four nucleobases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The others are adenine, guanine, and cytosine. Thymine is also known as 5-methyluracil, a pyrimidine nucleobase. As the name suggests, thymine may be derived by methylation of uracil at...
and cytosine
Cytosine
Cytosine is one of the four main bases found in DNA and RNA, along with adenine, guanine, and thymine . It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attached . The nucleoside of cytosine is cytidine...
, respectively. This is called complementary base pairing. In RNA
RNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
, the complement of adenine is uracil
Uracil
Uracil is one of the four nucleobases in the nucleic acid of RNA that are represented by the letters A, G, C and U. The others are adenine, cytosine, and guanine. In RNA, uracil binds to adenine via two hydrogen bonds. In DNA, the uracil nucleobase is replaced by thymine.Uracil is a common and...
instead of thymine.
Other notable purines are hypoxanthine
Hypoxanthine
Hypoxanthine is a naturally occurring purine derivative. It is occasionally found as a constituent of nucleic acids where it is present in the anticodon of tRNA in the form of its nucleoside inosine. It has a tautomer known as 6-Hydroxypurine. Hypoxanthine is a necessary additive in certain cell,...
(4), xanthine
Xanthine
Xanthine , is a purine base found in most human body tissues and fluids and in other organisms. A number of stimulants are derived from xanthine, including caffeine and theobromine....
(5), theobromine
Theobromine
Theobromine , also known as xantheose, is a bitter alkaloid of the cacao plant, with the chemical formula C7H8N4O2. It is found in chocolate, as well as in a number of other foods, including the leaves of the tea plant, and the kola nut...
(6), caffeine
Caffeine
Caffeine is a bitter, white crystalline xanthine alkaloid that acts as a stimulant drug. Caffeine is found in varying quantities in the seeds, leaves, and fruit of some plants, where it acts as a natural pesticide that paralyzes and kills certain insects feeding on the plants...
(7), uric acid
Uric acid
Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the formula C5H4N4O3. It forms ions and salts known as urates and acid urates such as ammonium acid urate. Uric acid is created when the body breaks down purine nucleotides. High blood concentrations of uric acid...
(8) and isoguanine
Isoguanine
Isoguanine or 2-hydroxyladenine is a purine base that is an isomer of guanine. It is a product of oxidative damage to DNA and has been shown to cause mutation. It is also used in combination with isocytosine in studies of unnatural nucleic acid analogues of the normal base pairs in DNA....
(9).

Functions
Aside from DNA and RNA, purines are biochemically significant components in a number of other important biomolecules, such as ATPAdenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
, GTP
Guanosine triphosphate
Guanosine-5'-triphosphate is a purine nucleoside triphosphate. It can act as a substrate for the synthesis of RNA during the transcription process...
, cyclic AMP, NADH, and coenzyme A
Coenzyme A
Coenzyme A is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All sequenced genomes encode enzymes that use coenzyme A as a substrate, and around 4% of cellular enzymes use it as a substrate...
. Purine (1) itself, has not been found in nature, but it can be produced by organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
.
They may also function directly as neurotransmitters, acting upon purinergic receptors. Adenosine activates adenosine receptor
Adenosine receptor
The adenosine receptors are a class of purinergic receptors, G protein-coupled receptors with adenosine as endogenous ligand.-Pharmacology:...
s.
History
The name 'purine' (purum uricum) was coined by the GermanGermany
Germany , officially the Federal Republic of Germany , is a federal parliamentary republic in Europe. The country consists of 16 states while the capital and largest city is Berlin. Germany covers an area of 357,021 km2 and has a largely temperate seasonal climate...
chemist
Chemist
A chemist is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties such as density and acidity. Chemists carefully describe the properties they study in terms of quantities, with detail on the level of molecules and their component atoms...
Emil Fischer
Hermann Emil Fischer
Hermann Emil Fischer, Emil Fischer was a German chemist and 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He developed the Fischer projection, a symbolic way of drawing asymmetric carbon atoms.-Early years:Fischer was born in Euskirchen, near Cologne,...
in 1884. He synthesized it for the first time in 1899. The starting material for the reaction sequence was uric acid
Uric acid
Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the formula C5H4N4O3. It forms ions and salts known as urates and acid urates such as ammonium acid urate. Uric acid is created when the body breaks down purine nucleotides. High blood concentrations of uric acid...
(8), which had been isolated from kidney stones by Scheele in 1776. Uric acid (8) was reacted with PCl5 to give 2,6,8-trichloropurine (10), which was converted with HI
Hydrogen iodide
Hydrogen iodide is a diatomic molecule. Aqueous solutions of HI are known as iohydroic acid or hydroiodic acid, a strong acid. Gas and aqueous solution are interconvertible...
and PH4I
Phosphonium salt
A phosphonium salt is a salt containing the phosphonium ion such as phosphonium iodide . More commonly, phosphonium refers to a quaternary organic derivative such as tetraphenylphosphonium chloride, 4P+ Cl- and tetramethylphosphonium iodide, [P4]+I−.Alkyltriphenylphosphonium salts are widely...
to give 2,6-diiodopurine (11). The product was reduced to purine (1) using zinc-dust. Purines are also much larger than pyrimidines.

Metabolism
Many organisms have metabolic pathwayMetabolic pathway
In biochemistry, metabolic pathways are series of chemical reactions occurring within a cell. In each pathway, a principal chemical is modified by a series of chemical reactions. Enzymes catalyze these reactions, and often require dietary minerals, vitamins, and other cofactors in order to function...
s to synthesize and break down purines.
Purines are biologically synthesized as nucleoside
Nucleoside
Nucleosides are glycosylamines consisting of a nucleobase bound to a ribose or deoxyribose sugar via a beta-glycosidic linkage...
s (bases attached to ribose
Ribose
Ribose is an organic compound with the formula C5H10O5; specifically, a monosaccharide with linear form H––4–H, which has all the hydroxyl groups on the same side in the Fischer projection....
).
Sources
Purines are found in high concentration in meat and meat products, especially internal organs such as liver and kidney. In general, plant-based diets are low in purines.Examples of high-purine sources include: sweetbread
Sweetbread
Sweetbreads or ris are culinary names for the thymus or the pancreas especially of the calf and lamb...
s, anchovies, sardine
Sardine
Sardines, or pilchards, are several types of small, oily fish related to herrings, family Clupeidae. Sardines are named after the Mediterranean island of Sardinia, around which they were once abundant....
s, liver, beef kidneys, brains, meat extract
Meat extract
Meat extract is highly concentrated meat stock, usually made from beef. It is used to add meat flavor in cooking, and to make broth for drinking....
s (e.g., Oxo, Bovril), herring, mackerel
Mackerel
Mackerel is a common name applied to a number of different species of fish, mostly, but not exclusively, from the family Scombridae. They may be found in all tropical and temperate seas. Most live offshore in the oceanic environment but a few, like the Spanish mackerel , enter bays and can be...
, scallop
Scallop
A scallop is a marine bivalve mollusk of the family Pectinidae. Scallops are a cosmopolitan family, found in all of the world's oceans. Many scallops are highly prized as a food source...
s, game meats, beer (from the yeast) and gravy.
A moderate amount of purine is also contained in beef, pork, poultry, other fish and seafood, asparagus, cauliflower, spinach, mushrooms, green peas, lentils, dried peas, beans, oatmeal, wheat bran, wheat germ, and hawthorn.
Higher levels of meat and seafood consumption are associated with an increased risk of gout
Gout
Gout is a medical condition usually characterized by recurrent attacks of acute inflammatory arthritis—a red, tender, hot, swollen joint. The metatarsal-phalangeal joint at the base of the big toe is the most commonly affected . However, it may also present as tophi, kidney stones, or urate...
, whereas a higher level of consumption of dairy products is associated with a decreased risk. Moderate intake of purine-rich vegetables or protein is not associated with an increased risk of gout.
In August 2011, a report, based on NASA
NASA
The National Aeronautics and Space Administration is the agency of the United States government that is responsible for the nation's civilian space program and for aeronautics and aerospace research...
studies with meteorites found on Earth
Earth
Earth is the third planet from the Sun, and the densest and fifth-largest of the eight planets in the Solar System. It is also the largest of the Solar System's four terrestrial planets...
, was published suggesting purine and related organic molecules (including the DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
and RNA
RNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
components, adenine
Adenine
Adenine is a nucleobase with a variety of roles in biochemistry including cellular respiration, in the form of both the energy-rich adenosine triphosphate and the cofactors nicotinamide adenine dinucleotide and flavin adenine dinucleotide , and protein synthesis, as a chemical component of DNA...
and guanine
Guanine
Guanine is one of the four main nucleobases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine . In DNA, guanine is paired with cytosine. With the formula C5H5N5O, guanine is a derivative of purine, consisting of a fused pyrimidine-imidazole ring system with...
) may have been formed extraterrestrially in outer space
Outer space
Outer space is the void that exists between celestial bodies, including the Earth. It is not completely empty, but consists of a hard vacuum containing a low density of particles: predominantly a plasma of hydrogen and helium, as well as electromagnetic radiation, magnetic fields, and neutrinos....
.
Laboratory synthesis
In addition to in vivoIn vivo
In vivo is experimentation using a whole, living organism as opposed to a partial or dead organism, or an in vitro controlled environment. Animal testing and clinical trials are two forms of in vivo research...
synthesis of purines in purine metabolism
Purine metabolism
-Biosynthesis:Purines are biologically synthesized as nucleotides and in particular as ribotides, i.e. bases attached to ribose 5-phosphate. A key regulatory step is the production of 5-phospho-α-D-ribosyl 1-pyrophosphate by PRPP synthetase, which is activated by inorganic phosphate and...
, purine can also be created artificially.
Purine (1) is obtained in good yield when formamide
Formamide
Formamide, also known as methanamide, is an amide derived from formic acid. It is a clear liquid which is miscible with water and has an ammonia-like odor. It is used primarily for manufacturing sulfa drugs and synthesizing vitamins and as a softener for paper and fiber...
is heated in an open vessel at 170 °C for 28 hours.

This remarkable reaction and others like it have been discussed in the context of the origin of life
Abiogenesis
Abiogenesis or biopoesis is the study of how biological life arises from inorganic matter through natural processes, and the method by which life on Earth arose...
.
Procedure:
Formamide (45 grams) was heated in an open vessel with a condenser for 28 hours in an oil bath at 170-190 °C. After removing excess formamide (32.1 grams) by vacuum distillation, the residue was refluxed with methanol. The methanol solvent was filtered, the solvent removed from the filtrate by vacuum distillation, and almost pure purine obtained; yield 4.93 grams (71% yield from formamide consumed). Crystallization from acetone afforded purine as colorless crystals; melting point 218 °C.
Oro, Orgel and co-workers have shown that four molecules of HCN tetramerize to form diaminomaleodinitrile (12), which can be converted into almost all natural-occurring purines.

The Traube purine synthesis (1900) is a classic reaction (named after Wilhelm Traube
Wilhelm Traube
Wilhelm Traube was a German chemist.- Biography :Traube was born at Ratibor in Prussian Silesia, a son of the famous private scholar Moritz Traube....
) between an amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
-substituted pyrimidine
Pyrimidine
Pyrimidine is a heterocyclic aromatic organic compound similar to benzene and pyridine, containing two nitrogen atoms at positions 1 and 3 of the six-member ring...
and formic acid
Formic acid
Formic acid is the simplest carboxylic acid. Its chemical formula is HCOOH or HCO2H. It is an important intermediate in chemical synthesis and occurs naturally, most notably in the venom of bee and ant stings. In fact, its name comes from the Latin word for ant, formica, referring to its early...
.


