
Vitamin B12 total synthesis
Encyclopedia
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
describes the total synthesis
Total synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
of the complex biomolecule
Biomolecule
A biomolecule is any molecule that is produced by a living organism, including large polymeric molecules such as proteins, polysaccharides, lipids, and nucleic acids as well as small molecules such as primary metabolites, secondary metabolites, and natural products...
vitamin B12
Vitamin B12
Vitamin B12, vitamin B12 or vitamin B-12, also called cobalamin, is a water-soluble vitamin with a key role in the normal functioning of the brain and nervous system, and for the formation of blood. It is one of the eight B vitamins...
. A method first reported by the groups of Robert Burns Woodward
Robert Burns Woodward
Robert Burns Woodward was an American organic chemist, considered by many to be the preeminent organic chemist of the twentieth century...
and Albert Eschenmoser
Albert Eschenmoser
Albert Eschenmoser is a Swiss chemist working at the ETH Zurich and The Scripps Research Institute.His work together with Lavoslav Ružička on terpenes and the postulation of squalene cyclization to form lanosterol improved the insight into steroid biosynthesis.In the early 1960s, Eschenmoser began...
in 1973 is considered a classic in this research field.
The Woodward publications on this topic starting in 1968 in the journal Pure and Applied Chemistry
Pure and Applied Chemistry
Pure and Applied Chemistry is the official journal for the International Union of Pure and Applied Chemistry. It is published monthly and contains recommendations and reports, and lectures from symposia....
are a transcript of a lecture Eschenmoser published an article in 1977 in Science
Science (journal)
Science is the academic journal of the American Association for the Advancement of Science and is one of the world's top scientific journals....
and is also a modification of a lecture. The X-ray crystal structure had already been determined by Dorothy Hodgkin in 1956. The total synthesis was also a scientific breakthrough because in one of its key steps the foundation was laid for what was to become known as the Woodward–Hoffmann rules formulated in 1982.
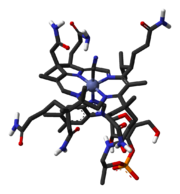
The molecule
The core of the molecule vitamin B12 (cobalamin) is a corrinCorrin
Corrin is an heterocyclic compound. It is the parent macrocycle related to substituted derivative that is found in vitamin B12. Its name reflects that it is the "core" of vitamin B12 .-Coordination chemistry:...
structure (depicted in red) with at its center a cobalt
Cobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
ion. Several vitamins exist with different cobalt ligands but the total synthesis concerned the one with a cyano ligand called cyanocobalamin
Cyanocobalamin
Cyanocobalamin is an especially common vitamer of the vitamin B12 family. It is the most famous vitamer of the family, because it is, in chemical terms, the most air-stable...
. The corrin rim is lined with methyl groups (8) and lined with amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
groups (6) linked through C1 and C2 spacers. A seventh amide group is N-alkylated by a large tail consisting of a isopropanol group, a phosphate
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
group, a ribose
Ribose
Ribose is an organic compound with the formula C5H10O5; specifically, a monosaccharide with linear form H––4–H, which has all the hydroxyl groups on the same side in the Fischer projection....
group and a dimethylbenzimidazole
Benzimidazole
Benzimidazole is a heterocyclic aromatic organic compound. This bicyclic compound consists of the fusion of benzene and imidazole. The most prominent benzimidazole compound in nature is N-ribosyl-dimethylbenzimidazole, which serves as an axial ligand for cobalt in vitamin B12. Benzimidazole, in...
group. One of the nitrogen atoms on the imidazole is a fifth nitrogen ligand for the cobalt atom. A total of nine carbon atoms on the corrin frame are chiral, adding an additional challenge to the synthesis.
| Vitamin B12 overview |
|---|
Retrosynthesis
In retrosynthesis step 1 was easy. It was already established by Bernhauer in 1960 that the tail can be removed from vitamin B12 by amide hydrolysis to cobyric acid and again replaced. The Woodward/Eschenmoser venture is strictly a formal synthesis because the ultimate target was cobyric acid and tail addition was not included.| Vitamin B12 retrosynthesis |
|---|
The methyl groups at C5 and C15 were added only after construction of the corrin core. This core was synthesised by joining an AD western part (III) with an BC eastern part (IV). Direct union was not considered feasible due to steric hindrance but both joins were made possible by a sulfur contraction method.
Ring A synthesis
Starting point for the synthesis of ring A was methoxydimethylindol 3 synthesised by condensation of the Schiff baseSchiff base
A Schiff base, named after Hugo Schiff, is a compound with a functional group that contains a carbon-nitrogen double bond with the nitrogen atom connected to an aryl or alkyl group, not hydrogen....
from m-anisidine
M-Anisidine
m-Anisidine one of the three isomers of anisidine. It is very poisonous for the blood leading to pink coloration of the skin and inner suffocation. Additionally its vapors are irritating to eyes, mucous membranes, the respiratory system and skin....
1 and acetoin 2. Reaction with the Grignard reagent of propargyl iodide 4 give the propargyl indolenine 5 and ring-closure to 7 was brought about by boron trifluoride
Boron trifluoride
Boron trifluoride is the chemical compound with the formula BF3. This pungent colourless toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.-Structure and bonding:...
and mercuric oxide in methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
through intermediate 6 (electrophilic addition) with the two methyl groups forced into a cis-relationship.
| Vitamin B12 AD ring part A |
|---|
This compound existed as a mixture of two enantiomers (racemic
Racemic
In chemistry, a racemic mixture, or racemate , is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule. The first known racemic mixture was "racemic acid", which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid.- Nomenclature :A...
) and chiral resolution
Chiral resolution
Chiral resolution in stereochemistry is a process for the separation of racemic compounds into their enantiomers. It is an important tool in the production of optically active drugs...
using (-)-alpha-phenylethylisocyanate was employed for the isolation of the (+)-enantiomer.
| Vitamin B12 ring A chiral resolution |
|---|
Ring D synthesis
The D ring was synthesized starting from chiral (S)-camphorCamphor
Camphor is a waxy, white or transparent solid with a strong, aromatic odor. It is a terpenoid with the chemical formula C10H16O. It is found in wood of the camphor laurel , a large evergreen tree found in Asia and also of Dryobalanops aromatica, a giant of the Bornean forests...
8 which was converted to oxime
Oxime
An oxime is a chemical compound belonging to the imines, with the general formula R1R2C=NOH, where R1 is an organic side chain and R2 may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds...
9 (oxidation / hydroxylamine
Hydroxylamine
Hydroxylamine is an inorganic compound with the formula NH2OH. The pure material is a white, unstable crystalline, hygroscopic compound. However, hydroxylamine is almost always provided and used as an aqueous solution. It is used to prepare oximes, an important functional group. It is also an...
), then to amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
10 (hydrolysis), lactam 11 (acid-amide condensation), N-nitroso compound 12, diazo
Diazo
Diazo refers to a type of organic compound called diazo compound that has two linked nitrogen atoms as a terminal functional group. The general formula is R2C=N2. The simplest example of a diazo compound is diazomethane...
compound 13 and to cyclopentene 14 (carbene, methyl group migration). Reduction (LiAlH4) gave alcohol 15, oxidation (chromic acid
Chromic acid
The term chromic acid is usually used for a mixture made by adding concentrated sulfuric acid to a dichromate, which may contain a variety of compounds, including solid chromium trioxide. This kind of chromic acid may be used as a cleaning mixture for glass. Chromic acid may also refer to the...
) gave aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
16, a Wittig reaction
Wittig reaction
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
with carbomethoxymethylenetriphenylphosphorane gave trans-alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
17 and hydrolysis gave carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
18.
| Vitamin B12 D ring synthesis |
|---|
AD coupling
Amine 7 and carboxylic acid 18 were condensed through the acid chloride to amideAmide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
19. treatment with potassium tert-butoxide
Potassium tert-butoxide
Potassium tert-butoxide is the chemical compound with the formula 3COK. This colourless solid is a strong base useful in organic synthesis. It exists as a tetrameric cubane-like cluster...
in tert-butanol
Tert-Butanol
tert-Butanol, or 2-methyl-2-propanol, is the simplest tertiary alcohol. It is one of the four isomers of butanol. tert-Butanol is a clear liquid with a camphor-like odor. It is very soluble in water and miscible with ethanol and diethyl ether...
then gave tricycle 20 in a Michael reaction
Michael reaction
The Michael reaction or Michael addition is the nucleophilic addition of a carbanion or another nucleophile to an alpha, beta unsaturated carbonyl compound. It belongs to the larger class of conjugate additions. This is one of the most useful methods for the mild formation of C-C bonds...
with hydrogen atoms in trans relationship. In anticipation to the partial reduction of the aromatic ring in the later compound protective groups were added: one of the carbonyl groups as the ketal in 21 and the other as an enol ether
Enol ether
An enol ether is an alkene with an alkoxy substituent. The general structure is R_1R_2C=CR_3-O-R_4 with R an alkyl or an aryl group. Enol ethers and enamines are so-called activated alkenes or electron rich alkenes because the oxygen atom donates electrons to the double bond by forming a resonance...
through the iminium
Iminium
An iminium salt or cation in organic chemistry has the general structure [R1R2C=NR3R4]+ and is as such a protonated or substituted imine. It is an intermediate in many organic reactions such as the Beckmann rearrangement, Vilsmeier-Haack reaction, Stephen reaction or the Duff reaction...
salt 22 (Triethyloxonium tetrafluoroborate) and the orthoamide 23 (sodium methoxide
Sodium methoxide
Sodium methoxide is a chemical compound, with formula CH3ONa. This colourless solid, which is formed by the deprotonation of methanol, is a widely used reagent in industry and the laboratory...
/ methanol). Enol ether 24 was obtained by heating in toluene expelling ethanol. Birch reduction
Birch reduction
The Birch Reduction is an organic reaction which is particularly useful in synthetic organic chemistry. The reaction was reported in 1944 by the Australian chemist Arthur Birch working in the Dyson Perrins Laboratory in the University of Oxford, building on earlier work by Wooster and Godfrey in...
provided tetraene 25 and acid treatment gave the dione 26 dubbed pentacyclenone.
| Vitamin B12 AD ring synthesis |
|---|
The second protective group in 26 (acetal, acid hydrolysis) was reconverted to the ketone in 27. The monooxime 28 (at the more hindered ketone group) was synthesised from the dioxime by selective hydrolysis (nitrous acid
Nitrous acid
Nitrous acid is a weak and monobasic acid known only in solution and in the form of nitrite salts.Nitrous acid is used to make diazides from amines; this occurs by nucleophilic attack of the amine onto the nitrite, reprotonation by the surrounding solvent, and double-elimination of water...
/ acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
). The new nitrogen atom is also the second nitrogen atom required for the AD building block. Both the cyclopentene ring and the cyclohexenone ring were oxidized next in an ozonolysis
Ozonolysis
Ozonolysis is the cleavage of an alkene or alkyne with ozone to form organic compounds in which the multiple carbon–carbon bond has been replaced by a double bond to oxygen...
(ozone
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
) forming triketone 29, an aldol condensation
Aldol condensation
An aldol condensation is an organic reaction in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by a dehydration to give a conjugated enone....
of the 1,5-dicarbonyl unit (pyrrolidine acetate) formed cyclohexene 30 with tosylation of the oxime group, a second oxidation with periodic acid
Periodic acid
Periodic acid, or iodic acid is an oxoacid of iodine having chemical formula HIO4 or H5IO6.In dilute aqueous solution, periodic acid exists as discrete hydronium and metaperiodate ions. When more concentrated, orthoperiodic acid, H5IO6, is formed; this dissociates into hydronium and...
cleaves the cyclohexene
Cyclohexene
Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes...
ring and diazomethane
Diazomethane
Diazomethane is the chemical compound CH2N2. It is the simplest of diazo compounds. In the pure form at room temperature, it is a extremely sensitive explosive yellow gas, thus it is almost universally used as a solution in diethyl ether...
esterifies the resulting carboxylic acid group in 31. A Beckmann rearrangement
Beckmann rearrangement
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann , is an acid-catalyzed rearrangement of an oxime to an amide...
(methanol, sodium polystyrene sulfonate
Sodium polystyrene sulfonate
Sodium polystyrene sulfonate is a type of polymer and ionomer based on polystyrene. It is the sodium salt of polystyrene sulfonic acid.-Chemical properties:...
2 hrs, 170°C) took place next to lactam 32 (not isolated) which reacted further to the tetracycle 33 called alpha-corrnorsterone in an amine-carbonyl condensation - aldol condensation
Aldol condensation
An aldol condensation is an organic reaction in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by a dehydration to give a conjugated enone....
cascade. This compound resisted ring-opening of the lactam group due to incorrect stereochemistry of the propionic ester side-chain. The alpha compound was therefore converted its epimer
Epimer
In chemistry, epimers are diastereomers that differ in configuration of only one stereogenic center. Diastereomers are a class of stereoisomers that are non-superposable, non-mirror images of one another....
34 by first equilibrating in excess base followed by reacidifiying and diazomethane treatment. This epimer was then converted to the 35 by the simultaneous action of methanol and thiophenol
Thiophenol
Thiophenol is an organosulfur compound with the formula C6H6S, and sometimes abbreviated as PhSH. This foul-smelling colourless liquid is the simplest aromatic thiol. The chemical structures of thiophenols are analogous to phenols except the oxygen atom in the hydroxyl group bonded to the...
. This ensured the differentiation of what will become the imidazole tail. the Ozonolysis gave aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
36 with ammonia converting the thioester into an amide group and aldehyde reduction (sodium borohydride
Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
) followed by mesylation and bromination (lithium bromide) gave the bromide 37 with conversion of the amide group into a nitrile
Nitrile
A nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called...
group as the completed AD section.
| Vitamin B12 AD ring synthesis II |
|---|
Ring C synthesis
The starting material for the synthesis of ring C was chiral (+)-camphorquinone 38 which can be converted to the acetoxy trimethylcyclohexene carboxylic acid ester 39 by addition of trifluoroborane in acetic anhydrideAcetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
, a reaction pioneered by Manasse & Samuel in 1902. Ester hydrolysis to carboxylic acid 40 and amidation to amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
41 was followed by ozonolysis
Ozonolysis
Ozonolysis is the cleavage of an alkene or alkyne with ozone to form organic compounds in which the multiple carbon–carbon bond has been replaced by a double bond to oxygen...
to peroxide
Organic peroxide
Organic peroxides are organic compounds containing the peroxide functional group . If the R' is hydrogen, the compound is called an organic hydroperoxide. Peresters have general structure RCOOR. The O-O bond easily breaks and forms free radicals of the form RO·...
42 that was reduced to succinimide
Succinimide
Succinimide is a cyclic imide with the formula C4H5NO2. It is used in a variety of organic syntheses, as well as in some industrial silver plating processes.-Succinimides:...
43 by zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
and methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
. treatment with methanolic hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
gave lactam
Lactam
A lactam is a cyclic amide. Prefixes indicate how many carbon atoms are present in the ring: β-lactam , γ-lactam , δ-lactam...
44 and pyrolysis
Pyrolysis
Pyrolysis is a thermochemical decomposition of organic material at elevated temperatures without the participation of oxygen. It involves the simultaneous change of chemical composition and physical phase, and is irreversible...
gave the complete C ring 45.
| Vitamin B12 C ring synthesis |
|---|
Ring B synthesis
The starting material for ring B was 3-methyl-4-oxo-2-pentenoic acid 46 which was reacted with butadiene in a Diels-Alder reactionDiels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
(stannic chloride) to racemic cyclohexene 47. This reaction is stereospecific with the methyl group and the carboxylic acid group ending up in a cis relationship (with hindsight a disrotatory
Disrotatory
In a conrotatory mode of an electrocyclic reaction the substituents located at the termini of a conjugated double bond system move in the same direction during ring opening or ring closure...
electrocyclic reaction). Chiral resolution
Chiral resolution
Chiral resolution in stereochemistry is a process for the separation of racemic compounds into their enantiomers. It is an important tool in the production of optically active drugs...
using alpha-phenylethylamine gave optically active 47. Oxidation of the double bond with chromic acid
Chromic acid
The term chromic acid is usually used for a mixture made by adding concentrated sulfuric acid to a dichromate, which may contain a variety of compounds, including solid chromium trioxide. This kind of chromic acid may be used as a cleaning mixture for glass. Chromic acid may also refer to the...
gave the triacid 48 as an intermediate which gave dilactone
Lactone
In chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
49 in two intramolecular esterifications. An Arndt–Eistert reaction elongated the carboxylic acid chain in 50, reaction with ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
gave lactam
Lactam
A lactam is a cyclic amide. Prefixes indicate how many carbon atoms are present in the ring: β-lactam , γ-lactam , δ-lactam...
51 and reaction with phosphorus pentasulfide
Phosphorus pentasulfide
Phosphorus pentasulfide is the inorganic compound with the formula P4S10. This yellow solid is the one of two phosphorus sulfides of commercial value...
give thiolactam 52.
| Vitamin B12 D ring synthesis |
|---|
BC coupling
Ring B (52) and ring C (45) were joined with benzoyl peroxideBenzoyl peroxide
Benzoyl peroxide is an organic compound in the peroxide family. It consists of two benzoyl groups bridged by a peroxide link. Its structural formula is [C6H5C]2O2. It is one of the most important organic peroxides in terms of applications and the scale of its production...
/HCl
HCL
HCL or HCl can stand for:* Hairy cell leukemia, an uncommon and slowly progressing B cell leukemia* Hardware compatibility list...
to sulfur bridged 53, the sulfur atom was extruded with triethylphosphite to enamine-imine 54 in a first of two sulfur contraction
Eschenmoser sulfide contraction
The Eschenmoser sulfide contraction is an organic reaction first described by Albert Eschenmoser for the synthesis of 1,3-dicarbonyl compounds from a thioester. The method requires a base and a tertiary phosphine...
s and the lactam group converted to the thiolactam 55 (trimethyloxonium fluoroborate / hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
)
| Vitamin B12 BC ring system synthesis |
|---|
AD BC coupling
The eastern part of the molecule BC (cyanobromide 37) and the western part AD (thiodextolin 55, with the propionic acid ester group racemized) were then joined using potassium t-butoxide to thioether 56 (through a sulfide ion intermediate). A second sulfur contractionEschenmoser sulfide contraction
The Eschenmoser sulfide contraction is an organic reaction first described by Albert Eschenmoser for the synthesis of 1,3-dicarbonyl compounds from a thioester. The method requires a base and a tertiary phosphine...
(cyanoethyl phosphine/ trifluoroacetic acid
Trifluoroacetic acid
Trifluoroacetic acid is the simplest stable perfluorinated carboxylic acid chemical compound, with the formula CF3CO2H. It is a strong carboxylic acid due to the influence of the electronegative trifluoromethyl group. TFA is almost 100,000-fold more acidic than acetic acid...
/sulfolane
Sulfolane
Sulfolane is a clear, colorless liquid commonly used in the chemical industry as an extractive distillation solvent or reaction solvent. Sulfolane was originally developed by the Shell Oil Company in the 1960s as a solvent to purify butadiene...
) yielded cyanocorrigenolide 57 with the propionic acid ester group of ring C also racemised. Due to the steric bulk of both reactants this contraction was the only successful method. The oxygen atoms in the lactam
Lactam
A lactam is a cyclic amide. Prefixes indicate how many carbon atoms are present in the ring: β-lactam , γ-lactam , δ-lactam...
and the lactone
Lactone
In chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
group were replaced by sulfur (phosphorus pentasulfide
Phosphorus pentasulfide
Phosphorus pentasulfide is the inorganic compound with the formula P4S10. This yellow solid is the one of two phosphorus sulfides of commercial value...
/4-methylpyridine
4-Methylpyridine
4-Methylpyridine is the organic compound with the formula CH3C5H4N. It is one of the three isomers of methylpyridine. This colourless pungent liquid is a building block for the synthesis of other heterocyclic compounds...
) in dithiocyanocorrigenolide 58 and the S-methyl derivate 59 was formed by reaction with trimethyloxonium fluoroborate. Dimethylamine
Dimethylamine
Dimethylamine is an organic compound with the formula 2NH. This secondary amine is a colorless, flammable liquified gas with an ammonia-like odor. Dimethylamine is generally encountered as a solution in water at concentrations up to around 40%...
addition opened the thiolactone
Thiolactone
Thiolactones are a class of heterocyclic compounds in organic chemistry. They are analogs of the more common lactones in which an oxygen atom is replaced with a sulfur atom. The sulfur atom is within the ring system and adjacent to a carbonyl group....
ring with an exocyclic alkene group in 60 by elimination of the sulfide anion from the methyl group. In an early example of template-directed synthesis this compound was isolated as the cobalt adduct.
| Vitamin B12 BCAD construction |
|---|
The final cyclisation reaction of 60 to 61 was facilitated by the central cobalt ion (placing the ends in close proximity) and consisted of another type of sulfur contraction employing basic conditions (diazabicyclononane / dimethylacetamide
Dimethylacetamide
Dimethylacetamide is the organic compound with the formula CH3CN2. This colorless, water miscible, high boiling liquid is commonly used as a polar solvent in organic chemistry. DMAc is miscible with most other solvents, although it is poorly soluble in aliphatic hydrocarbons.The chemical reactions...
). This reaction takes place with racemisation of the propionic acid ester group of ring C. Oxidation (iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
/acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
) formed lactone 62 and restored the correct stereochemistry at the ring B propionic acid ester tail.
The final efforts were directed at placing methyl groups at position 5 and 15. With position 10 sufficiently shielded reaction with chloromethyl benzyl ether produced the di(chloromethyl) adduct which was further converted to the dithiophenyl compound 63 using thiophenol
Thiophenol
Thiophenol is an organosulfur compound with the formula C6H6S, and sometimes abbreviated as PhSH. This foul-smelling colourless liquid is the simplest aromatic thiol. The chemical structures of thiophenols are analogous to phenols except the oxygen atom in the hydroxyl group bonded to the...
, isolation of which required plate chromatography. Desulfurisation took place with Raney nickel
Raney nickel
Raney nickel is a solid catalyst composed of fine grains of a nickel-aluminium alloy, used in many industrial processes. It was developed in 1926 by American]] engineer Murray Raney as an alternative catalyst for the hydrogenation of vegetable oils in industrial processes...
and the reduction reaction also opened the lactone ring to the carboxylic acid which was converted to the ester 64 by reaction with diazomethane
Diazomethane
Diazomethane is the chemical compound CH2N2. It is the simplest of diazo compounds. In the pure form at room temperature, it is a extremely sensitive explosive yellow gas, thus it is almost universally used as a solution in diethyl ether...
. At this stage the number of isomers in the mixture was reduced by HPLC
High-performance liquid chromatography
High-performance liquid chromatography , HPLC, is a chromatographic technique that can separate a mixture of compounds and is used in biochemistry and analytical chemistry to identify, quantify and purify the individual components of the mixture.HPLC typically utilizes different types of stationary...
to just two with the racemic propionic acid ester group at C13 (ring C) remaining in 65. Reaction with sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
converted the cyano group to the amide group in 66, again destroying stereochemistry at C13. The correct isomer 67 (minor yield) was isolated again by HPLC.
| Vitamin B12 BCAD construction part II |
|---|
The amide group was converted to the carboxylic acid group in 68 by action of the cyclohexylnitrone derived from chloroacetaldehyde
Chloroacetaldehyde
Chloroacetaldehyde is the organic compound with the formula ClCH2CHO. Like some related compounds, it is highly electrophilic reagent and a potentially dangerous alkylating agent. The compound is not normally encountered in the anhydrous form, but rather as the hydrate , ClCH2CH2. ...
in combination with silver tetrafluoroborate
Silver tetrafluoroborate
Silver tetrafluoroborate sometimes referred to "silver BF-4" is an inorganic compound commonly encountered in inorganic and organometallic chemistry. Similar to silver hexafluorophosphate, it is commonly used to replace halide anions or ligands with the weakly coordinating tetrafluoroborate anions...
and in the final step 6 ester groups were converted to the amide groups in cobyric acid 69 by reaction with ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
and ammonium chloride
Ammonium chloride
Ammonium chloride NH4Cl is an inorganic compound with the formula NH4Cl. It is a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. Sal ammoniac is a name of natural, mineralogical form of ammonium chloride...
.
| Vitamin B12 BCAD construction part III |
|---|

