Phosphorus pentasulfide
Encyclopedia
Phosphorus pentasulfide is the inorganic compound
with the formula P4S10. This yellow solid is the one of two phosphorus sulfide
s of commercial value. Samples often appear greenish-gray due to impurities.
and is almost identical to the structure of phosphorus pentoxide
.
Phosphorus pentasulfide is obtained by the reaction of liquid white phosphorus (P4) with sulfur above 300 °C. The first synthesis of P4S10 by Berzelius in 1843 was by this method. Alternatively, P4S10 can be formed by reacting elemental sulfur or pyrite
, FeS2, with ferrophosphorus
, impure Fe2P (a byproduct of P4 production from phosphate rock
):
and Malathion
. It is also a component of some amorphous solid electrolyte
s (e.g. 2S-P2S5) for some types of lithium batteries
.
Phosphorus pentasulfide is a dual-use material, as it can be used for manufacture of the VX
nerve agent
.
of P4S10 gives phosphoric acid
:
Other mild nucleophile
s react with P4S10, including alcohol
s and amine
s. Aromatic compounds such as anisole
, ferrocene
and 1-methoxynaphthalene
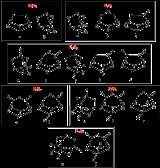
react to form 1,3,2,4-dithiadiphosphetane 2,4-disulfides
such as Lawesson's reagent
.
In organic chemistry P4S10 is used as a thionation reagent. Reactions of this type require refluxing solvents such as benzene
, dioxane or acetonitrile
with P4S10 dissociating into P2S5. P2S5 can be trapped
for example as the pyridine
complex. Ketones are converted to thioketone
s. In esters, imide
s and lactones the oxygen atom can also be replaced by sulfur. With amides the reaction product is a thioamide
. With 1,4-diketones the reagent forms a thiophene
. Compared to the better known Lawesson's reagent
P4S10 suffers from reduced yields.
Inorganic compound
Inorganic compounds have traditionally been considered to be of inanimate, non-biological origin. In contrast, organic compounds have an explicit biological origin. However, over the past century, the classification of inorganic vs organic compounds has become less important to scientists,...
with the formula P4S10. This yellow solid is the one of two phosphorus sulfide
Phosphorus sulfide
The Phosphorus sulfides comprise a family of inorganic compounds containing only phosphorus and sulfur. These compounds have the formula P4Sx where x is less than or equal to 10...
s of commercial value. Samples often appear greenish-gray due to impurities.
Structure and synthesis
Its tetrahedral molecular structure is related to that of adamantaneAdamantane
Adamantane is a colorless, crystalline chemical compound with a camphor-like odor. With a formula C10H16, it is a cycloalkane and also the simplest diamondoid. Adamantane molecules consist of three cyclohexane rings arranged in the "armchair" configuration. It is unique in that it is both rigid...
and is almost identical to the structure of phosphorus pentoxide
Phosphorus pentoxide
Phosphorus pentoxide is a chemical compound with molecular formula P4O10 . This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant.-Structure:...
.
Phosphorus pentasulfide is obtained by the reaction of liquid white phosphorus (P4) with sulfur above 300 °C. The first synthesis of P4S10 by Berzelius in 1843 was by this method. Alternatively, P4S10 can be formed by reacting elemental sulfur or pyrite
Pyrite
The mineral pyrite, or iron pyrite, is an iron sulfide with the formula FeS2. This mineral's metallic luster and pale-to-normal, brass-yellow hue have earned it the nickname fool's gold because of its resemblance to gold...
, FeS2, with ferrophosphorus
Iron phosphide
Iron phosphide can be used as a semiconductor. It has use in high power, high frequency applications, such as laser diodes....
, impure Fe2P (a byproduct of P4 production from phosphate rock
Apatite
Apatite is a group of phosphate minerals, usually referring to hydroxylapatite, fluorapatite, chlorapatite and bromapatite, named for high concentrations of OH−, F−, Cl− or Br− ions, respectively, in the crystal...
):
- 4 Fe2P + 18 S → P4S10 + 8 FeS
- 4 Fe2P + 18 FeS2 + heat → P4S10 + 26 FeS
Applications
Approximately 150,000 tons of P4S10 are produced annually. The compound is mainly converted to other derivatives for use as lubrication additives such zinc dithiophosphates. It is also used in the production of pesticides such as ParathionParathion
Parathion, also called parathion-ethyl or diethyl parathion, is an organophosphate compound. It is a potent insecticide and acaricide. It was originally developed by IG Farben in the 1940s. It is highly toxic to non-target organisms, including humans. Its use is banned or restricted in many...
and Malathion
Malathion
Malathion is an organophosphate parasympathomimetic which binds irreversibly to cholinesterase. Malathion is an insecticide of relatively low human toxicity, however one recent study has shown that children with higher levels of organophosphate pesticide metabolites in their urine are more likely...
. It is also a component of some amorphous solid electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
s (e.g. 2S-P2S5) for some types of lithium batteries
Lithium battery
Lithium batteries are disposable batteries that have lithium metal or lithium compounds as an anode. Depending on the design and chemical compounds used, lithium cells can produce voltages from 1.5 V to about 3.7 V, over twice the voltage of an ordinary zinc–carbon battery or alkaline battery...
.
Phosphorus pentasulfide is a dual-use material, as it can be used for manufacture of the VX
VX (nerve agent)
VX, IUPAC name O-ethyl S-[2-ethyl] methylphosphonothioate, is an extremely toxic substance whose only application is in chemical warfare as a nerve agent. As a chemical weapon, it is classified as a weapon of mass destruction by the United Nations in UN Resolution 687...
nerve agent
Nerve agent
Nerve agents are a class of phosphorus-containing organic chemicals that disrupt the mechanism by which nerves transfer messages to organs...
.
Reactivity
Due to hydrolysis by atmospheric moisture, P4S10 evolves H2S, thus P4S10 is associated with a rotten egg odour. Aside from H2S, hydrolysisHydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of P4S10 gives phosphoric acid
Phosphoric acid
Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way...
:
- P4S10 + 16 H2O → 4 H3PO4 + 10 H2S
Other mild nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s react with P4S10, including alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s and amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s. Aromatic compounds such as anisole
Anisole
Anisole, or methoxybenzene, is the organic compound with the formula CH3OC6H5. It is a colorless liquid with a smell reminiscent of anise seed, and in fact many of its derivatives are found in natural and artificial fragrances...
, ferrocene
Ferrocene
Ferrocene is an organometallic compound with the formula Fe2. It is the prototypical metallocene, a type of organometallic chemical compound consisting of two cyclopentadienyl rings bound on opposite sides of a central metal atom. Such organometallic compounds are also known as sandwich compounds...
and 1-methoxynaphthalene
Naphthalene
Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
react to form 1,3,2,4-dithiadiphosphetane 2,4-disulfides
1,3,2,4-Dithiadiphosphetane 2,4-disulfides
1,3,2,4-Dithiadiphosphetane 2,4-disulfides are a class of four-membered ring compounds which contain a P2S2 ring, many of these compounds are able to act as sources of the dithiophosphine ylides...
such as Lawesson's reagent
Lawesson's reagent
Lawesson's reagent, or LR, is a chemical compound used in organic synthesis as a thiation agent. Lawesson's reagent was first made popular by Sven-Olov Lawesson, who did not, however, invent it. Lawesson's reagent was first made in 1956 during a systematic study of the reactions of arenes with...
.
In organic chemistry P4S10 is used as a thionation reagent. Reactions of this type require refluxing solvents such as benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
, dioxane or acetonitrile
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
with P4S10 dissociating into P2S5. P2S5 can be trapped
Chemical trap
In chemistry, a chemical trap is a chemical compound that is used to detect a certain molecule when* The concentration of this molecule is very small and below detection limit...
for example as the pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
complex. Ketones are converted to thioketone
Thioketone
Thioketones are organosulfur compounds related to conventional ketones. Instead of the formula R2C=O, thioketones have the formula R2C=S. Unhindered alkylthioketones are typically unstable; such compounds tend to form polymers or rings.-Preparative methods:Thiones are usually prepared from ketones...
s. In esters, imide
Imide
In organic chemistry, an imide is a functional group consisting of two carbonyl groups bound to nitrogen. These compounds are structurally related to acid anhydrides. The relationship between esters and amides and between imides and anhydrides is analogous, the amine-derived groups are less reactive...
s and lactones the oxygen atom can also be replaced by sulfur. With amides the reaction product is a thioamide
Thioamide
Thioamide is a functional group with the general structure R-CS-NR'R, where R, R', and R are organic groups. They are analogous to amides but they exhibit greater multiple bond character along the C-N bond, resulting in a larger rotational barrier...
. With 1,4-diketones the reagent forms a thiophene
Thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a flat five-membered ring, it is aromatic as indicated by its extensive substitution reactions. Related to thiophene are benzothiophene and dibenzothiophene, containing the thiophene ring fused with one and two benzene...
. Compared to the better known Lawesson's reagent
Lawesson's reagent
Lawesson's reagent, or LR, is a chemical compound used in organic synthesis as a thiation agent. Lawesson's reagent was first made popular by Sven-Olov Lawesson, who did not, however, invent it. Lawesson's reagent was first made in 1956 during a systematic study of the reactions of arenes with...
P4S10 suffers from reduced yields.

