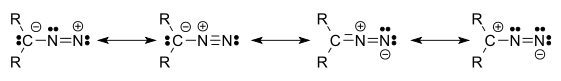
Diazo
Encyclopedia
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
called diazo compound that has two linked nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
atoms (azo
Azo compound
Azo compounds are compounds bearing the functional group R-N=N-R', in which R and R' can be either aryl or alkyl. IUPAC defines azo compounds as: "Derivatives of diazene , HN=NH, wherein both hydrogens are substituted by hydrocarbyl groups, e.g. PhN=NPh azobenzene or diphenyldiazene." The more...
) as a terminal functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
. The general formula is R2C=N2. The simplest example of a diazo compound is diazomethane
Diazomethane
Diazomethane is the chemical compound CH2N2. It is the simplest of diazo compounds. In the pure form at room temperature, it is a extremely sensitive explosive yellow gas, thus it is almost universally used as a solution in diethyl ether...
. The electronic structure of diazo compounds involves a positive charge on the central nitrogen and negative charge distributed between the terminal nitrogen and the carbon. Some of the most stable diazo compounds are α-diazoketones and α-diazoesters since the negative charge is delocalized into the carbonyl. In contrast, most alkyldiazo compounds are explosive. A commercially relevant diazo compound is ethyl diazoacetate
Ethyl diazoacetate
Ethyl diazoacetate is a diazo compound and a reagent in organic chemistry. It was discovered by Theodor Curtius in 1883. The compound can be prepared by reaction of the ethyl ester of glycine with sodium nitrite and sodium acetate in water....
(N2CHCOOEt). A group of isomeric compounds with similar properties are the diazirine
Diazirine
Diazirines are a class of organic molecules consisting of a carbon bound to two nitrogen atoms, which are double-bonded to each other, forming a cyclopropene-like ring...
s, where the carbon and two nitrogens are linked as a ring.
Four resonance structures can be drawn:
Diazo compounds should not be confused with azo compound
Azo compound
Azo compounds are compounds bearing the functional group R-N=N-R', in which R and R' can be either aryl or alkyl. IUPAC defines azo compounds as: "Derivatives of diazene , HN=NH, wherein both hydrogens are substituted by hydrocarbyl groups, e.g. PhN=NPh azobenzene or diphenyldiazene." The more...
s of the type R-N=N-R or with diazonium compound
Diazonium compound
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group R-N2+ X- where R can be any organic residue such alkyl or aryl and X is an inorganic or organic anion such as a halogen...
s of the type R-N2+.
From amines
Alpha-acceptor-substituted primary aliphatic amineAmine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s R-CH2-NH2 (R = COOR, CN, CHO, COR) react with nitrous acid
Nitrous acid
Nitrous acid is a weak and monobasic acid known only in solution and in the form of nitrite salts.Nitrous acid is used to make diazides from amines; this occurs by nucleophilic attack of the amine onto the nitrite, reprotonation by the surrounding solvent, and double-elimination of water...
to generate the diazo compound.
From diazomethyl compounds
An example of an electrophilic substitutionElectrophilic substitution
Electrophilic substitution reactions are chemical reactions in which an electrophile displaces a group in a compound, typically but not always hydrogen. Electrophilic aromatic substitution is characteristic of aromatic compounds and is an important way of introducing functional groups onto benzene...
using a diazomethyl compound is that of a reaction between an acyl halide
Acyl halide
An acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group....
and diazomethane
Diazomethane
Diazomethane is the chemical compound CH2N2. It is the simplest of diazo compounds. In the pure form at room temperature, it is a extremely sensitive explosive yellow gas, thus it is almost universally used as a solution in diethyl ether...
, for example the first step in the Arndt-Eistert synthesis
Arndt-Eistert synthesis
The Arndt-Eistert synthesis is a series of chemical reactions designed to convert a carboxylic acid to a higher carboxylic acid homologue and is considered a homologation process...
.
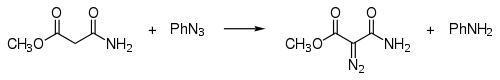
By diazo transfer
In diazo transfer certain carbon acids can be reacted with tosyl azideTosyl azide
Tosyl azide is a reagent used in organic synthesis.-Uses:Tosyl azide is used for the introduction of azide and diazo functional groups. It is also used as a nitrene source and as a substrate for [3+2] cycloaddition reactions.-Preparation:...
:
This reaction is also called the Regitz diazo transfer. Examples are the synthesis of tert-butyl diazoaceate and di-tert-butyl diazomalonate.
From N-alkyl-N-nitroso compounds
Diazo compounds can be obtained in an elimination reactionElimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
of N-alkyl-N-nitroso compounds, such as in the synthesis of diazomethane
Diazomethane
Diazomethane is the chemical compound CH2N2. It is the simplest of diazo compounds. In the pure form at room temperature, it is a extremely sensitive explosive yellow gas, thus it is almost universally used as a solution in diethyl ether...
from Diazald or MNNG:
From hydrazones
HydrazoneHydrazone
Hydrazones are a class of organic compounds with the structure R1R2C=NNH2. They are related to ketones and aldehydes by the replacement of the oxygen with the NNH2 functional group...
s are Oxidized (dehydrogenation
Dehydrogenation
Dehydrogenation is a chemical reaction that involves the elimination of hydrogen . It is the reverse process of hydrogenation. Dehydrogenation reactions may be either large scale industrial processes or smaller scale laboratory procedures....
) for example with silver oxide or mercury oxide
Mercury oxide
Mercury oxide can refer to:* Mercury oxide , Hg2O* Mercury oxide , HgO...
for example the synthesis of 2-diazopropane from acetone hydrazone. Other oxidizing reagents are lead tetraacetate, manganese dioxide and the Swern reagent. Tosylhydrazones RRC=N-NHTs are reacted with base for example triethylamine
Triethylamine
Triethylamine is the chemical compound with the formula N3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine, for which TEA is also a common abbreviation....
in the synthesis of crotyl diazoacetate and in the synthesis of phenyldiazomethane from PhCHNHTs and sodium methoxide
Sodium methoxide
Sodium methoxide is a chemical compound, with formula CH3ONa. This colourless solid, which is formed by the deprotonation of methanol, is a widely used reagent in industry and the laboratory...
.
By fragmentation
1,3-disubstituted alkyl aryl triazeneTriazene
Triazene, also known as triazanylene, is an unsaturated inorganic compound having the chemical formula N3H3. It has one double bond, and is the second simplest member of the azene class of hydronitrogen compounds, and is not found in nature. It is also the name given to the functional group...
s can be fragmentated to form diazo compounds. These triazenes (ArN=NNH-CH2R) result from coupling of aromatic diazonium salts with primary amines but the reaction type is rare.
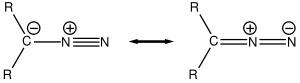
From azides
One method is described for the synthesis of diazo compounds from azideAzide
Azide is the anion with the formula N3−. It is the conjugate base of hydrazoic acid. N3− is a linear anion that is isoelectronic with CO2 and N2O. Per valence bond theory, azide can be described by several resonance structures, an important one being N−=N+=N−...
s using phosphines:
In cycloadditions
Diazo compounds react as 1,3-dipoles in diazoalkane 1,3-dipolar cycloadditionDiazoalkane 1,3-dipolar cycloaddition
The Diazoalkane 1,3-dipolar cycloaddition is a 1,3-dipolar cycloaddition between a 1,3-dipole diazo compound and a dipolarophile. When the dipolarphile is an alkene, the reaction product is a pyrazoline....
s.
As carbene precursors
Diazo compounds are used as precursors to carbeneCarbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
s, which are generated by thermolysis or photolysis, for example in the Wolff rearrangement
Wolff rearrangement
The Wolff rearrangement is a rearrangement reaction converting a α-diazo-ketone into a ketene. This reaction was first reported by Ludwig Wolff in 1912....
. As such they are used in cyclopropanation for example in the reaction of ethyl diazoacetate
Ethyl diazoacetate
Ethyl diazoacetate is a diazo compound and a reagent in organic chemistry. It was discovered by Theodor Curtius in 1883. The compound can be prepared by reaction of the ethyl ester of glycine with sodium nitrite and sodium acetate in water....
with styrene.. Certain diazo compounds can couple to form alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s in a formal carbene dimerization
Carbene dimerization
Carbene dimerization is a type of organic reaction in which two carbene or carbenoid precursors react in a formal dimerization to an alkene. This reaction is often considered an unwanted side-reaction but it is also investigated as a synthetic tool. In this reaction type either the two carbenic...
reaction.
Diazo compounds are intermediates in the Bamford-Stevens reaction
Bamford-Stevens reaction
The Bamford–Stevens reaction is a chemical reaction whereby treatment of tosylhydrazones with strong base gives alkenes. It is named for the British chemist William Randall Bamford and the Scottish chemist Thomas Stevens Stevens...
of tosylhydrazone
Tosylhydrazone
A tosylhydrazone in organic chemistry is a functional group with the general structure RR'C=NH-Ts where Ts is a tosyl group. Organic compounds having this functional group can be accessed by reaction of an aldehyde or ketone with tosylhydrazine.-Synthesis:...
s to alkenes, again with a carbene intermediate:
In the Doyle-Kirmse reaction
Doyle-Kirmse reaction
The Doyle-Kirmse reaction is an organic reaction in which in the original scope an allyl sulfide reacts with trimethylsilyldiazomethane to form the homoallyl sulfide compound. The reaction was first reported by W. Kirmse in 1968 and modified by M.P. Doyle in 1981.The Kirmse protocol required a...
certain diazo compounds react with allyl sulfides to the homoallyl sulfide. Intramolecular reactions of diazocarbonyl compounds
Intramolecular reactions of diazocarbonyl compounds
Intramolecular reactions of diazocarbonyl compounds include addition to carbon–carbon double bonds to form fused cyclopropanes and insertion into carbon–hydrogen bonds or carbon–carbon bonds.-Introduction:...
provide access to cyclopropanes.
As nucleophile
The Buchner-Curtius-Schlotterbeck Reaction yields ketones from aldehydes and aliphatic diazo compounds:The reaction type is nucleophilic addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
.
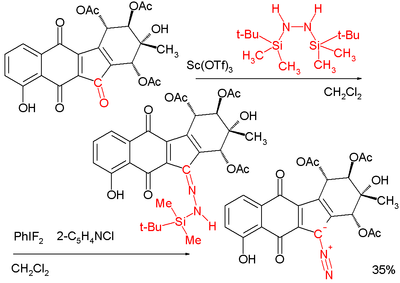
Diazo biomolecules
An unusual biomoleculeBiomolecule
A biomolecule is any molecule that is produced by a living organism, including large polymeric molecules such as proteins, polysaccharides, lipids, and nucleic acids as well as small molecules such as primary metabolites, secondary metabolites, and natural products...
sporting a diazo group was synthesized in 2006. In the final stage of the synthesis, the reaction of a carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group with the hydrazine
Hydrazine
Hydrazine is an inorganic compound with the formula N2H4. It is a colourless flammable liquid with an ammonia-like odor. Hydrazine is highly toxic and dangerously unstable unless handled in solution. Approximately 260,000 tons are manufactured annually...
1,2-bis(tert-butyldimethylsilyl)hydrazine to form the hydrazone
Hydrazone
Hydrazones are a class of organic compounds with the structure R1R2C=NNH2. They are related to ketones and aldehydes by the replacement of the oxygen with the NNH2 functional group...
is followed by reaction with the periodinane
Periodinane
Periodinanes are chemical compounds containing hypervalent iodine. These iodine compounds are hypervalent because the iodine atom in it contains more than the 8 electrons in the valence shell required for the octet rule...
difluoroiodobenzene to yield the diazo compound:
See also
- Azo compoundAzo compoundAzo compounds are compounds bearing the functional group R-N=N-R', in which R and R' can be either aryl or alkyl. IUPAC defines azo compounds as: "Derivatives of diazene , HN=NH, wherein both hydrogens are substituted by hydrocarbyl groups, e.g. PhN=NPh azobenzene or diphenyldiazene." The more...
- Diazoalkane 1,3-dipolar cycloadditionDiazoalkane 1,3-dipolar cycloadditionThe Diazoalkane 1,3-dipolar cycloaddition is a 1,3-dipolar cycloaddition between a 1,3-dipole diazo compound and a dipolarophile. When the dipolarphile is an alkene, the reaction product is a pyrazoline....
- Diazonium compoundDiazonium compoundDiazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group R-N2+ X- where R can be any organic residue such alkyl or aryl and X is an inorganic or organic anion such as a halogen...
- ReprographyReprographyReprography is the reproduction of graphics through mechanical or electrical means, such as photography or xerography. Reprography is commonly used in catalogs and archives, as well as in the architectural, engineering, and construction industries....
- WhiteprintWhiteprintWhiteprint is the commercial terminology to describe document reproduction using the diazo chemical process. It is also known as the blue-line or blue-line process...