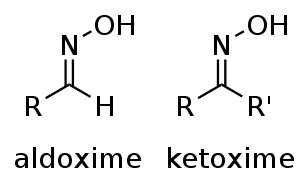
Oxime
Encyclopedia
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
belonging to the imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
s, with the general formula R1R2C
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
=N
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
O
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
H
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, where R1 is an organic
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
side chain
Side chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called "main chain" or backbone. The placeholder R is often used as a generic placeholder for alkyl group side chains in chemical structure diagrams. To indicate other non-carbon...
and R2 may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of hemiaminal
Hemiaminal
A hemiaminal is a functional group or type of chemical compound that has a hydroxyl group and an amine attached to the same carbon atom: -C-. R can be hydrogen or an alkyl group...
s with general structure RC(=NOH)(NRR').
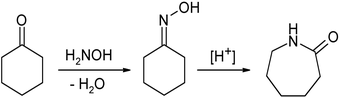
Oximes are usually generated by the reaction of hydroxylamine
Hydroxylamine
Hydroxylamine is an inorganic compound with the formula NH2OH. The pure material is a white, unstable crystalline, hygroscopic compound. However, hydroxylamine is almost always provided and used as an aqueous solution. It is used to prepare oximes, an important functional group. It is also an...
and aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s or ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s. The term oxime dates back to the 19th century, a portmanteau of the words oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and imide
Imide
In organic chemistry, an imide is a functional group consisting of two carbonyl groups bound to nitrogen. These compounds are structurally related to acid anhydrides. The relationship between esters and amides and between imides and anhydrides is analogous, the amine-derived groups are less reactive...
.
Structure and properties
Oximes exist as two geometric stereoisomers: a syn isomer and an anti isomer. Aldoximes, except for aromatic aldoximes, exist only as a syn isomer, while ketoximes can be separated almost completely and obtained as a syn isomer and an anti isomer.Oximes have three characteristic bands in the infrared spectrum
Infrared spectroscopy
Infrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
, at wavenumbers 3600 (O-H), 1665 (C=N) and 945 (N-O).
Preparation
Oximes can be synthesized by condensationCondensation reaction
A condensation reaction is a chemical reaction in which two molecules or moieties combine to form one single molecule, together with the loss of a small molecule. When this small molecule is water, it is known as a dehydration reaction; other possible small molecules lost are hydrogen chloride,...
of an aldehyde or a ketone with hydroxylamine
Hydroxylamine
Hydroxylamine is an inorganic compound with the formula NH2OH. The pure material is a white, unstable crystalline, hygroscopic compound. However, hydroxylamine is almost always provided and used as an aqueous solution. It is used to prepare oximes, an important functional group. It is also an...
. The condensation of aldehydes with hydroxylamine gives aldoxime, and ketoxime is produced from ketones and hydroxylamine. Generally, oximes exist as colorless crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
s and are poorly soluble in water. Therefore, oximes can be used for the identification of ketone or aldehyde.
Oximes can also be obtained from reaction of nitrite
Nitrite
The nitrite ion has the chemical formula NO2−. The anion is symmetric with equal N-O bond lengths and a O-N-O bond angle of ca. 120°. On protonation the unstable weak acid nitrous acid is produced. Nitrite can be oxidised or reduced, with product somewhat dependent on the oxidizing/reducing agent...
s such as isoamyl nitrite with compounds containing an acidic hydrogen atom. Examples are the reaction of ethyl acetoacetate
Ethyl acetoacetate
The organic compound ethyl acetoacetate is the ethyl ester of acetoacetic acid. It is mainly used as a chemical intermediate in the production of a wide variety of compounds, such as amino acids, analgesics, antibiotics, antimalarial agents, antipyrine and aminopyrine, and vitamin B1; as well as...
and sodium nitrite
Sodium nitrite
Sodium nitrite is the inorganic compound with the chemical formula NaNO2. It is a white to slight yellowish crystalline powder that is very soluble in water and is hygroscopic...
in acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
, the reaction of methyl ethyl ketone with ethyl nitrite
Ethyl nitrite
The chemical compound ethyl nitrite is an alkyl nitrite. It may be prepared from ethanol.Ethyl nitrite is the main ingredient in a traditional ethanol-based South African remedy for colds and flu known as Witdulsies and sold in pharmacies...
in hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
. and a similar reaction with propiophenone
Propiophenone
Propiophenone is an aryl ketone. It is a clear liquid that is insoluble in water, but miscible with methanol, ethanol, diethyl ether, benzene and toluene....
, the reaction of phenacyl chloride, the reaction of malononitrile
Malononitrile
Malononitrile, also propanedinitrile, is a nitrile, with formula CH22. Malononitrile is relatively acidic, with an pKa of 11 in water...
with sodium nitrite in acetic acid
A conceptually related reaction is the Japp-Klingemann reaction
Japp-Klingemann reaction
The Japp-Klingemann reaction is a chemical reaction used to synthesize hydrazones from β-keto-acids and aryl diazonium salts. The Reaction is named after the chemists Francis Robert Japp and Felix Klingemann....
.
Reactions
The hydrolysisHydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of oximes proceeds easily by heating in the presence of various inorganic acids, and the oximes decompose into the corresponding ketones or aldehydes, and hydroxylamines. The reduction of oximes by sodium amalgam
Sodium amalgam
Sodium amalgam, commonly denoted Na, is an alloy of mercury and sodium. The term amalgam is used for alloys, intermetallic compounds, and solutions involving mercury as a major component. Sodium amalgam is often used in reactions as strong reducing agents with better handling properties compared...
or hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
produces amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s. The reduction of aldoximes gives both primary amines and secondary amines.
Generally oximes can be changed to the corresponding amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
derivatives by treatment with various acids. This reaction is called Beckmann rearrangement
Beckmann rearrangement
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann , is an acid-catalyzed rearrangement of an oxime to an amide...
. In this reaction, a hydroxyl group is exchanged with the group that is in the anti position of the hydroxyl group. The amide derivatives that are obtained by Beckmann rearrangement can be transformed into a carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
by means of hydrolysis (base or acid catalyzed).And an amine by hoffman degradation of the amide in the presence of alkali hypoclorites at 80 degrees Celsius, the degradation is itself prone to side reactions namely, the formation of biurets or, cyanate polymers, To avoid this side reaction strict temperature control is necessary, the reaction must be conducted at sufficient temperature to isomerise the cyanate to the isocyante.
also, good solvation is also crucial to be successful. Beckmann rearrangement is used for the industrial synthesis of caprolactam
Caprolactam
Caprolactam is an organic compound with the formula 5CNH. This colourless solid is a lactam or a cyclic amide of caproic acid. Approximately 2 billion kilograms are produced annually...
(see applications below).
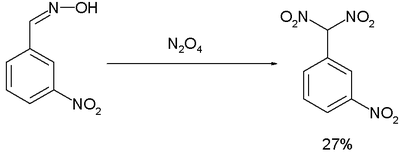
The Ponzio reaction (1906) concerning the conversion of m-nitrobenzaldoxime to m-nitrophenyldinitromethane with dinitrogen tetroxide
Dinitrogen tetroxide
Dinitrogen tetroxide is the chemical compound N2O4. It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide; some call this mixture dinitrogen tetroxide, while some call it nitrogen dioxide.Dinitrogen tetroxide is a powerful oxidizer, making it highly...
, was the result of research into TNT-like high explosives:
In the Neber rearrangement
Neber rearrangement
The Neber rearrangement is an organic reaction in which an oxime is converted into an alpha-aminoketone in a rearrangement reaction.The oxime is first converted to a ketoxime tosylate by reaction with tosyl chloride...
certain oximes are converted to the corresponding alpha-amino ketones.
Certain amidoximes react with benzenesulfonyl chloride to substituted urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
s in the Tiemann rearrangement
Uses
In their largest application, an oxime is an intermediate in the industrial production of caprolactamCaprolactam
Caprolactam is an organic compound with the formula 5CNH. This colourless solid is a lactam or a cyclic amide of caproic acid. Approximately 2 billion kilograms are produced annually...
, a precursor to Nylon 6
Nylon 6
Nylon 6 or polycaprolactam is a polymer developed by Paul Schlack at IG Farben to reproduce the properties of nylon 6,6 without violating the patent on its production. Unlike most other nylons, nylon 6 is not a condensation polymer, but instead is formed by ring-opening polymerization. This makes...
. About half of the world's supply of cyclohexanone
Cyclohexanone
Cyclohexanone is the organic compound with the formula 5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oil has an odor reminiscent of peardrop sweets as well as acetone. Over time, samples assume a yellow color due to oxidation...
, more than a billion kilograms annually, is converted to the oxime. In the presence of sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
catalyst, the oxime undergoes the Beckmann rearrangement
Beckmann rearrangement
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann , is an acid-catalyzed rearrangement of an oxime to an amide...
to give the cyclic amide caprolactam:
Other applications
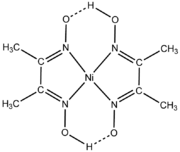
DimethylglyoximeDimethylglyoxime
Dimethylglyoxime is a chemical compound described by the formula CH3CCCH3. This colourless solid is the dioxime derivative of the diketone diacetyl . DmgH2 is used in the analysis of palladium or nickel. Its coordination complexes are of theoretical interest as models for enzymes and as catalysts...
(dmgH2) is a reagent for the analysis of nickel and a popular ligand in its own right. Typically a metal reacts with two equivalents of dmgH2 concomitant with ionization of one proton.
Pralidoxime
Pralidoxime or 2-PAM, usually as the chloride or methiodide salts, belongs to a family of compounds called oximes that bind to organophosphate-inactivated acetylcholinesterase. It is used to combat poisoning by organophosphates or acetylcholinesterase inhibitors , in conjunction with atropine and ...
(also known as 2-PAM), obidoxime
Obidoxime
Obidoxime is a member of the oxime family used to treat nerve gas poisoning. Oximes are drugs known for their ability to reverse the binding of organophosphorus compounds to the enzyme acetylcholinesterase ....
, methoxime, HI-6, Hlo-7, and TMB-4. The effectiveness of the oxime treatment depends on the particular nerve agent used. Perillartine
Perillartine
Perillartine, also known as perillartin and perilla sugar, is a sweetener that is about 2000 times as sweet as sucrose. It is mainly used in Japan.Perillartine is the oxime of perillaldehyde,which is found in Perilla frutescens ....
, the oxime of perillaldehyde
Perillaldehyde
Perillaldehyde, or perilla aldehyde, is a natural organic compound found most abundantly in the annual herb perilla, but also in a wide variety of other plants and essential oils...
is used as an artificial sweetener in Japan, as it is 2000 times sweeter than sucrose
Sucrose
Sucrose is the organic compound commonly known as table sugar and sometimes called saccharose. A white, odorless, crystalline powder with a sweet taste, it is best known for its role in human nutrition. The molecule is a disaccharide composed of glucose and fructose with the molecular formula...
. Salicylaldoxime
Salicylaldoxime
Salicylaldoxime is a chemical compound described by the formula C6H4CH=NOH-2-OH. It is the oxime of salicylaldehyde. This crystalline solid is a chelator and sometimes used in the analysis of samples containing transition metal ions, with which it often forms brightly-coloured coordination...
is a chelator. Glyoxime, produced via the condensation of glyoxal
Glyoxal
Glyoxal is an organic compound with the formula OCHCHO. This yellow colored liquid is the smallest dialdehyde . Its tautomer acetylenediol is unstable.-Production:...
with hydroxylamine
Hydroxylamine
Hydroxylamine is an inorganic compound with the formula NH2OH. The pure material is a white, unstable crystalline, hygroscopic compound. However, hydroxylamine is almost always provided and used as an aqueous solution. It is used to prepare oximes, an important functional group. It is also an...
, forms highly energetic copper, lead and silver salts (copper, lead and silver glyoximate respectively). However these compounds are too unstable to be of any commercial value. Diaminoglyoxime, a glyoxime derivative, is a key synthetic precursor, used to prepare various compounds, containing the highly reactive furazan ring.
Methyl Ethyl Ketoxime is a skin-preventing additive in many oil-based paints.